Abstract
Although regeneration is widespread among metazoa, the molecular mechanisms have been studied in only a handful of taxa. Of these taxa, fewer still are amenable to studies of embryogenesis. Our understanding of the evolution of regeneration, and its relation to embryogenesis, therefore remains limited. Using β-catenin as a marker, we investigated the role of canonical Wnt signaling during both regeneration and embryogenesis in the cnidarian Nematostella vectensis. The canonical Wnt signaling pathway is known to play a conserved role in primary axis patterning in triploblasts. Induction of Wnt signaling with alsterpaullone results in ectopic oral tissue during both regeneration and embryogenesis by specifically upregulating β-catenin expression, as measured by qRTPCR. Our data indicate that canonical Wnt signaling is sufficient for oral patterning during Nematostella regeneration and embryogenesis. These data also contribute to a growing body of literature indicating a conserved role for patterning mechanisms across various developmental modes of metazoans.
Keywords: Nematostella, Wnt, beta-catenin, cnidaria, regeneration
INTRODUCTION
Regeneration has captivated biologists for over 250 years, and yet remains one of the least understood developmental processes. Our understanding of regeneration has grown rapidly over the past decade, due in part to technological enhancements and utilization of model organisms (e.g., planarians, hydra, and select vertebrates) tractable for studying regeneration. Yet, as is the case with embryogenesis, studying a limited number of taxa can obscure the true variety of developmental mechanisms employed across diverse animal lineages. Therefore, to gain a better understanding of the fundamental aspects of regeneration shared across metazoa with this capacity, it will be fruitful to broaden the number of taxa in which regeneration is studied. Moreover, the relationship between the developmental processes underlying embryogenesis and regeneration is poorly understood, primarily because model systems for one mode of development offer significant hurdles to investigating the other mode.
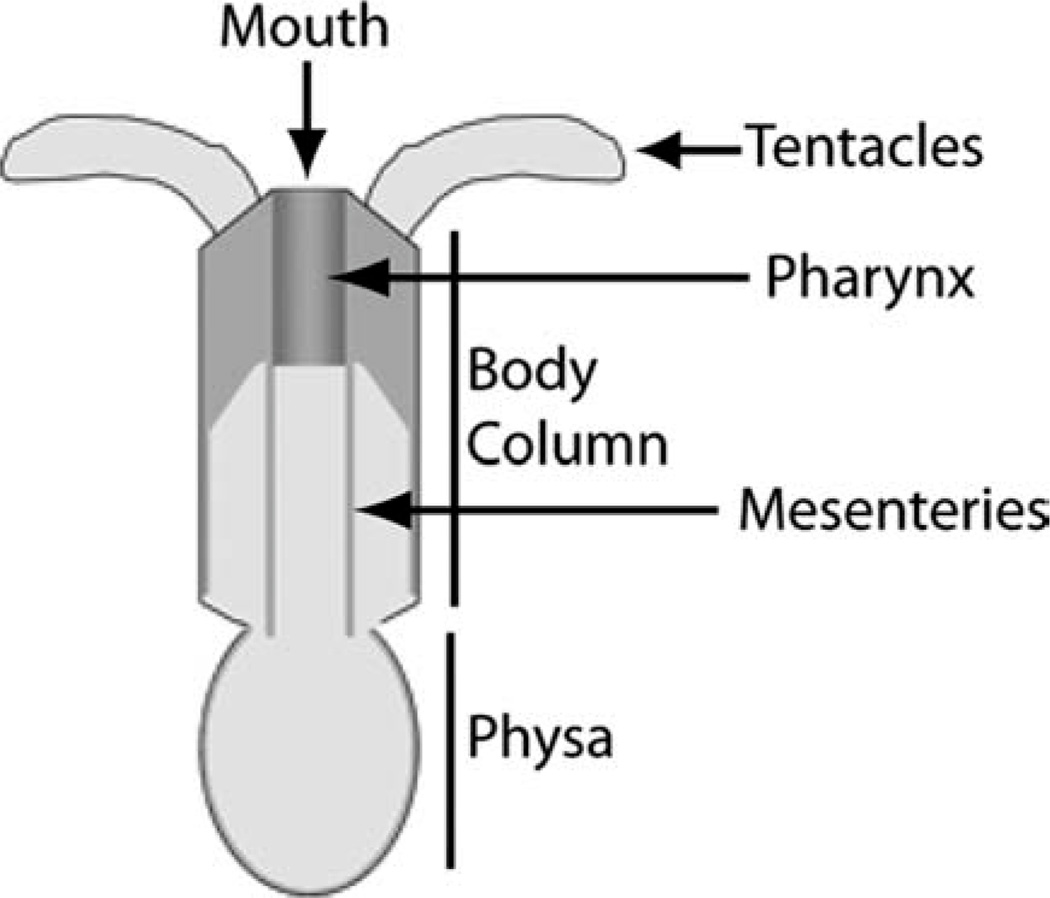
The sea anemone Nematostella vectensis is a member of the phylum Cnidaria (Class: Anthozoa). Nematostella has two body axes. The primary (oralaboral or O-A) axis extends from the mouth to the aboral pole of the adult. A secondary axis extends perpendicular to the primary axis. This secondary axis is apparent from both anatomical structures (by the presence of a siphonoglyph, a ciliated feeding structure, within the pharynx; Stephenson, 1926) and gene expression patterns (for instance, asymmetric expression of a number of developmental regulatory genes including Nv-dpp along this secondary axis) (Finnerty et al., 2004; Matus et al., 2006). Nematostella has a relatively simple body plan consisting of several major structures (Fig. 1). The main body column terminates at the oral pole with the mouth.
