Abstract
Scarring of the kidney is a major public health concern, directly promoting loss of kidney function. In order to understand the role of microRNA (miRNA) in the progression of kidney scarring in response to injury, we investigated changes in miRNA expression in two kidney fibrosis models, and identified 24 commonly upregulated miRNAs. Among them, miR-21 was highly elevated in both animal models and human transplant kidney nephropathy. Deletion of miR-21 in mice resulted in no overt abnormality. However, miR-21-/- mice suffered far less interstitial fibrosis in response to kidney injury, which was pheno-copied in wild-type mice treated with anti-miR-21 oligonucleotides. Surprisingly, global de-repression of miR-21 target messenger RNAs was only readily detectable in miR-21-/- kidneys after injury. Analysis of gene expression profiles identified groups of genes involved in metabolic pathways that were up-regulated in the absence of miR-21, including the lipid metabolism pathway regulated by Peroxisome proliferator activated receptor-α (Pparα), a direct miR-21 target. Over-expression of Pparα prevented UUO-induced injury and fibrosis. Pparα deficiency abrogated the anti-fibrotic effect of anti-miR21 oligonucleotides. miR-21 also regulates the redox metabolic pathway. The mitochondrial inhibitor of reactive oxygen species generation, Mpv17l, was repressed by miR-21, correlating closely with enhanced oxidative kidney damage. These studies demonstrate that miR-21 contributes to fibrogenesis and epithelial injury in the kidney in two mouse models and is a candidate target for anti-fibrotic therapies.
Keywords: kidney, fibrosis, microRNA-21, PPAR-α, chronic allograft dysfunction, chronic kidney disease
Introduction
Scarring of the internal organs caused by microscopic injury is known as fibrosis. It is characterized by deposition of matrix and basement membrane structural proteins in an uncontrolled manner and in inappropriate places, often in the virtual spaces between functioning units of the organ. Tissue fibrosis is widespread in industrialized nations and contributes to morbidity and mortality (1-3). Organ fibrosis, a final common pathway of chronic or iterative tissue injury, is an inappropriate wound healing response and is frequently associated with inflammation (inflammatory cells), loss of organ function, and tissue ischemia resulting from abnormal angiogenesis. Increasing evidence indicates that fibrosis per se contributes both directly and indirectly to organ demise and that the cells that lay down matrix, known as myofibroblasts, perpetuate the fibrotic process (4,5). Organ fibrosis is seen in many common and rare diseases including diabetes mellitus, ischemic heart disease, hypertension, and chronic diseases of lung, liver, kidney, gut, heart and brain. Despite the current burden of fibrosis-related human disease, there are currently few therapies to specifically treat fibrosis.
The kidney is particularly susceptible to fibrosis perhaps because of its highly unusual vascular bed and predisposition to tissue ischemia. Many disparate diseases including diabetes mellitus, hypertension, acute injuries to the kidney (acute kidney injury [AKI]) and sequelae of organ transplantation of the kidney result in the development of either glomerular or interstitial fibrosis and are thus classified as chronic kidney diseases (CKD) or chronic allograft dysfunction (CAD) (6,7). In response to injury, the kidney epithelium, endothelium and inflammatory leukocytes can all contribute indirectly to fibrogenesis by releasing factors that signal to resident perivascular fibroblasts and pericytes, which then differentiate into scar-forming myofibroblasts, (8). Identification of factors that regulate the activation and proliferation of pericytes and perivascular fibroblasts either directly or indirectly is important for ultimately yielding new therapies for this disease.
MicroRNAs (miRNAs) are endogenously encoded, evolutionarily conserved small RNAs (∼22 base-pairs) that regulate gene expression predominantly by facilitating degradation and inhibiting protein translation of target mRNAs (9,10). To date, more than 1000 miRNAs have been identified in the human genome. MicroRNA can recognize several hundred different mRNA targets through sequence complementarity between the miRNA and binding sites in the 3′ untranslated regions (3′ UTRs) of the target mRNAs. Dysregulated miRNA expression has been identified in a wide variety of human diseases (11,12) and such dysregulation is also readily observed in animal models. Modulation of dysregulated miRNAs in vivo can attenuate the manifestation of disease suggesting that the aberrant miRNA expression contributes to disease pathogenesis(5).
In the present study, we identified dysregulated miRNAs in kidney injury and fibrosis in both animal models and human disease. Among the dysregulated miRNAs, we focused on investigating the role of miR-21 in kidney injury and fibrosis because it was upregulated consistently in human kidney fibrosis and in animal models of fibrosis and because studies in models of heart disease suggested it may play a role in cardiac fibrosis (5).
Results
MicroRNA-21 is upregulated in Kidney injury and fibrosis
To study the role of miRNAs in fibrosis we used two well-characterized models of kidney injury that result in progressive interstitial fibrosis: the unilateral ureteral obstruction (UUO) and the unilateral ischemia reperfusion injury (IRI) models in mice. The former is induced by mechanical obstruction of the flow of urine, and is characterized by a slow initial injury that accelerates with time; the latter is an excellent model of chronic kidney fibrosis resulting from the initial insult post reperfusion that causes severe injury with only partial repair and subsequent chronic injury with fibrosis. IRI leading to chronic injury with fibrosis is commonly seen in humans. In rodents, it is induced by placing a temporary occlusive clamp on the renal artery for approximately 30 minutes followed by restoration of the flow prior to surgical closure (3,13). Using Agilent microRNA microarrays, we identified a common miRNA signature in these models (Fig. 1A-B, Fig. S1A-B, Table S1) that suggested that these miRNAs were specifically regulated in response to kidney injury and fibrosis. Based on observations in other organs (5) and preliminary studies to test the efficacy of silencing several of these candidate miRNAs, we selected miR-21 as the primary target for detailed investigation. MiR-21 was highly expressed in normal kidney but was significantly upregulated in response to either UUO or IRI. The dysregulation of miR-21 was confirmed by quantitative RT-PCR (Fig. 1C). Consistent with findings reported by others (14), miR-21 was up-regulated soon after ischemia in the IRI mouse model and prior to the appearance of fibrosis suggesting its upregulation was an early response to injury. Prospectively collected biopsies from normal human kidneys and patients with acute kidney injury (AKI) or chronic allograft dysfunction (CAD) that may occur after kidney transplantation indicated normal human kidneys express miR-21 at a high level that was upregulated in both patient populations (Fig. 1D). CAD biopsies had interstitial fibrosis ranging from 11% and 65% of the area of the kidney.
Fig. 1.
MicroRNA 21 is upregulated in injury with fibrosis in mouse and human kidney. (A-B) Plots showing significantly upregulated microRNAs [red box, P < 0.001] detected on Agilent microRNA microarrays (A) after d10 of murine kidney UUO compared to normal kidney and (B) after 10d of murine unilateral kidney IRI compared with normal kidney. (C) Time course Q-PCR of whole kidney total RNA for miR-21 normalized to Sno234, from mice with UUO or IRI [** P < 0.01, * P< 0.05]. (D) RT-qPCR for miR-21 normalized to RNU19 in human kidney biopsies from patients with kidney transplantation (n= 5/group). Biopsies from healthy donor kidneys were normal or patients with CAD or transplant AKI was determined by histological assessment. (E) Q-PCR for miR-21 normalized to Sno234 from total RNA of purified endothelial cells, pericyte/myofibroblasts, macrophages and proximal epithelial cells from normal mouse kidney or d2 or d7 of UUO kidney. (F) Comparative Q-PCR from purified cells from normal kidney. (G) In situ hybridization of normal or post IRI murine kidney sections from miR-21+/+ or miR-21-/- mice for miR-21. Purple stain shows the presence of miR-21. Note in normal kidney some epithelium in medulla and papilla and some perivascular cells have miR-21 but in post IRI kidney expression of miR-21 is widespread in the kidney. [v = venule, a = arteriole, g = glomerulus, bar = 50μm] (n = 3-7/group. * P<0.05, **P<0.01).
