Abstract
In nature, organic matrix macromolecules play a critical role in enhancing the mechanical properties of biomineralized composites such as bone and teeth. Designing artificial matrix analogues is promising but challenging because relatively little is known about how natural matrix components function. Therefore, in lieu of using natural components, we created biomimetic matrices using genetically engineered elastin-like polypeptides (ELPs) then used them to construct mechanically robust ELP-hydroxyapatite (HAP) composites. ELPs were engineered with well-defined backbone charge distributions by periodic incorporation of negative, positive, or neutral side chains or with HAP-binding octaglutamic acid motifs at one or both protein termini. ELPs exhibited sequence-specific capacities to interact with ions, bind HAP, and disperse HAP nanoparticles. HAP-binding ELPs were incorporated into calcium phosphate cements resulting in materials with improved mechanical strength, injectability, and anti-washout properties. The results demonstrate that rational design of genetically engineered polymers is a powerful system for determining sequence-property relationships and for improving the properties of organic-inorganic composites. Our approach may be used to further develop novel, multifunctional bone cements and expanded to the design of other advanced composites.
Keywords: elastin-like polypeptide, calcium phosphate cement, hydroxyapatite, biocomposite
Introduction
Biomacromolecules play a critical role in generating the remarkable mechanical properties found in load-bearing, biomineralized composites such as bone, teeth, and nacre.1-4 Organic phase polymers, in combination with hierarchically organized structures, result in materials with exceptional combinations of high stiffness and toughness,5-7 properties which are challenging to achieve simultaneously with conventional synthetic approaches.8,9 Despite being present at small weight percents, organic phase macromolecules play several roles: as biomineralization modulators through amorphous calcium phosphate stabilization, mineralization acceleration, and mineralization inhibition10-15; as cell-binding mediators16; and as nanoscale adhesives3,7. As adhesives, macromolecules, such as the non-collagenous proteins in bone, bind the mineral phase, transfer strain, and dissipate energy through the breaking of “sacrificial bonds”. These bonds form through non-covalent intra-chain, inter-chain, and interfacial interactions.3,17,18 Considering their importance in nature, it would be extremely beneficial to further explore the rational design of synthetic organic phase analogues. Calcium phosphate cement (CPC) bone defect fillers are commonly used biomedical materials that could benefit from incorporation of an organic phase. CPCs are synthesized by mixing of calcium orthophosphate precursor powders and an aqueous phase. After powder dissolution and reprecipitation, they form purely inorganic, brittle materials composed predominantly of hydroxyapatite (HAP), the mineral component of bones and teeth.19 They are valued for their osteoconductivity, biocompatibility, and moldability into complex defect sites.19,20 However, due to their insufficient strength, CPCs are only FDA approved for filling non-load bearing defects.21 Therefore, improvements to CPCs could expand their medically suitable application sites and reduce the need for autografts, which are limited in supply, and allografts, which can be immunogenic.22
We endeavored to emulate the mechanical enhancements observed in nature by designing biomimetic polymers and incorporating them into CPCs. The design goals were to control the polymers’ backbone-backbone and backbone-inorganic interactions which may be crucial determinants of organic phase functionality.3 Having such control allows for systematic determination of sequence-property relationships. Conventional approaches for fabricating inorganic-organic CPC composites have relied on incorporating off-the-shelf polymers or biomolecules such as gelatin or albumin.20,23-25 However, chemically synthesized polymers are limited in the complexity of primary structures that can be conveniently and accurately prepared. Meanwhile, naturally derived proteins have complex primary and tertiary structures making it difficult to deduce their sequence-property relationships. As an alternative, advanced materials can be created using genetically engineered protein based polymers (PBPs).26-30 Genetic engineering of PBPs is a remarkable toolkit which allows for full control over a polymer’s primary sequence. Thus, PBPs can have reduced sequence complexity compared to natural proteins and more tunable sequence architectures compared to chemically synthesized polymers. In addition PBPs have uniform properties