Fig. 1.
Nematostella body plan.
Immediately aboral to the mouth is the pharynx. Mesenteries, which contain the digestive and reproductive cells, extend from the pharynx along the body column terminating at the physa, which constitutes the aboral end of the anemone. Tentacles extend from the oral pole and function in prey capture. Nematostella is capable of both sexual and asexual reproduction as well as regeneration of the primary axis. Investigation into Nematostella development has led to important insights into the evolution of a variety of developmental processes, conserved regulatory genes, and signaling pathways during both embryogenesis (e.g., Finnerty, 2003; Wikramanayake et al., 2003; Lee et al., 2007; Wijesena et al., 2011) and regeneration (Burton and Finnerty, 2009). Thus, Nematostella is an excellent model for investigating regeneration and its relationship to embryogenesis.
The canonical Wnt signaling pathway plays a conserved role in patterning the primary axis (Anterior-Posterior or A-P) of a variety of triploblasts during both regeneration (Gurley et al., 2008) and embryogenesis (Huelsken et al., 2000). Despite no clear homology between the A-P axis of triploblasts and the O-A axis of cnidarians, canonical Wnt signaling also appears to play a similarly fundamental role in establishing the primary body axis in cnidarians. Indeed, the role of canonical Wnt signaling in patterning the cnidarian primary axis is supported by studies in both major cnidarian branches, the anthozoa and medusozoa. In medusozoans, β-catenin (a downstream component of the canonical Wnt pathway) has been implicated in O-A patterning during embryogenesis and/or regeneration in Hydra (Hobmayer et al., 2000; Broun et al., 2005; Gee et al., 2010), Hydractinia (Duffy et al., 2010), and Clytia (Momose et al., 2008). In anthozoans, our understanding of the role of Wnt signaling comes primarily from studies on embryogenesis in Nematostella, in which it plays a role in establishing axial polarity and germ layer specification. Specifically, in Nematostella, β-catenin proteins are differentially stabilized along the O-A axis leading to selective degradation of Nv-β-catenin at one pole, and stabilization and nuclear localization at the other pole (Wikramanayake et al., 2003). Nuclear localization of Nv-β-catenin occurs at the site of gastrulation, which marks the future oral end of the animal, and is observed in cells that form the entoderm. Induction of Nv-β-catenin nuclear localization with lithium chloride results in elongated planulae defective for the ability to make the ectodermal components of the pharynx and tentacles. LiCl-treated planulae, therefore, exhibit overproliferation of entodermal tissue (Wikramanayake et al., 2003).
We investigated the role of the canonical Wnt pathway during anthozoan regeneration by exposing regenerating adult Nematostella to alsterpaullone, a potent inhibitor of the Wnt pathway component Glycogen Synthase Kinase-3-β (GSK3β) across taxa (Leost et al., 2000). Thus, alsterpaullone acts on the canonical Wnt signaling pathway through inhibiting the cytosolic degradation of β-catenin. Our results indicate that β-catenin is sufficient to generate an ectopic oral pole in regenerating tissue. We also observed a similar effect during embryogenesis. In contrast, uninjured adults showed no response to alsterpaullone. These results indicate that the canonical Wnt signaling pathway plays a consistent role in oral entodermal patterning across Nematostella developmental modes. Our results also demonstrate the tractability of performing functional assays using a small molecule inhibitor across developmental modes in Nematostella.