Because recent studies have identified miR-21 as a regulator of fibrosis in the heart (5), we purified cell populations from normal and UUO-induced fibrotic kidneys to determine which cell populations upregulated miR-21 (Fig. 1E, Fig. S2). The pericyte precursors that become scar-forming myofibroblasts in kidney showed markedly upregulated miR-21. Similarly, inflammatory macrophages throughout the course of the disease model had elevated miR-21 compared with resident macrophages. MiR-21 was not increased in the injured proximal tubule epithelium and endothelial cells of the peritubular capillaries. In normal kidneys, normal proximal epithelium express miR-21 at higher basal levels than pericytes (Fig. 1F). We also performed in situ hybridization in mouse kidney sections (Fig. 1G) which showed widespread up-regulation of miR-21 throughout the kidney in response to IRI, particularly in interstitial compartments, but also in glomerular cells. These findings were similar in the UUO model, although up-regulation in the proximal epithelial compartment was less striking than in IRI (Fig. S1C). To try to reconcile the difference in the two methods of detection, we tested whether direct stimulation of mouse epithelial cells in culture would modulate miR-21. When subjected to 24h of hypoxia, a known mediator of epithelial injury, miR-21 did not change. In contrast, chronic exposure to TGFβ1, a cytokine known to drive pro-fibrotic responses in the kidney, triggered a 2-fold increase in miR-21 levels in mouse cells (Fig. S1D-E) and more than 3-fold increase in primary human tubule epithelial cells (Fig. S1F). Unlike pericytes therefore kidney epithelial cells express high levels of miR-21 basally and the absolute increase in levels in response to cell stress or injury are modest.
MicroRNA-21 targets kidney genes but only during injury
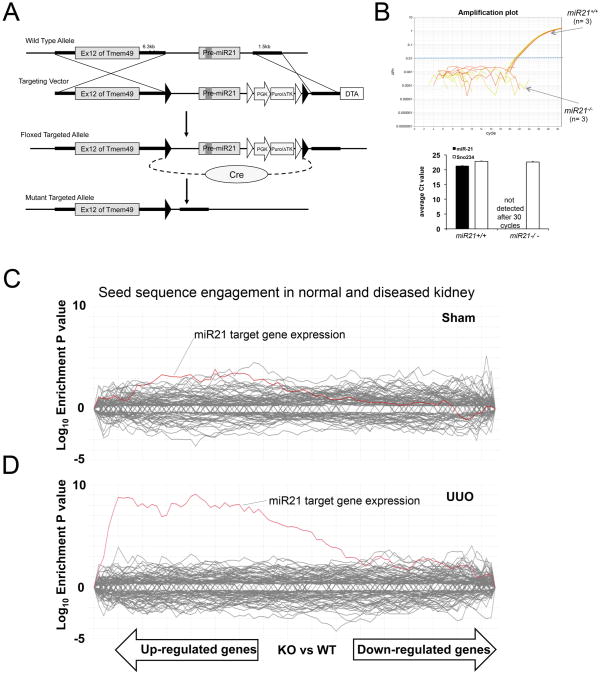
To study the role of miR-21 in the kidney, we generated mice with a targeted mutation at the miR-21 locus. MiR-21 is located in the 3′ UTR of Tmem49 (Vacuole membrane protein 1 [Vmp1]) gene (Fig. 2A-B). LoxP sites were introduced flanking Pre-miR-21, and Cre recombinase was expressed transiently in embryonic stem cells to trigger recombination prior to generation of mouse embryos. We confirmed miR-21 was not expressed (Fig. 2B) and Tmem49 transcripts were not affected by the deletion of miR-21 (Fig. S3A). MiR-21 deficient mice were healthy with normal fertility, body weight and lifespan of at least 6 months of age when housed in standard sterile conditions. Deletion of specific miRNAs can result in transcriptome wide de-repression of seed-matched target transcripts and this can be detected by analyzing global gene expression profiles (15,16) (Seed-matched segments of a transcript have Watson-Crick complementarity to positions 2-7 (known as the seed) of a miRNA). The gene expression profiles can be tissue and cell-type specific, and are used to define molecular pathways through which a miRNA functions. Surprisingly, the gene expression profiles of normal healthy kidneys from both miR21-/- and miR+/+ mice were similar and did not show a global de-repression (over-representation) of transcripts that contain putative miR-21 binding sites in their 3′ UTR (Fig. 2C). When mRNA was isolated from kidneys of miR-21+/+ and miR-21-/- littermates that had experienced tissue injury, however, the gene expression profiles showed a robust signature when miR-21 was absent (UUO, Fig. 2D) (IRI, Fig. S3B). Taken together these findings suggest that despite a high expression level in normal kidney, miR-21 regulates a limited set of target mRNAs in normal unstressed conditions. In response to kidney injury, miR-21 increases, and suppresses expression of an expanded network of target mRNAs.
Fig. 2.
Development and characterization of miR-21 targeted mutation in mice. (A) Schema showing targeting strategy for miR-21 in intron 12 of the Tmem49 gene. LoxP sites [black triangles] were inserted around Pre-miR-21 and the inserted puromycin resistance gene. Cre mediated excision was performed in ES cells prior to generation of mutant mice. (B) Taqman Amplification plot (upper) showing amplification of miR-21 in RNA extracted from miR21+/+ kidney but not miR21-/- kidney, and graph (lower) showing expression level of mature miR-21 from miR-21+/+ and miR-21-/- kidneys. The small nucleolar RNA Sno234 was used as a control. (C-D) SylArray analyses (16) showing whole kidney microarray data [30,000 genes] from miR-21-/- compared with miR-21+/+ kidneys. Each line shows the relative expression intensity [miR-21-/- relative to miR-21+/+] for all mRNA transcripts that contain a target (seed) sequence for a particular microRNA in their 3′ untranslated region. The red line identifies all mRNA transcripts with seed sequence for miR-21. In normal miR-21-/- kidney (C), there is no significant enrichment of miR-21 target genes, but in miR-21-/- kidney d7 after UUO (D) there is marked and highly significant enrichment of expression of miR-21 target gene transcripts in the microarray.
MicroRNA-21 amplifies injury and fibrosis in kidney
To investigate the role of miR-21 in kidney injury and fibrosis, we subjected miR-21-/- mice and their age and sex-matched miR-21+/+ littermates to either UUO or unilateral IRI. We compared the development of interstitial fibrosis (Fig. 3). In both cases (UUO or IRI), miR-21+/+ mice develop significantly more interstitial fibrosis than miR-21-/- mice, as detected by Sirius red staining of the kidneys (Fig. 3A-B, K-L) and by mRNA expression of major pathological matrix proteins Col1a1 and Col3a1 (Fig. 3C, M). In addition we quantified the presence of apoptotic cells in kidney injury, and these were significantly higher in miR-21+/+ injured kidneys, mainly the in renal tubule epithelium (Fig. 3A, D). Consistent with this finding, the tubule injury scores for kidney epithelium, detection of proximal tubule brush border and the detection of the proximal tubule epithelial marker, all suggested that miR-21 promotes epithelial injury (Fig. 3A, E). Similarly, the epithelial injury marker, Kim1, was consistently expressed at higher levels (Fig. 3F) and the number of myofibroblasts in miR-21+/+ kidneys was increased although the number of inflammatory macrophages in the tissues was not different (Fig. 3G, J). Consistent with the recently recognized link between fibrosis and loss of microvasculature in the kidney (4), microvascular density was reduced in diseased miR-21+/+ kidneys (Fig. 3H). These findings show that miR-21 both amplifies injury in the epithelium and stimulates myofibroblast expansion and their capacity to deposit fibrous matrix.