owing to their monodispersity.31 These factors make PBPs ideal for achieving our design goals. This study utilized a particularly well characterized class of PBPs known as elastin-like polypeptides (ELPs). The class of ELPs we used are based on a repeating pentapeptide sequence derived from tropoelastin, (Val-Pro-Gly-Xaa-Gly), where Xaa is a guest amino acid (excluding proline).32 ELPs are elastic, resilient, biocompatible, and can be purified in high yield at low cost.33-36 ELPs exhibit a tunable thermoresponsive behavior known as the inverse temperature transition (ITT) that allows them to transition between a soluble form and an insoluble ELP-rich aggregated form when they are raised above a certain transition temperature (Tt). The Tt varies with ELP sequence and solution conditions.33,37 Furthermore, ELPs are relatively unstructured and have fairly inert backbone sequence moieties making them a blank canvas on which to modularly impart functionalities.26,27,29,34,38,39 Due to these unique, tunable properties, ELPs are promising for use as components in composite biomaterials. Here we developed novel ELP-based functional biopolymers to construct mechanically enhanced CPC-ELP nanocomposites. By controlling ELP primary structures at the molecular level through genetic engineering, we synthesized PBPs to mimic the adhesive proteins of biocomposites. We incorporated octaglutamic acid, a previously characterized HAP binding motif40, at the protein termini and positively, negatively, or neutrally charged amino acids along the polymer backbone to control the intramolecular, intermolecular, and interfacial interactions with HAP as depicted in Figure 1. Our results showed that mineral binding had the largest effect on ELP-CPC composites, altering their mechanical, rheological, and cohesive properties. ELPs that did not bind HAP had no effect despite exhibiting the expected protein-protein interactions. This study provides useful information for the future rational design of ELPs and other polymers, for further improvement of CPC properties, for synthesis of novel hard-tissue biomaterials, and for use in other engineered composites.
Figure 1. Schematic of possible ELP-ELP and ELP-HAP interactions.

A) Backbone-backbone van der Waals or hydrogen bonding. B) ELP-HAP binding C) Backbone-backbone ionic interactions. D) Ion-mediated crosslinks.
Materials and Methods
Nomenclature
The ELP backbones were composed of repeating sequences of the form [(VPGVG)2VPGXG(VPGVG)2]n where X was either valine (V) glutamate (E), or lysine (K). The backbones were named after the one letter amino acid code corresponding to the X position followed by the number of elastin-like pentapeptides in the sequence. For example, E15 corresponds to the sequence [(VPGVG)2VPGEG(VPGVG)2]3. Functional protein terminal sequences were indicated by the addition of a descriptor to the protein’s name (e.g. E8).
Genetic engineering
Cloning was performed in XL-1 Blue E. coli (Agilent Technologies, Santa Clara, CA) and constructed genes were confirmed by DNA sequencing. DNA sequences corresponding to the sequences for V5, E5, and K5 were created by annealing and ligation of synthetic oligonucleotides (IDT, Coralville, IA) (Supporting Table 1). The oligonucleotides were ligated into a pJ54 derived vector (DNA 2.0, Menlo Park, CA) (Supporting Figure 1) prepared by reaction with BamHI and Eco31I (V5) or BpiI and Eco31I (E5, K5) (Restriction enzymes from Fermentas, Glen Burnie, MD). In a typical round of cloning, the ELP insert was prepared by digestion with BamHI and Eco31I and the ELP vector was prepared with BamHI and Eco31I (Figure 2). The products were ligated by T4 DNA Ligase (New England Biolabs, Ipswich, MA) and transformed into E. coli. Cloning rounds were repeated until the V/E/K125 sequences were generated. The ELP sequences were transferred into Eco31I digested, modified pET28b vectors (EMD, Gibbstown, NJ) containing specific N-terminal and C-terminal DNA sequences. The modified vectors were created by ligation of oligonucleotides into NcoI and BamHI digested pET28b (Supporting Table 2). Detailed methods are available in the supporting information.
Figure 2. Synthesis of ELPs.

A) Schematic of ELP gene synthesis. The scheme allows for seamless gene-lengthening and reconstitution of the enzyme architecture for future analogous rounds of cloning B) Agarose gel electrophoresis of ELP genes of various defined lengths (arrows). “n” refers to the length of the ELP gene in terms of the number of pentapeptides it codes for. C) From left to right, the MALDI-MS peaks with observed and expected molecular weights in kilodaltons in parenthesis for V125 (51.76, 51.62), E125 (52.48, 52.43), V125-E8 (52.88, 52.81), and V125-E8E8 (53.91, 53.84). The K125 (52.48, 52.26) spectrum was omitted for clarity.