RESULTS AND DISCUSSION
Nv-β-Catenin Induces Oral Fates During Cnidarian Regeneration
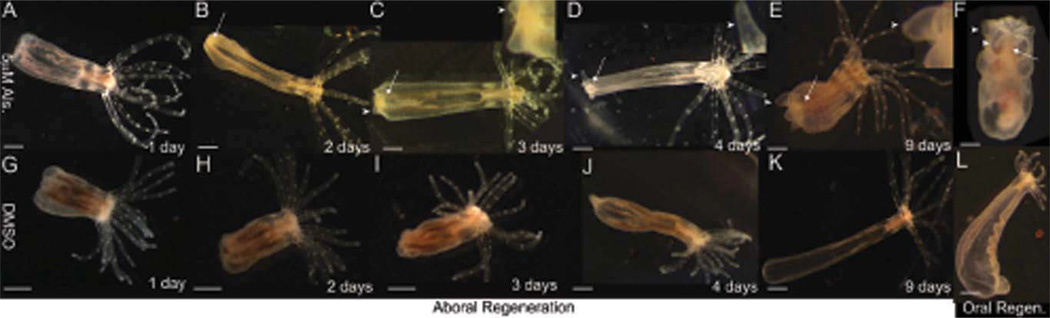
To determine the effect of alsterpaullone on regeneration of the physa, we bisected adult polyps midway along the primary axis and incubated the oral half in either 5 µM alsterpaullone or DMSO controls. Rather than regenerating the physa as observed in controls (Fig. 2g–k), polyps incubated in alsterpaullone developed ectopic oral poles at the site of bisection (Fig. 2a–e). A mouth and pharynx were visible as early as 2 days post-amputation (dpa) (Fig. 2b) with tentacle buds apparent 3–4 dpa (Fig. 2c,d, see insets). Tentacle buds continued to grow throughout the experiment, but failed to elongate properly and instead increased in width (Fig. 2d–f, see insets).
Fig. 2.
Canonical Wnt signaling induces oral fates during regeneration. Adult polyps were bisected and placed in 1/3× artificial sea water containing either 5 µM alsterpaullone (A–E: oral halves; F: aboral) or DMSO (G–K: oral halves; L: aboral) for 1–9 days. A–E: Polyps undergoing aboral regeneration in the presence of alsterpaullone developed an ectopic oral pole complete with a mouth, pharynx (arrows), and tentacle buds (arrowheads) rather than a physa (G–K). The tentacle buds fail to elongate (compare to L). F: Polyps undergoing oral regeneration in the presence of alsterpaullone formed an oral pole similar to controls (L) but also failed to elongate their tentacles. Aboral regeneration refers to regeneration of lost aboral structures. Scale bar = ≈ 1 mm.
The abnormal tentacle development described above could be the result of either alsterpaullone exposure, or a retardation of cell re-specification due to the ectopic location of these tentacles. To distinguish between these hypotheses, we investigated the effect of alsterpaullone on the aboral halves of polyps following bisection. These individuals would normally regenerate oral structures in the absence of alsterpaullone. While such individuals regenerated normally in DMSO (Fig. 2l), the tentacle buds failed to elongate in samples continually incubated in alsterpaullone (Fig. 2f), similar to those above (Fig. 2e), indicating that the alsterpaullone is the cause of abnormal tentacle development rather than the ectopic location.
To confirm that alsterpaullone was repressing tentacle elongation through continual exposure, we induced secondary oral pole development by incubation in alsterpaullone for 2 days, then allowed further development to occur in its absence. Polyps developing in this fashion formed complete secondary oral poles with morphologically normal tentacles within 9 days (not shown, but see Fig. 5a). Thus, alsterpaullone promotes mouth, pharynx, and tentacle bud formation but represses tentacle elongation during regeneration.
Fig. 5.
Effects of alsterpaullone during wound healing and adult growth. A,B: Adults Nematostella exposed to alsterpaullone for 48 hr following injury midway along their primary axis develop ectopic oral poles at the site(s) of injury (A, one injury; B, two injuries). C: Uninjured adults exposed to alsterpaullone show no morphological response. Scale bar = ≈ 1 mm.