Fig. 3.
Gene targeted mutation indicates that microRNA 21 amplifies injury and fibrosis in the kidney. (A) Panels showing low power images of d10 UUO injured or sham treated kidneys from miR-21-/- or miR-21+/+ matched mice. (B) Quantification of Sirius red stained fibrosis in kidneys. (C) Q-PCR quantification of Coll1a1 and Coll3a1 transcripts normalized to Gapdh. (D) Number of apoptotic cells per high power field on TUNEL stained kidney sections. (E) Epithelia injury scores; either number of epithelial cells with brush border or number of tubules with cells containing brush border. (F) Western Blot showing Kim1 protein levels in miR-21-/- or miR-21+/+ UUO kidneys. (G-J) Morphometric quantification of (G) αSMA stained myofibroblasts (H) endothelial density (J) macrophages. (K) Panels showing low power images of d10 post IRI or sham treated kidneys from miR-21-/- or miR-21+/+ matched mice. (L) Quantification of Sirius red stained fibrosis in kidneys. (M) Q-PCR quantification of Coll1a1 and Coll3a1 transcripts normalized to Gapdh (n = 6-9/group. * P<0.05, **P<0.01 ***P<0.001. Bar = 100μm).
Inhibition of miR-21 in wild-type mice ameliorates injury and fibrosis
Given the attenuation of kidney injury and fibrosis in miR-21-deficient mice we sought to determine whether inhibition of miR-21 with stable, non-toxic backbone-modified oligonucleotides complementary to miR-21 (anti-miR-21) could effectively improve injury and prevent fibrosis. In vitro these anti-miR oligos effectively enhance expression of a miR-21 luciferase reporter construct with a perfectly-matched miR-21 binding site, indicating robust inhibition of miR-21 (Fig. S4). These oligonucleotides are known to have long half-lives (17) exemplified by detection of fluorescently tagged anti-miRs several days after intraperitoneal injection. By far, the greatest fluorescence intensity in the kidney was in proximal tubule epithelium, but the endothelium, pericytes, myofibroblasts and macrophages all contained detectable amounts of anti-miRs (Fig. 4A). The glomerulus, in particular podocytes, did not appear to take up significant levels of anti-miRs consistent with the known distribution of chemically-modified oligonucleotides (18) (Fig. 4A). We delivered anti-miRs systemically before and during kidney injury in a study designed to prevent fibrosis. Compared with either control anti-miR or vehicle, anti-miR-21 administration led to less kidney fibrosis in both the UUO and IRI models (Fig. 4B-C, E). Consistent with the reduction of Sirius red staining of fibrosis in kidneys of anti-miR-21-treated animals, the upregulation of fibrillar collagens Col1a1 and Col3a1 known to occur in both models was consistently attenuated specifically by anti-miR-21 (Fig. 4D, F).
Fig. 4.
Silencing microRNA 21 in vivo ameliorates fibrosis and albuminuria in the kidney. (A) Panels of normal and diseased kidney showing detection of Cy3 tagged anti-miR given by a single IP injection (20mg/kg) 7 days previously, and co-labeled for pericytes/myofibroblasts with anti-Pdgfrβ antibodies (green). Note intense red color in epithelium but also in vascular and perivascular cells (arrowheads) [g=glomerulus]. Prevention Study (panels B-F): (B-C) Low power images (B) and morphometric quantification (C) of sirius red stained kidneys for fibrosis at d10 of UUO model, in wild-type mice given anti-miR-21 or a control anti-miR by IP injection. (D) Q-PCR results for pathological collagen transcripts normalized to Gapdh in the UUO model. (E-F) Morphometric quantification (E) and Q-PCR (F) for collagen transcripts in kidneys subjected to unilateral IRI and given anti-miR-21 or a control anti-miR by IP injection. Reversal Study (panels G-K): (G-J) Fibrosis and collagen transcripts in mice subjected to unilateral IRI 5 days prior to delivery of anti-miR-21 or a control anti-miR by IP injection, and assessed at d14 after IRI. (K) Urinary Albumin:Creatinine ratio in mice after unilateral IRI and given anti-miR-21 or a control anti-miR by IP injection, followed at 7 days by removal of the healthy kidney and urine assessed at one day later (n= 7-16/group. * P<0.05, **P<0.01. Bar = 100μm).
To determine whether anti-miR-21 could also reverse an ongoing fibrotic program, we induced kidney fibrosis using IRI on day 0 and waited until day 5, 6 and 7 to treat with the anti-miR-21 or control anti-miR. On day 14, kidneys were harvested and the extent of fibrosis was assessed. Anti-miR-21 was also effective at limiting fibrosis in ongoing disease (Fig. 4G-J). To explore the functional consequences of miR-21 inhibition on injured and/or fibrotic kidneys we generated a model where blood flow was diverted to the injured kidney by removing the healthy contralateral kidney one week after triggering injury in the unilateral IRI model. The reserve of the remaining diseased kidney was examined one day later by assessing albuminuria. Mice treated with anti-miR during the first week on d2, 3 and 4, (prior to nephrectomy) showed substantially less leak of plasma albumin into urine (albuminuria) than mice treated with vehicle, at d15 (Fig. 4K). Since proteinuria after IRI is largely a result of tubular dysfunction these observations complement the findings from genetic deficiency of miR-21 showing tubular function protection (Fig. 3) and indicate that anti-miR21 administration also protects tubular function.
MicroRNA 21 regulates metabolic pathways in the injured kidney
To investigate the mechanism by which miR-21 amplifies injury and fibrosis in the injured kidney, we probed the renal gene expression profiles from the miR-21-/- and miR-21+/+ mice in response to UUO. About 700 genes were significantly de-repressed (P < 0.05) in miR-21-/- kidneys compared to those of miR-21+/+ littermates; approximately 130 of these genes contained complementary sequences to seed region of miR-21 in their 3′ UTRs (Table S2). Among the 700 de-repressed genes, we identified several major gene ontology processes (GO) (Fig. 5A). Unexpectedly, genes involved in metabolic pathways but not in immune response, cell activation, or proliferation pathways were highly enriched. The fatty acid and lipid oxidation pathways were highly enriched. Parenchymal and immune cells become activated in response to stress, injury or infection and respond by phenotypic changes which include migration, de-differentiation, production of chemokines and cytokines, and activation of novel programs of gene expression that govern function. The ERK/MAPK signaling cascade is one such pathway that governs cell activation. miR-21-/- kidneys showed significantly reduced activation of the ERK/MAPK pathway compared with miR-21+/+ kidneys, and anti-miR-21 in wild-type mice specifically inhibited kidney ERK activation (Fig. S5A-C). In particular the single pERK1 form (Fig. S5C) was reduced. These observations suggest that miR-21 drives cell activation and it does so by regulating metabolic pathways. Metabolic pathways and cell activation are therefore linked.
Fig. 5.
The lipid metabolism pathway and Pparα are miR-21 targets in kidney injury. (A) De-repressed genes from microarray analysis of UUO kidneys from miR-21-/- or miR-21+/+ mice by biological process. Green bars highlight lipid metabolism (B) Heat map (blue-low, red, high) comparing lipid metabolism genes that are over-represented in Pparα transgenic mouse muscle with miR-21 deficient UUO kidneys (C) Photomicrogaphs showing expression of Pparα in normal and UUO kidney. (D) Q-PCR for Ppara transcripts normalized to Gapdh in miR-21-/- or miR-21+/+ mice. (E-F) Western blot (F) and band intensity quantification (E) for Pparα in UUO kidneys from miR-21-/- or miR-21+/+ mice. (G-H) Q-PCR and Western blot of confluent monolayers of primary epithelial cultures for Pparα from kidneys from miR-21-/- or miR-21+/+ mice, in normal culture conditions or following 24h of hypoxia. Schema (J) and characterization (K-N) of the response of kidneys to UUO injury during over-expression of Pparα in androgen sensitive conditional KAP2-Ppara transgenic female mice. (K) Ppara expression in normal kidneys 5d after implantation of testosterone (t) or sham. (L) Q-PCR quantification of Mcad transcripts, (M) Western Blot and quantification of αSMA in sham or UUO kidneys of Kap2-Ppara or WT mice treated with testosterone and (N) Quantification of Sirius red stained fibrosis. (P-R) Characterization of the response of Ppara-/- kidneys to anti-miR21 administration. Graphs quantifying Sirius red stained fibrosis (P) and Collagen transcript quantification in UUO or sham surgery kidneys (Q), or epithelial injury scores (R) in UUO kidneys. (n= 3-7/group. * P<0.05, **P<0.01. Bar = 100μm).
In previous studies of cardiac hypertrophy, miR-21 was reported to activate ERK/MAPK signaling by repressing the tyrosine kinase inhibitor, Sprouty1 (Spry1) in cardiac fibroblasts (5). Our mRNA profiles did not suggest Spry1 as a miR-21 de-repressed gene in the UUO-injured kidney. Spry1 expression is restricted to kidney pericytes and myofibroblasts, which showed increased miR-21 levels in the UUO model (Fig. S5D-E). We tested the potential role of Sprouty1 as a target in miR-21 amplification of fibrosis by generating kidney myofibroblast cell cultures and treated them with anti-miR-21, but did not see evidence of de-repression of Spry1 (Fig. S5F). Pericytes/myofibroblasts purified from kidneys treated with anti-miR-21 did not show changes in Spry1. (Fig. S5G). Moreover, we did not see evidence of global de-repression of miR-21 target mRNAs in these purified myofibroblasts. Similarly primary myofibroblast cultures treated with anti-miR-21 did not show de-repression of target genes (Fig. S5H). These results suggest that the myofibroblast population in kidney is not a major site of miR-21 activity, despite the high level of upregulation of miR-21 in these cells (Fig. 1).
Peroxisome proliferator-activated receptor-α is an important microRNA 21 target in kidney injury
Among the many genes that were de-repressed in miR-21-/- UUO kidneys, we focused further investigation on Ppara. Pparα is a major transcription factor that regulates a number of lipid oxidation and metabolism pathways (Fig 5A, Fig. S6). Furthermore, there is a conserved octamer sequence complementary to miR-21 seed region in the 3′ UTR of Ppara (19). Consistent with the recent report that miR-21 directly regulates Ppara transcripts in endothelial cells (20), Ppara was specifically de-repressed in UUO kidneys with either miR-21 genetic deletion or pharmacological silencing. (Fig. 5B-H, Fig. S7). Moreover, Ppara was increased in epithelial cell cultures with anti-miR-21 silencing (Fig. S7A, D). We have previously demonstrated that as little as three-fold over-expression of Pparα in kidney epithelia protects against acute kidney injury as does pharmacological stimulation of Pparα activity (21). To determine whether miR-21 results in a similar regulation of signaling pathways downstream of Pparα, we compared the gene signature of over-expression of Pparα in muscle, with the gene signature of miR-21 deficiency (Fig. 5B, Fig. S6) and focused on genes involved in the lipid metabolism pathway. Nine of the genes regulated by Pparα over-expression were similarly modulated by miR-21 deletion (Fig. 5B, Fig. S6), Taken together, this links miR-21 to regulation of Ppara, which together regulate downstream lipid metabolic pathways. Pparα is highly expressed in normal kidney in both the epithelium and interstitial cells (likely myofibroblast precursors [pericytes]) (Fig. 5C, D), and in response to injury it was robustly down-regulated (Fig. 5C-D). Ppara transcripts and Pparα protein were de-repressed in miR-21-/- injured kidneys (Fig. 5D-F) and in injured wild-type kidneys treated with anti-miR-21 (Fig. S7B-C). To test the site of action of miR-21 we subjected primary cultures of miR-21-/- and miR-21+/+ kidney epithelial cells to hypoxia. Pparα was more highly expressed in miR-21-/- epithelium in both normoxic and hypoxic conditions (Fig. 5G-H). The regulation of Ppara in control (normoxic) conditions may reflect the mild activation of stress/injury pathways as a result of cell culture that we have previously reported(22). Further, under conditions of chronic TGFβ1 stimulation kidney Ppara was de-repressed in primary miR21-/- epithelial cells and primary human epithelial cells treated with anti-miR-21 (Fig. S7D).
To test the importance of Pparα directly in kidney fibrosis, we used a conditional system (testosterone-sensitive) to over-express Pparα specifically in kidney epithelium during UUO injury (Fig. 5J) (21). Administration of testosterone to transgenic mice but not control mice lead to a four-fold increase in Pparα in kidneys with UUO (Fig. 5K). This increase in Pparα caused an increase in expression of medium chain acylCoA dehydrogenase (MCAD) in UUO kidney, a fatty acid oxidation gene target of Pparα (Fig. 5L). This increase in Pparα inhibited the appearance of myofibroblasts as detected by αSMA (Fig. 5M), and inhibited the development of interstitial fibrosis as demonstrated by Sirius red staining (Fig. 5N), confirming a central role for Pparα in controlling epithelial signals to the interstitium that promote fibrosis. To understand the overall importance of Ppara as a miR-21 target in kidney pathology, we silenced miR-21 in vivo with anti-miR in Ppara-/- mutant mice. In the absence of Ppara, anti-miR-21 administration no longer protected against kidney fibrosis and proximal epithelial cell injury in UUO injury (Fig. 5P-R). Taken together, these results provide evidence that Pparα-mediated fatty acid oxidation is a key downstream effector of miR-21. In response to kidney injury, miR-21 mediated down-regulation of Pparα and alteration of lipid metabolism pathways leads to kidney injury and fibrosis.