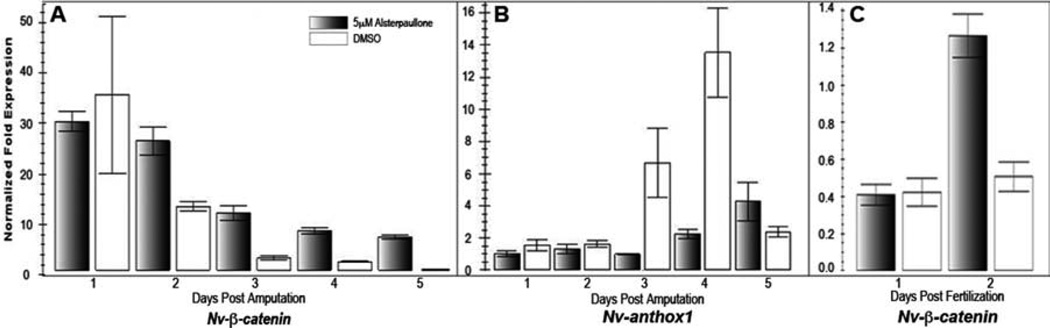
As previously indicated, alsterpaullone is a potent inhibitor of GSK3β, and therefore an activator of the canonical Wnt pathway (Leost et al., 2000). Since a hallmark of canonical Wnt signaling is nuclear translocation of β-catenin, we attempted to confirm that this molecular effect is conserved in Nematostella upon exposure to alsterpaullone. We attempted to visualize Nv-β-Catenin localization during regeneration and embryogenesis. Unfortunately, privately developed antibodies previously employed in cnidarians (Wikramanayake, 2003; Broun et al., 2005) were not available and a commercial antibody proved non-specific in both Western and whole mount application (data not shown). However, the expression of several components of the Wnt signaling pathway has been shown to be autoregulated (e.g., fz, Muller et al., 1999; arrow, Wehrli et al., 2001; tcf1, Roose et al., 1999; gsk3, Zhang et al., 2003). Therefore, we assessed Nv-β-catenin expression levels as a proxy for measuring Wnt activity. Beginning 2dpa, Nv-β-catenin expression levels were at least two-fold higher in regenerating polyps exposed to alsterpaullone as compared to controls (Fig. 3a). These results were consistent across three independent experiments (data not shown). Likewise, expression of the aboral marker Nv-anthox1 (Finnerty et al., 2004) was repressed in alsterpaullone treated samples 3–4dpa (Fig. 3a), consistent with a transition from an aboral to oral pole in the presence of alsterpaullone.
Fig. 3.
Alsterpaullone specifically promotes Nv-β-catenin expression. A: Regenerating polyps exposed to alsterpaullone express Nv-β-catenin at levels at least two-fold higher than DMSO controls after 2 days of regeneration. B: Regenerating polyps exposed to alsterpaullone show decreased expression of the aboral marker Nv-anthox1 after 3–4 days of regeneration. C: Similar to regenerating adults, embryos exposed to alsterpaullone also display increased Nv-β-catenin expression after 2 days of development. For all experiments, expression levels are normalized to Nv-hsp70 expression. Individual bars represent mean expression across three qRTPCR reactions. Error bars are one standard error. Results were consistent across three independent experiments.
The role of canonical Wnt signaling as an inducer of oral fates is conserved across non-embryonic developmental modes in cnidarians. Canonical Wnt signaling is involved in oral tissue formation during asexual budding in Hydra (Hobmayer et al., 2000; Lengfeld et al., 2009), and is sufficient to induce oral tissue formation during regeneration (Lengfeld et al., 2009). Additionally, activation of Wnt downstream events is sufficient to induce oral tissue development and repress aboral structures during metamorphosis and regeneration in Hydractinia (Duffy et al., 2010). Taken together, these results indicate that the ectopic oral tissue development during regeneration in Nematostella, Hydractinia and Hydra is due to canonical Wnt signaling that is activated through alsterpaullone-induced inhibition of GSK3β. Therefore, these data support a conserved role for the canonical Wnt signaling pathway in oral patterning of adult developmental modes (regeneration and asexual reproduction) across Cnidaria.
Nv-β-Catenin Induces Oral Fates During Embryogenesis
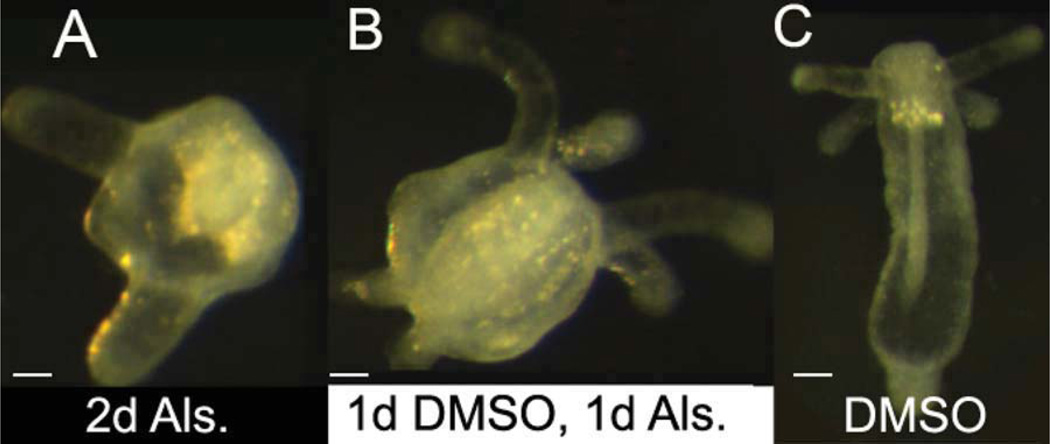
The effects of canonical Wnt signaling during Nematostella embryogenesis have been previously reported (Wikramanayake et al., 2003). The authors used LiCl (a semi-specific inhibitor of GSK3β) to promote Nv-β-catenin function. They found that LiCl-treated planulae exhibit overproliferation of entodermal tissue (Wikramanayake et al., 2003). We confirmed that alsterpaullone has similar effects by exposing zygotes for the first 48 hr of development. Relative to controls, these embryos developed enlarged tentacle buds and delayed metamorphosis (Table 1, Fig. 4a,c). Thus, embryos exposed to GSK3β inhibitors during cleavage exhibit overproliferation of entodermal tissue at the expense of ectoderm, similar to regeneration.
TABLE 1.
Effects of Alsterpaullone (Als.) on Nematostella Embryogenesis
| Initial treatment | |||
|---|---|---|---|
| 2 days, Als. (n = 22) |
1 day DMSO, 1 day ALS (n = 102) |
2 days, DMSO (n = 97) |
|
| % Metamorphosis | 50 | 40 | 83 |
| % Ectopic oral pole | 5 | 60 | 0 |
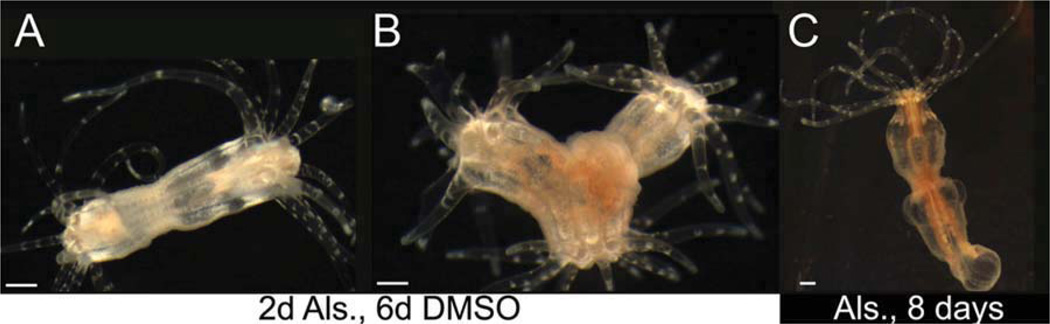
Fig. 4.
Canonical Wnt signaling induces oral, entodermal structures. A: Embryos exposed to alsterpaullone for the first 48 hr of embryogenesis develop excess entoderm after 10 days of development, including increased tentacle bud size as well as delayed metamorphosis relative to controls (C). B: Sixty percent of embryos exposed to alsterpaullone for one day, beginning 24 hr post-fertilization, develop an ectopic oral pole with a continuous physa by the tenth day of development (see also Table 1). All embryos are 10 days old. Times listed indicate initial treatment. Scale bar = ≈ 50 µm.
Although the embryos above exhibited increased entoderm, this initial experiment did not induce ectopic tentacles as predicted. We wondered whether this was related to the timing of Nv-β-catenin nuclear localization, which normally occurs during early cleavage (Wikramanayake et al., 2003). To test this, we exposed embryos to alsterpaullone beginning 24 hr post-fertilization, which is later than published reports of asymmetric nuclear localization (Wikramanayake et al., 2003). In contrast to embryos exposed immediately following fertilization, 60% of embryos exposed to alsterpaullone 24 hr after fertilization developed an ectopic oral pole (Table 1, Fig. 4b). These results are consistent with regeneration, where ectopic poles are observed in the aboral portion of the regenerating polyp, a region where Nv-β-catenin nuclear localization would not normally occur.
We also confirmed the molecular effect of alterpaullone in embryos. Despite lower overall gene expression, embryos exposed to alsterpaullone showed increased Nv-β-catenin expression levels after 2 days relative to controls (Fig. 3c), as was the case for regenerating adults.
These data are consistent with previous reports of components of the canonical Wnt pathway patterning the O-A axis in cnidarian embryogenesis. Wnt and Tcf are asymmetrically localized in Hydractinia oocytes (Plickert et al., 2006), as are CheFz1, CheFz3, and CheWnt3 in Clytia embryos (Momose and Houliston, 2007; Momose et al., 2008). In Nematostella, multiple Wnts are expressed in overlapping domains along the oral-aboral axis of the embryo in a tissue layer–specific manner (Kusserow et al., 2005).