Mpv17-like and Reversion-inducing cysteine-rich protein with Kazal Motifs (Reck) are microRNA 21 targets in kidney injury
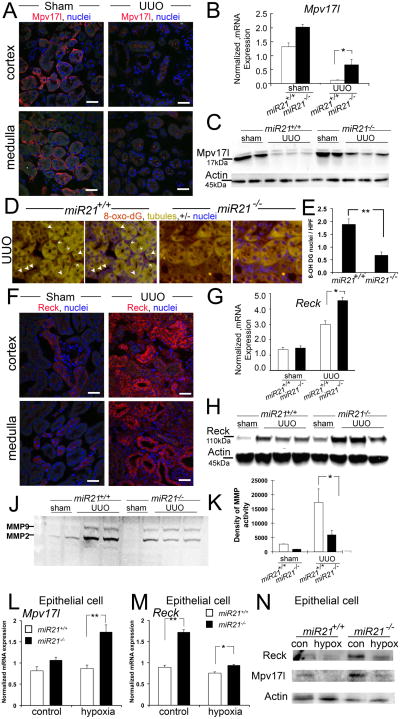
In addition to genes in lipid metabolism pathways, microarray analysis identified miR-21 target genes that regulate the redox status of the kidney (Fig. 5A, Table S1, Fig. S6). Generation of reactive oxygen species (ROS), particularly by kidney epithelium, is believed to prevent regeneration and to exacerbate injury and possibly fibrosis (23,24). Among the genes highly-regulated by miR-21 were transcripts for the protein Mpv17-like (Mpv17l also known as M-LP). Mpv17l is believed to prevent the formation of ROS (25,26). A homolog of Mpv17l, named Mpv17, was identified by random insertional mutagenesis of embryonic stem cells by the recombinant retrovirus MPVneo to identify functionally important genes. Mpv17 mutant mice have severe chronic kidney disease with 90% mortality at 4 months (27). Mpv17 is similar to peroxisomal membrane proteins, but is found in the inner mitochondrial membrane where it plays roles in metabolism of ROS (28,29). Mpv17l shares 70% sequence identity to Mpv17(30). It has been detected in peroxisomal and mitochondrial membranes and inhibits ROS generation by activating the serine protease OMI in mitochondria (26). We detected Mpv17l expression restricted to kidney epithelium (Fig. 6A), located in intracellular compartments, consistent with epithelial mitochondria, not peroxisomes. Its expression was restricted to the proximal segments of the epithelium. Like Ppara, Mpv17l transcripts and protein level were markedly down regulated after kidney injury and Mpv17l became scattered within the cytoplasm, consistent with mitochondrial expression (Fig. 6A-C). Compared with miR-21+/+ injured kidneys, miR-21-/- injured kidneys had substantially more Mpv17l (Fig. 6B-C), a finding recapitulated by injection of anti-miR-21 into wild type mice with kidney disease (Fig. S7C). Tissue sections from miR-21-/- injured kidneys showed significantly lower levels of the oxidized nucleotide, 8-hydroxy-2′-deoxyguanosine (8-oxo-DG), a marker for ROS generation (Fig. 6D-E). To confirm that epithelial cells were the cellular site of regulation of Mpv17l by miR-21, primary cultures of kidney epithelium were subjected to hypoxia (Fig. 6L, N). Mpv17l transcript and protein level were found to be regulated in epithelial cells. Collectively these studies suggest that Mpv17l may also be an important miR-21 target in kidney injury responses, and provide evidence that miR-21 amplifies ROS generation in injured kidney epithelium.
Fig. 6.
The mitochondrial redox regulator Mpv17-like and the metalloproteinase inhibitor Reck are miR-21 targets in kidney injury and are important endogenous inhibitors of injury and fibrosis. (A) Photomicrographs showing expression of Mpv17-like. (B) Q-PCR for Mpv17l transcripts normalized to Gapdh in miR-21-/- or miR-21+/+ matched mice. (C) Western blot Mpv17l in UUO kidneys from miR-21-/- or miR-21+/+ matched mice. (D-E) Images (D) and quantification (E) of ROS production as detected by the oxidized derivative of deoxyguanosine in nuclei of kidney cells in miR-21-/- or miR-21+/+matched mice. (F) Photomicrographs showing expression of Reck. (G-H) Q-PCR for Reck transcripts normalized to Gapdh (G) Western blot for Reck (H) in normal or UUO kidneys from miR-21-/- or miR-21+/+ matched mice. (J-K) Gelatin zymography (J) and band density quantification (K) of whole cell lysates from UUO kidneys from miR-21-/- or miR-21+/+ matched mice. (L-N) Hypoxia studies on primary kidney epithelial cells cultures showing Q-PCR (L-M) and Western blots (N) for Mpv17l and Reck. (n= 3-7/group. * P<0.05, **P<0.01. Bar = 100μm).
In addition to metabolic genes, the ontology data suggested that miR-21 plays a role in regulating cell migration (Table S1). A major inhibitor of cell migration, invasion and angiogenesis is the membrane anchored, metalloproteinase inhibitor Reck (31,32). There is a conserved octamer sequence complementary to miR-21 seed region in the 3′ UTR of Reck (19). Although it is structurally distinct from tissue inhibitors of metalloproteinases (Timp), it shares functions with Timp2 and Timp3 and may synergize with these inhibitors (33). Timp3 is a known miR-21 target (33), but it is not engaged significantly in the injured kidney (Fig. S5). Reck was identified intracellularly and at the brush border of proximal epithelial cells in healthy kidneys (Fig. 6F) and was readily detected in epithelial and interstitial cells in diseased kidneys (Fig. 6F). Unlike many of the target genes of miR-21, Reck is upregulated in response to kidney injury at both the transcriptional and translational level (Fig. 6G-H). In the absence of miR-21 (Fig. 6G-H) or after silencing of miR-21 in vivo (Fig. S7C), there was a substantial increase in Reck protein levels after kidney injury. The differences in protein level exceed those in transcript level. To determine the significance of this response we performed whole kidney zymography to determine metalloproteinase activity and identify major MMP forms (Fig. 6J-K). As expected, compared with normal kidneys metalloproteinase activity was increased in fibrotic disease, but the absence of miR-21 lead to decreased MMP activity, particularly MMP2. MMPs have numerous functions that contribute to disease progression (34). To confirm that epithelial cells were a significant cellular site of regulation of Reck by miR-21, primary cultures of kidney epithelium were subjected to normoxia or hypoxia, or chronic TGFβ stimulation (Fig. 6M-N, Fig. S7D). Reck was regulated at both transcript and protein level in epithelium. Under control conditions primary epithelial cultures exhibit some cell stress, which may explain the significant de-repression under these control conditions. One consequence of Reck de-repression might be enhanced inhibition of cell migration. Migration studies on primary epithelial cell cultures from miR-21-/- or miR-21+/+ kidneys showed no difference in migratory capacity in vitro in a scratch assay (miR-21-/- 57.7± 5.8% vs miR-21+/+ 63.4±6.9% wound closure p=NS). Reck is an additional, potentially important, miR-21 target gene that may inhibit kidney injury and fibrosis, but further studies are required to determine whether it plays a functional role.
Discussion
The studies presented here systematically demonstrate the involvement of miR-21 in the deleterious response of kidney injury that leads to organ fibrosis and functional demise. Using global gene expression profiling approaches we identified several major metabolic pathways as targets for the action of miR-21, in particular inhibition of lipid metabolism and enhanced oxygen radical production. Our studies identified key molecular factors in these two metabolic pathways, Pparα and Mpv17l, respectively, and showed that they are critical miR-21 targets. In addition, miR-21 down-regulates inhibitors of migration and angiogenesis, in particular the atypical MMP inhibitor, Reck resulting in enhanced MMP activity in kidney injury. Systemic administration of modified oligonucleotides that specifically silence miR-21 effectively reversed the deleterious effects of miR-21 in kidney injuries. In addition to its involvement in kidney fibrosis (35,36), several studies indicate that miR-21 may play an important role in stimulating fibrosis in cardiac and pulmonary injury (5,37). Although PPARα-regulated lipid metabolism has not previously been shown to be protective against fibrosis in direct studies, the transcription factor PPARγ has been shown in several organs to protect against fibrosis(38), implying that regulation of lipid and fatty acid oxidation is critical in fibrotic responses to chronic organ injury. The generation of ROS in kidney injury is widely held to promote organ fibrosis and demise and is a target for therapeutics(23).
MiR-21 is widely expressed in normal tissues at significant concentrations, but targeted mutation in mice as shown here and by others (39) does not lead to any obvious developmental or adult pathology suggesting that miR-21 is not important in normal tissue homoeostasis. Consistent with these findings, we showed that there is minimal downstream signal for miR-21 deficiency (lack of predicted target mRNA de-repression in miR-21-/- tissues) in healthy animals, a finding that would not be explained by compensatory mechanisms in the kidney. In contrast, we detected global de-repression of miR-21 target mRNAs in miR-21-/- kidneys in response to injury. These observations indicate that either miR-21 is sequestered and therefore inactive, or a high threshold number of copies of miR-21 in cells must be achieved before it can engage targets. Several other lines of investigation in these studies indicate that miR-21 concentrations have a non-linear relationship with functional activity. In cell types including epithelial cells or conditions where there are relatively small changes in miR-21 levels, we showed evidence of significant de-novo engagement of target genes, whereas in other cell-types including pericytes, despite large increases in miR-21 levels there is little engagement of target genes. These observations suggest that miR-21 may be compartmentalized within cells, rendering it inaccessible to target mRNA transcripts despite high total intracellular levels. Further detailed analysis is required to characterize this putative compartmentalization and sequestration of miR-21.
We, like others, found that in kidney, miR-21 also stimulates ERK/MAPK activity (5). The mechanism by which miR-21 stimulates this pathway has been investigated in cardiac injury and in cancers with disparate findings (5,39,40). Although Sprouty1 is substantially expressed by kidney pericytes (precursors of myofibroblasts), Sprouty1 is not silenced by miR-21 during kidney injury, unlike the heart. Moreover, although miR-21 is highly upregulated and expressed in kidney pericytes when they differentiate into myofibroblasts, we did not find evidence of significant gene target engagement by miR-21 in these cells in vivo or in vitro. It is likely therefore that ERK/MAPK activation by miR-21 occurs mainly in kidney epithelium, and that ERK/MAPK activation is a consequence of suppression of metabolic pathways.
These studies identified major metabolic pathways, that are highly regulated following cell stress/injury, as targets for miR-21. The miR-21 locus is within the Tmem49 (Vmp1) gene. Although Vmp1 is not a target for miR-21, Vmp1 is nevertheless a crucial transmembrane stress response protein playing roles in organelle formation, endosome formation and the development and maturation of autophagosomes (41-44). MiR-21 in kidney, targets many genes involved in peroxisome formation, and mitochondrial function, and is itself upregulated by cell stress. These studies therefore provide a clear link between the miR-21 locus and its function. Several miRNAs have already been identified within the introns of genes that are highly functionally linked (45,46).
Although metabolic pathways have not been associated with inflammation and fibrosis, a number of recent independent studies identify metabolic pathways and the genes regulating them as significantly and independently linked with the progression of chronic kidney disease in humans (47,48). The oxidation and metabolism of lipids and fatty acids by kidney epithelium is a major source of metabolic fuel for energy production in kidney tissue (49). The epithelium derives large amounts of ATP from this oxidative pathway and is likely therefore to be cytoprotective. In addition, lipid oxidation is able to de-activate a number of biologically active lipids such as eicosanoids, that may play deleterious roles.
MiR-21 regulates many genes in the lipid oxidative pathway. We identified PPARα as a major upstream regulator of lipid metabolism that is targeted by miR-21, and our findings suggest that miR-21 and PPARα coordinate activity of the same metabolic pathway. In mice deficient in Pparα, anti-miR-21 was no longer capable of ameliorating fibrogenesis, suggesting that the PPARα-dependent oxidative pathway is critical to kidney fibrogenesis and that it is upstream of other mechanisms of miR-21 action. PPARα, a member of the steroid hormone family of intracellular receptors, senses free fatty acids and their derivatives, translocates to the nucleus where it activates several genes involved in fatty acid oxidation in peroxisomes and mitochondrial compartments (50). In addition to regulating lipid metabolism, PPARα regulates glucose homoeostasis (50), interferes negatively with other nuclear signaling pathways, including AP1 and NFκB, and inhibits other pro-inflammatory genes, It may therefore be directly anti-inflammatory, as has been shown in AKI models (51). Several agonists of PPARα of the fibrate class of pharmacological agents have been used to reduce lipotoxicity in humans, and several pharmacological agents of the glitazone family that stimulate both PPARγ and PPARα are used to treat diabetes and its complications. Furthermore, in animal models of kidney disease associated with aging in rats, these agents reduce inflammation and scarring (52). Here, we show that Pparα expression three times above normal in kidney epithelium is highly protective against the development of kidney fibrosis. Moreover in IRI mouse models, a similar level of Pparα over-expression is highly protective against organ dysfunction and epithelial injury (21). Given the limitations of Pparα agonists and the side effects of currently available PPAR ligands, direct inhibition of miR-21 may be a highly desirable avenue to directly inhibit renal fibrosis.
Several studies point to PPARα in the inhibition of the generation of ROS (53). The mechanisms of this action are obscure but ROS generation occurs in peroxisomes and mitochondria where PPARα-regulated lipid oxidation occurs. The generation of ROS by injured epithelial cells is deleterious to the kidney and attempts to limit ROS generation can limit kidney injury, in circumstances where delivery of therapeutics has been possible (23) (Most anti-oxidants are extremely weak and have proven ineffective as therapeutics in humans due to inadequate delivery (54)). It is striking therefore that in our studies, preventing miR-21 activation either genetically or by use of silencing oligonucleotides also limits ROS mediated damage and injury amplification in the kidney. Mpv17l may be an endogenous gene product that inhibits ROS formation by mitochondria and is a target for miR-21. Further studies will be required to understand how important Mpv17l is as a miR-21 target and understand whether Mpv17l is regulated indirectly by Pparα signaling. Nevertheless, Mpv17l is protective in aging and age-associated kidney functional deterioration; this is due to its downregulation, which leads to greater ROS generation (28,55). We do not know whether miR-21 becomes increasingly active in aging and whether it is therefore a factor in age-related kidney loss.
Metalloproteinases (MPs) are important in basement membrane degradation and matrix degradation that are necessary for cell migration, cell invasion, cell activation and angiogenesis. All of these are features of injury responses, but persistent (inappropriate) activation of these responses is at the heart of chronic inflammation and fibrosis. Therefore although protease activity may be desirable in early injury responses, it is mostly deleterious if persistently and aberrantly activated in chronic inflammation. This runs counter to the notion that MPs are necessary for matrix degradation and therefore degradation of interstitial fibrosis. We identified the MP inhibitor Reck as a major target for miR-21. Reck is recognized for its capacity to revert transformed cell lines by preventing their migration and invasiveness. It plays crucial roles in matrix turnover and as an inhibitor of angiogenesis. In our studies, kidneys from miR-21-/- mice showed reduced MMP, activity suggesting that it was indeed functioning to limit cell activation, basement membrane degradation, dysfunctional angiogenesis and cell invasiveness but further studies will required to dissect its importance as a miR-21 target in injury with fibrosis (32).