Wnt Signaling and Nematostella Life History
Based on the above results, we hypothesized that promoting canonical Wnt signaling is sufficient to induce oral fates at any point during Nematostella life history. We, therefore, examined the morphological response to alsterpaullone during two additional developmental stages: wound healing and adult growth. Consistent with this hypothesis, adults wounded midway along their primary axis and exposed to alsterpaullone for 48 hr developed ectopic oral poles at the site of wound healing (Fig. 5a,b). In contrast, Nematostella undergoing homeostatic adult growth failed to respond morphologically, even after 8 days of continuous alsterpaullone exposure (Fig. 5c). Unlike Hydra, adult Nematostella do not contain a growth zone and are, therefore, not in a constant state of body patterning (Burton, unpublished data). It is possible that uninjured adult Nematostella do not possess cells of the appropriate developmental identity to respond to canonical Wnt pathway signals. We hypothesize that such Wnt-competent cells are generated during the initial response to injury.
Evolution of Wnt Signaling Across Developmental Modes
The molecular mechanisms underlying primary axis patterning during embryogenesis and, more recently, regeneration are well known. However, the model systems used for embryogenesis (e.g., mice, flies, nematodes) and regeneration (e.g., Hydra, planarians) are generally refractory to studies of the alternate developmental mode. Thus, comparisons of the molecular mechanisms underlying axial patterning across developmental modes rely on interspecies comparisons, which are complicated by evolutionary divergence in morphology and genome content (e.g., no clear relationship between the primary axis of triploblasts and cnidarians has been established).
Within species comparisons of Wnt signaling across non-embryonic developmental modes have occurred in at least three species. During planarian regeneration and adult growth, canonical Wnt signaling promotes posterior development (Gurley et al., 2008). In the acoel genus Convolutriloba, inhibition of Wnt signaling is essential in establishing primary axis polarity during regeneration, asexual fission, and adult growth (Sikes and Bely, 2010). In Hydra, Wnt signaling promotes oral fates during regeneration, budding, and adult growth (Hobmayer et al., 2000; Broun et al., 2005; Guder et al., 2006; Lengfeld et al., 2009; Gee et al., 2010). Unfortunately, embryonic mechanisms of axial patterning have not been investigated in any of these taxa.
The data presented here are the first comparing the role of canonical Wnt signaling between regeneration and embryogenesis within a single species. These data support a role for canonical Wnt signaling in primary axis patterning across regeneration and embryogenesis in the cnidarian common ancestor. Wnt signaling is implicated in embryonic primary axis patterning in triploblasts (Petersen and Reddien, 2009), as well as a variety of non-triploblastic taxa including sponges (Adamska et al., 2007), cnidarians (Wikramanayake et al., 2003; Plickert et al., 2006; Lee et al., 2007; Momose et al., 2008; Wijesena et al., 2011), and ctenophores (Pang et al., 2010). This is also true for non-embryonic developmental modes (Hobmayer et al., 2000; Broun et al., 2005; Duffy et al., 2010). The role of the canonical Wnt pathway across developmental modes and phyla is consistent with the hypothesis of an ancestral role in primary axis patterning in the common ancestor of Metazoa.
EXPERIMENTAL PROCEDURES
Animal Husbandry
Anemones were cultured in non-circulating 1/3 strength artificial sea water (ASW) at room temperature with weekly water changes. Adults were fed frozen sea scallops daily. Embryos were acquired following observed spawning events.
Phenotypic Response Experiments
Adult Nematostella vary in size from 0.5 cm to well over 10 cm in length. Because we wanted to use the same size polyps for both observation of morphology and molecular studies, we used only polyps of 1.0 cm in relaxed length or less. Thus, regenerating tissue comprised approximately half of each resulting fragment.
For induction of oral fates, individuals were placed in 1/3× ASW containing 5 µM alsterpaullone dissolved in DMSO. Control samples were incubated in equal concentrations of DMSO only. Development was allowed to proceed for up to 2 weeks. Solutions were changed every 72 hr. Samples were observed daily for up to 2 weeks and photographed with a Zeiss Axiocam ICc 1 mounted on a Zeiss Axioskop 40 and Zeiss Discovery.V8 (Zeiss, Thornwood, NY).
To observe regeneration, each polyp was bisected at the body column/physa boundary along the O-A axis. The oral and aboral fragments were separated and randomly assigned to control or experimental treatments. In each experiment, five polyps were used for each treatment. Observational experiments were independently performed three times, with identical results.
To observe wound healing, equally sized polyps were partially (~ 50%) bisected midway along the primary axis. Wound healing experiments were performed as above, but repeated only once.
Embryogenesis
Embryos were acquired following induced spawning events as described (Fritzenwanker and Technau, 2002). Embryos were divided into control or experimental treatments and observed for 10 days (see Table 1).
Unfertilized eggs and/or dead embryos were removed from the culture medium at the time of transition from alsterpaullone exposure to normal 1/3 ASW.
qPCR
Molecular experiments on regenerating adults were performed using the equally sized polyps bisected in the same manner as above. Individuals were allowed to regenerate for 1–5 days. For each time point, three polyps were used for each treatment. RNA was isolated from whole individuals (of which approximately 50% consisted of regenerating tissue) and pooled together to ensure adequate amounts of RNA. This experiment was independently performed three times, with consistent results. We also repeated this experiment twice, using only the regenerating tissue of the polyps. These results were consistent with whole polyp experiments, though expression levels of all genes showed more variance presumably due to a rapid molecular response to injury associated with the dissection to isolate regenerating tissue (data not shown).