These studies indicate that in mice miR-21 is a potentially important post-transcriptional regulator of a number of stress and injury response pathways that are initially appropriate for cell survival and permissive for inflammation but when chronically silenced, promote secondary injury. To target such pathways we have developed oligonucleotides that are safe, non-toxic and easy to administer. They are taken up in all non-glomerular kidney cells with the highest concentration in kidney epithelial cells, where they effectively silence miR-21. In mouse studies these silencing oligonucleotides were effective at limiting injury and fibrosis. Further studies are required to understand whether simple application of such silencing oligonucleotides can benefit humans at risk for the development or progression of kidney diseases.
Although previous studies in the heart indicated that miR-21 engaged target genes in the pericyte/fibroblast/myofibroblast populations (5), we were surprised to find that in the kidney, although miR-21 is highly expressed in the equivalent pericyte/fibroblast/myofibroblast cell populations, gene targets were not significantly engaged by miR-21 in these cells. In addition our studies suggest that miR-21 did not engage gene targets in inflammatory macrophages in the kidney either. The epithelial cell was a major cell site for target engagement, pointing to kidney epithelial injury (as have other studies) as a major stimulus for organ fibrosis. Since recent studies strongly suggest that epithelial cells in the kidney do not become myofibroblasts(56), further studies should identify the genes that signal from injured epithelium to the microvasculature of the kidney where the myofibroblast precursors lie.
To conclude, these studies identify miR-21 as post-transcriptional regulator in the kidney that amplifies injury responses, resulting in increased fibrosis. The major miR-21 targets in the kidney are epithelial metabolic pathways, particularly the PPARα-regulated lipid metabolic signaling pathway. Silencing of miR-21 in mice with kidney disease by systemic administration of oligonucleotides effectively limits injury and fibrosis in the kidney. Anti-miR-21 therapy is potentially useful as a treatment of chronic kidney disease and chronic allograft dysfunction in humans.
Materials and Methods
Transgenic mice
MiR-21-/- mice were generated by targeted mutation of C57BL6:129 ES cells at the Dana Farber Cancer Institute core. Coll-GFP transgenic mice were generated and validated as described on the C57BL/6 background (3). Ppara-/- mice were generated as described (57) and purchased from Jackson Laboratories. Transgenic expression of Pparα in skeletal muscle of mice (MCK-Ppara) was achieved as previously described (58). Transgenic expression of Pparα under regulation of the kidney proximal epithelial restricted and androgen sensitive KAP (kidney androgen-regulated protein) promoter was achieved with Kap2-Ppara mice which were generated and characterized as previously described (21). All studies were carried out under approved IACUC protocols held at Regulus, Harvard Medical School, University of Washington, and University of Arkansas for Medical Sciences.
Anti-microRNA administration
Adult (8-12wk) C57BL6, sex, age and weight matched WT mice or Ppara-/- mice received 20mg/kg of anti-miR-21 or 20mg/kg control anti-miR in PBS carriage medium, by IP injection. Anti-miR21 is a high affinity oligonucleotide complementary to the active site of miR-21 with a phosphorothioate backbone containing modifications at the sugar 2′ position. For disease models stopping by d7, mice received injections on d-5, d-2 and d+1 with surgery to the kidney on d0. For disease models continuing to d10 the third dose of anti-miR-21 or control was given on d+4. In some experiments Cy3-conjugated anti-miR were injected IP and kidneys were examined 7 days later.
Statistical analysis
Error bars are SE of mean. Statistical analyses were carried out with GraphPad Prism (GraphPad Software). The statistical significance was evaluated by one-way ANOVA. All miRNA and mRNA array data were analyzed using Array Studio (OmicSoft Corporation). The mRNA array data were Robust Multichip Average (RMA) normalized and then log2 transformed. ANOVA comparisons were performed on the probeset-level data and ranked from highest to lowest fold-change. An ordered list of 45101 probeset IDs was generated and submitted to SylArray (16,59), a web-based tool for detection of miRNA signatures in large-scale expression datasets. Gene Ontology (GO) analysis was performed using the DAVID bioinformatics resource (60), with the Mouse Genome 430 2 Array as background set against which enrichment of GO biological process categories was determined.
Supplementary Material
Supplementary Materials and Methods
Fig. S1. Upregulation of miRNAs and miR-21 in kidney injury models.
Fig S2: Purification of kidney cells by FACS sorting
Fig. S2. Vmp1 expression and target engagement in miR21-/- mice
Fig. S3. Inhibition of miR-21 by oligos in vitro in HELA cells detected by luciferase reporter assay.
Fig. S4. Erk phosphorylation in mir-21 knockout and anti-miR-21 treated mice, and examination of miR-21 target genes.
Fig. S5. Lipid metabolic genes are upregulated in Ppara transgenic mice.
Fig. S6. Lipid metabolic proteins are upregulated after miR-21 administration.
Tables S1. List of upregulated miRNAs.
Table S2. List of seed target matched de-repressed mRNA in UUO kidneys from miR21+/+ compared with miR21-/- mice.
Table S3. Primers used for Q-PCR.
Supplementary References
Acknowledgments
We wish to thank Dr. Victor Kotelianski (Alnylam Inc) for advice, Dr. Erwin Bottinger (Mount Sinai School of Medicine) for kindly sharing anti-Mpv17l polyclonal antibodies, Dr. Sidharth Jogani (SUNY, Upstate Medical University) for assistance with detecting fluorescently tagged anti-miR, Dr. Claudia Schrimpf (Harvard Medical School) for assistance with ischemia reperfusion experiments and Tom Hudson (University of Washington) for help with IEF & manuscript preparation. Chris Shaffer and Siegfried Hirschmann (Regulus) for providing anti-miR-21 and control oligonucleotides.
Funding: The Duffield Lab is funded by NIH Grants DK73299, DK84077, DK8739, Genzyme GRIP Award, University of Washington, and a Research agreement from Regulus therapeutics.
Footnotes
Author contributions: BNC, JSD, DAM, DP designed experiments, BNC, JSD, DAM, CX, SR, APC, GL, JL, PTT, SL, VK, XH, ANC, DP performed experiments and analyzed data, JH and SHO generated mutant mice, AK, MG collected and analyzed human samples.
Competing interests: All authors from Regulus Therapeutics have stock options in the company. JSD is on the Scientific Advisory Board at Regulus Therapeutics and the Duffield Lab has a Sponsored Research Agreement with Regulus Therapeutics. JSD is on the Scientific Advisory Board at, and has stock options in, Promedior Inc.