Total RNA was isolated from three pooled polyps using the standard PureZOL (Bio-Rad, Hercules, CA) extraction protocol with the following exceptions. Prior to processing, samples were placed in 250 µl of PureZOL for 1 hr at 4°C, then homogenized via centrifugation through a BioMasher (Cartagen, San Carlos, CA) column. RNA precipitation was performed overnight at −20°C using 250 µl of ice-cold isopropanol, 125 µl of 1.2M NaCl, and 125 µl of 0.8M Na citrate. RNA was stored at −80°C.
Two-step qRT-PCR was performed as follows. One hundred and fifty nanograms of total RNA was reverse transcribed using the ImProm-II Reverse Transcription System (Promega, Madison, WI). The resulting cDNA was amplified using iTaq Fast SYBR Green Supermix with ROX (Bio-Rad) in a CFX96 Real-Time System (Bio-Rad). Individual reactions contained 0.04 pmol of each primer and were performed in triplicate. Injury and subsequent regeneration result in variation in overall gene expression, requiring normalization to compare samples across time. Standard reference genes (e.g., gapdh and actin) proved unreliable, as previously reported (Reitzel and Tarrant, personal communication). Results were, therefore, normalized by comparing the level of target gene expression (Nv-β-catenin, Nv-anthox1) to the level of Nv-hsp70 expression for each sample, as previously performed (Reitzel et al., 2010). We also confirmed that Nv-hsp70 expression was not affected by alsterpaullone (data not shown). Each complete experiment was performed in triplicate. Primers: Nv-β-catenin, forward GCCCTGGTTAAGCTGCTTGG, reverse AGC AAGCCGAACAGCCATCT; Nv-Hsp70, forward TCGATGATCCTGGGGTAAAG, reverse CCTGCCTCGTTCACTACCTC; Nv-anthox1, forward AGGCGTCGTGGAGTTGTTCATA, reserve GCCCTGACAAAAACCTCCAAGT.
For embryonic gene expression analysis, embryos from a single spawning event were divided equally into two groups (5 µM alsterpaullone and DMSO controls) of approximately 60 embryos immediately following fertilization. For each treatment, RNA was isolated from half of the embryos at 24 hr and the remaining at 48 hr of development. All subsequent steps were performed as described above.
ACKNOWLEDGMENTS
We thank Dave Shire for his work on preliminary experiments, Sarah McAnulty for her help with embryo manipulations, and John Finnerty for supplying Nematostella vectensis, use of lab space, and general advice. We also thank Adam Reitzel for advice on qPCR experiments.
REFERENCES
- Adamska M, Degnan SM, Green KM, Adamski M, Craigie A, Larroux C, Degnan BM. Wnt and TGF-beta expression in the sponge Amphimedon queenslandica and the origin of metazoan embryonic patterning. PLoS ONE. 2007;21:e1031. doi: 10.1371/journal.pone.0001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broun M, Gee L, Reinhardt B, Bode HR. Formation of the head organizer in hydra involves the canonical Wnt pathway. Development. 2005;132:2907–2916. doi: 10.1242/dev.01848. [DOI] [PubMed] [Google Scholar]
- Burton PM, Finnerty JR. Conserved and novel gene expression between regeneration and asexual fission in Nematostella vectensis. Dev Genes Evol. 2009;219:79–87. doi: 10.1007/s00427-009-0271-2. [DOI] [PubMed] [Google Scholar]
- Duffy DJ, Plickert G, Kuenzel T, Tilmann W, Frank U. Wnt signaling promotes oral but suppresses aboral structures in Hydractinia metamorphosis and regeneration. Development. 2010;137:3057–3066. doi: 10.1242/dev.046631. [DOI] [PubMed] [Google Scholar]
- Finnerty JR. The origins of axial patterning in the metazoa: how old is bilateral symmetry? Int J Dev Biol. 2003;47:523–529. [PubMed] [Google Scholar]
- Finnerty JR, Pang K, Burton P, Paulson D, Martindale MQ. Origins of bilateral symmetry: Hox and dpp expression in a sea anemone. Science. 2004;304:1335–1337. doi: 10.1126/science.1091946. [DOI] [PubMed] [Google Scholar]
- Fritzenwanker JH, Technau U. Induction of gametogenesis in the basal cnidarian Nematostella vectensis (Anthozoa) Dev Genes Evol. 2002;212:99–103. doi: 10.1007/s00427-002-0214-7. [DOI] [PubMed] [Google Scholar]
- Gee L, Hartig J, Law L, Wittlieb J, Khalturin K, Bosch TCG, Bode HR. [beta]-catenin plays a central role in setting up the head organizer in hydra. Dev Biol. 2010;340:116–124. doi: 10.1016/j.ydbio.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Guder C, Philipp I, Lengfeld T, Watanabe H, Hobmayer B, Holstein TW. The Wnt code: cnidarians signal the way. Oncogene. 2006;25:7450–7460. doi: 10.1038/sj.onc.1210052. [DOI] [PubMed] [Google Scholar]