References and Notes
- 1.Lupher ML, Jr, Gallatin WM. Regulation of fibrosis by the immune system. Adv Immunol. 2006;89:245–288. doi: 10.1016/S0065-2776(05)89006-6. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 3.Castano AP, et al. Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci Transl Med. 2009;1:5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin SL, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 6.Racusen LC, Regele H. The pathology of chronic allograft dysfunction. Kidney Int Suppl. 2010:S27–32. doi: 10.1038/ki.2010.419. [DOI] [PubMed] [Google Scholar]
- 7.Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:353–365. doi: 10.1053/j.ackd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2010;20:297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 9.Jinek M, Doudna JA. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457:405–412. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- 10.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 11.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 12.Gao P, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin SL, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godwin JG, et al. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci U S A. 2010;107:14339–14344. doi: 10.1073/pnas.0912701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dongen S, Abreu-Goodger C, Enright AJ. Detecting microRNA binding and siRNA off-target effects from expression data. Nat Methods. 2008;5:1023–1025. doi: 10.1038/nmeth.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://www.ebi.ac.uk/enright-srv/sylarray/
- 17.Geary RS, Yu RZ, Siwkowski A, Levin AA. Pharmacokinetic/pharmacodynamic properties of phosphorothioate 2′-O-(2-Methoxyethyl)-modified antisense oligonucleotides in Animals and Man. In: Crooke ST, editor. Antisense Drug Discovery: Principles, Strategies, and Applications. CRC Press; Boca Raton: 2008. [Google Scholar]
- 18.Masarjian L, de Peyster A, Levin AA, Monteith DK. Distribution and excretion of a phosphorothioate oligonucleotide in rats with experimentally induced renal injury. Oligonucleotides. 2004;14:299–310. doi: 10.1089/oli.2004.14.299. [DOI] [PubMed] [Google Scholar]
- 19.http://www.targetscan.org
- 20.Zhou J, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, et al. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int. 2009;76:1049–1062. doi: 10.1038/ki.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichimura T, et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009;297:F461–470. doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- 24.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spinazzola A, et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet. 2006;38:570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- 26.Krick S, et al. Mpv17l protects against mitochondrial oxidative stress and apoptosis by activation of Omi/HtrA2 protease. Proc Natl Acad Sci U S A. 2008;105:14106–14111. doi: 10.1073/pnas.0801146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiher H, Noda T, Gray DA, Sharpe AH, Jaenisch R. Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell. 1990;62:425–434. doi: 10.1016/0092-8674(90)90008-3. [DOI] [PubMed] [Google Scholar]
- 28.Binder CJ, Weiher H, Exner M, Kerjaschki D. Glomerular overproduction of oxygen radicals in Mpv17 gene-inactivated mice causes podocyte foot process flattening and proteinuria: A model of steroid-resistant nephrosis sensitive to radical scavenger therapy. Am J Pathol. 1999;154:1067–1075. doi: 10.1016/S0002-9440(10)65359-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iida R, et al. Human Mpv17-like protein is localized in peroxisomes and regulates expression of antioxidant enzymes. Biochem Biophys Res Commun. 2006;344:948–954. doi: 10.1016/j.bbrc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Kaldi K, et al. Membrane topology of the 22 kDa integral peroxisomal membrane protein. FEBS Lett. 1993;315:217–222. doi: 10.1016/0014-5793(93)81167-x. [DOI] [PubMed] [Google Scholar]
- 31.Noda M, Takahashi C. Recklessness as a hallmark of aggressive cancer. Cancer Sci. 2007;98:1659–1665. doi: 10.1111/j.1349-7006.2007.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh J, et al. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001;107:789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 33.Gabriely G, et al. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeisberg M, et al. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 2006;3:e100. doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong X, Chung AC, Chen HY, Meng XM, Lan HY. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol. 2011;22:1668–1681. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zarjou A, Yang S, Abraham E, Agarwal A, Liu G. Identification of a microRNA signature in renal fibrosis: role of miR-21. Am J Physiol Renal Physiol. 2011;301:F793–801. doi: 10.1152/ajprenal.00273.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu G, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulkarni AA, et al. PPAR-gamma ligands repress TGFbeta-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: implications for therapy of fibrosis. PLoS One. 2011;6:e15909. doi: 10.1371/journal.pone.0015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patrick DM, et al. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 120:3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatley ME, et al. Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell. 2010;18:282–293. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calvo-Garrido J, Carilla-Latorre S, Lazaro-Dieguez F, Egea G, Escalante R. Vacuole membrane protein 1 is an endoplasmic reticulum protein required for organelle biogenesis, protein secretion, and development. Mol Biol Cell. 2008;19:3442–3453. doi: 10.1091/mbc.E08-01-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaccaro MI, Ropolo A, Grasso D, Iovanna JL. A novel mammalian trans-membrane protein reveals an alternative initiation pathway for autophagy. Autophagy. 2008;4:388–390. doi: 10.4161/auto.5656. [DOI] [PubMed] [Google Scholar]
- 43.Sauermann M, et al. Reduced expression of vacuole membrane protein 1 affects the invasion capacity of tumor cells. Oncogene. 2008;27:1320–1326. doi: 10.1038/sj.onc.1210743. [DOI] [PubMed] [Google Scholar]
- 44.Dusetti NJ, et al. Cloning and expression of the rat vacuole membrane protein 1 (VMP1), a new gene activated in pancreas with acute pancreatitis, which promotes vacuole formation. Biochem Biophys Res Commun. 2002;290:641–649. doi: 10.1006/bbrc.2001.6244. [DOI] [PubMed] [Google Scholar]
- 45.Najafi-Shoushtari SH, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Rooij E, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 47.Ju W, et al. Renal gene and protein expression signatures for prediction of kidney disease progression. Am J Pathol. 2009;174:2073–2085. doi: 10.2353/ajpath.2009.080888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Susztak K, et al. Molecular profiling of diabetic mouse kidney reveals novel genes linked to glomerular disease. Diabetes. 2004;53:784–794. doi: 10.2337/diabetes.53.3.784. [DOI] [PubMed] [Google Scholar]
- 49.Portilla D. Energy metabolism and cytotoxicity. Semin Nephrol. 2003;23:432–438. doi: 10.1016/s0270-9295(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 50.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Gokden N, Okusa MD, Bhatt R, Portilla D. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am J Physiol Renal Physiol. 2005;289:F469–480. doi: 10.1152/ajprenal.00038.2005. [DOI] [PubMed] [Google Scholar]
- 52.Yang HC, et al. The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol. 2009;20:2380–2388. doi: 10.1681/ASN.2008111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta. 2006;1763:1755–1766. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Nanayakkara PW, et al. Randomized placebo-controlled trial assessing a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on plasma asymmetric dimethylarginine concentration in mild to moderate CKD. Am J Kidney Dis. 2009;53:41–50. doi: 10.1053/j.ajkd.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Iida R, et al. A novel alternative spliced Mpv17-like protein isoform localizes in cytosol and is expressed in a kidney- and adult-specific manner. Exp Cell Res. 2005;302:22–30. doi: 10.1016/j.yexcr.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 56.Humphreys BD, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SS, et al. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finck BN, et al. A potential link between muscle peroxisome proliferator- activated receptor-alpha signaling and obesity-related diabetes. Cell Metab. 2005;1:133–144. doi: 10.1016/j.cmet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Bartonicek N, Enright AJ. SylArray: a web server for automated detection of miRNA effects from expression data. Bioinformatics. 26:2900–2901. doi: 10.1093/bioinformatics/btq545. [DOI] [PubMed] [Google Scholar]
- 60.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods
Fig. S1. Upregulation of miRNAs and miR-21 in kidney injury models.
Fig S2: Purification of kidney cells by FACS sorting
Fig. S2. Vmp1 expression and target engagement in miR21-/- mice
Fig. S3. Inhibition of miR-21 by oligos in vitro in HELA cells detected by luciferase reporter assay.
Fig. S4. Erk phosphorylation in mir-21 knockout and anti-miR-21 treated mice, and examination of miR-21 target genes.
Fig. S5. Lipid metabolic genes are upregulated in Ppara transgenic mice.
Fig. S6. Lipid metabolic proteins are upregulated after miR-21 administration.
Tables S1. List of upregulated miRNAs.
Table S2. List of seed target matched de-repressed mRNA in UUO kidneys from miR21+/+ compared with miR21-/- mice.
Table S3. Primers used for Q-PCR.
Supplementary References