- Gurley KA, Rink JC, Alvarado AS. beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319:323–327. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobmayer B, Rentzsch F, Kuhn K, Happel CM, von Laue CC, Snyder P, Rothbacher U, Holstein TW. WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature. 2000;407:186–189. doi: 10.1038/35025063. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusserow A, Pang K, Sturm C, Hrouda M, Lentfer J, Schmidt HA, Technau U, von Haeseler A, Hobmayer B, Martindale MQ, Holstein TW. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. 2005;433:156–160. doi: 10.1038/nature03158. [DOI] [PubMed] [Google Scholar]
- Lee PN, Kumburegama S, Marlow HQ, Martindale MQ, Wikramanayake AH. Asymmetric developmental potential along the animal-vegetal axis in the anthozoan cnidarian, Nematostella vectensis, is mediated by Dishevelled. Dev Biol. 2007;310:169–186. doi: 10.1016/j.ydbio.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Lengfeld T, Watanabe H, Simakov O, Lindgens D, Gee L, Law L, Schmidt HA, Ozbek S, Bode H, Holstein TW. Multiple Wnts are involved in Hydra organizer formation and regeneration. Dev Biol. 2009;330:186–199. doi: 10.1016/j.ydbio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zaharevitz DW, Gussio R, Senderowicz AM, Sausville EA, Kunick C, Meijer L. Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur J Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- Matus DQ, Pang K, Marlow H, Dunn CW, Thomsen GH, Martindale MQ. Molecular evidence for deep evolutionary roots of bilaterality in animal development. Proc Natl Acad Sci USA. 2006;103:11195–11200. doi: 10.1073/pnas.0601257103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose T, Houliston E. Two oppositely localised frizzled RNAs as axis determinants in a cnidarian embryo. PLoS Biol. 2007;5:e70. doi: 10.1371/journal.pbio.0050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose T, Derelle R, Houliston E. A maternally localised Wnt ligand required for axial patterning in the cnidarian Clytia hemisphaerica. Development. 2008;135:2105–2113. doi: 10.1242/dev.021543. [DOI] [PubMed] [Google Scholar]
- Muller HAJ, Samanta R, Wieschaus E. Wingless signaling in the Drosophila embryo: zygotic requirements and the role of the frizzled genes. Development. 1999;126:577–586. doi: 10.1242/dev.126.3.577. [DOI] [PubMed] [Google Scholar]
- Pang K, Ryan JF, Mullikin JC, Baxevanis AD, Martindale MQ. Genomic insights into Wnt signaling in an early diverging metazoan, the ctenophore Mnemiopsis leidyi. Evodevo. 2010;1:10. doi: 10.1186/2041-9139-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Plickert G, Jacoby V, Frank U, Muller WA, Mokady O. Wnt signaling in hydroid development: formation of the primary body axis in embryogenesis and its subsequent patterning. Dev Biol. 2006;298:368–378. doi: 10.1016/j.ydbio.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Reitzel AM, Behrendt L, Tarrant AM. Light entrained rhythmic gene expression in the sea anemone Nematostella vectensis: The evolution of the animal circadian clock. Plos One. 2010;5 doi: 10.1371/journal.pone.0012805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, Logtenberg T, Cleavers H. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- Sikes JM, Bely AE. Making heads from tails: development of a reversed anterior-posterior axis during budding in an acoel. Dev Biol. 2010;338:86–97. doi: 10.1016/j.ydbio.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Stephenson TA. British sea anemones. London: Ray Society; 1926. [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2001;410:847–847. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wijesena N, Kumburegama S, Xu R, Wikramanayake A. Wnt signaling in the cnidarian Nematostella vectensis: insights into the evolution of gastrulation. Integr Comp Biol. 2011;51:E149–E149. [Google Scholar]
- Wikramanayake AH, Hong M, Lee PN, Pang K, Byrum CA, Bince JM, Xu R, Martindale MQ. An ancient role for nuclear beta-catenin in the evolution of axial polarity and germ layer segregation. Nature. 2003;426:446–450. doi: 10.1038/nature02113. [DOI] [PubMed] [Google Scholar]
- Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium: eEvidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]