Protein Synthesis and Purification
The ELP plasmids were transformed into BLR(DE3) E. Coli (EMD, Gibbstown, NJ) for expression. ELP expression was performed in shake flasks of Terrific Broth supplemented with 30 μg/mL kanamycin with shaking at 225RPM and 37°C for 24 hours.36 The cells were isolated by centrifugation, resuspended in 10mM Tris-Cl, 2mM EDTA, pH 8.0 and lysed by probe sonication (Sonicator 3000, Misonix, Farmingdale, NY). The cell lysate was centrifuged at 30,000xg for 30 minutes and the soluble fraction was mixed with poly(ethlyeneimine) (PEI Mw ~ 750,000, Sigma-Aldrich) to form a 0.5% PEI solution. The PEI solution was centrifuged at 20,000xg for 15 minutes and the supernatant was subjected to 3-4 rounds of inverse transition cycling for purification.41 NaCl or ammonium sulfate was used as the precipitating salt and resuspension was performed in phosphate buffered saline, pH 7.4, during each round.37 A modified method was used for purification of V125-E8E8 (Details available in supporting information). The proteins were dialyzed into deionized water then lyophilized. MALDI-TOF MS was performed using a sinapinic acid matrix in a 30% acetonitrile, 0.1% TFA solution on an Applied Biosystems Voyager DE system.
Transition temperature measurements
ELP transition temperatures were measured using ELPs dissolved to a concentration of 10mg/mL in 20mM Tris-Cl, pH 7.4 with either 150mM NaCl or 120mM NaCl and 10mM CaCl2. 50uL solutions of each ELP were placed in an MJ Mini thermocycler (Bio-Rad, Hercules, CA) and ramped up from 10°C to 95°C in 1°C increments. At each temperature, the solutions were inspected for bulk solution turbidity. After the onset of turbidity, the temperature at which no further increases in turbidity were perceptible was designated as the transition temperature.
Hydroxyapatite binding assays
For hydroxyapatite (HAP) binding assays, each ELP was mixed with polycrystalline HAP particles (Alfa Aesar) to form 1.6mg/mL ELP, 40mg/mL HAP solutions in 20mM Tris-Cl pH 7.4. The solutions were rotated end-over-end for 24 hours at room temperature. The soluble fraction was isolated after centrifugation at 10000xg for 10 minutes. The concentration of ELP in the soluble fraction was determined using a bicinchoninic acid assay (Thermo-Scientific, Rockford, IL). Each protein was used to create its own standard curve.
Hydroxyapatite dispersion
For HAP dispersion assays, each ELP was mixed with nanoscale hydroxyapatite (nHAP) crystals. The nHAP crystals were hydrothermally synthesized from a solution of linoleic acid, sodium linoleate, ethanol, CaNO3 and Na3PO4. as previously described42 and washed thoroughly with several rounds of ethanol and 400mM sodium phosphate, pH 9 solutions to remove surfactant. 1.0mg/mL solutions of nHAP in 20mM Tris-Cl, pH 7.4 were bath sonicated for half an hour. An equal volume of 2.0mg/mL ELP solutions in the same buffer were then added to the nHAP solutions. The mixtures were sonicated for an additional 10 minutes then transferred to a tabletop for monitoring of sedimentation.
Calcium Phosphate Cement Synthesis
The components of the calcium phosphate cements (CPC) are tetracalcium phosphate (TTCP) and dicalcium phosphate anhydrous (DCPA). TTCP with an average particle size of ~15μm was purchased (Ensail Beijing Co. Ltd., Beijing, China). DCPA was synthesized from a solution of CaCO3 and H3PO4 as previously described except the reaction time was extended to 24 hours.43 The powders were thoroughly mixed at a 1:1 molar ratio. To fabricate cements, the powders were mixed in PTFE mixing bowls with ice-cold water or ELP-solutions at powder to liquid weight ratios ranging from 3:1 to 4.25:1. Washout tests were performed by hand molding the cements into spheres and immediately immersing them into a simulated physiological solution (1.15 mM CaCl2, 1.2 mM Na2HPO4, 133 mM NaCl, 50 mM HEPES, pH 7.4)) at 37°C.20 Injection testing was performed by immediately placing the CPC mixtures into a chilled syringe with an attached 16 gauge needle.
Mechanical Testing of ELP-CPC composites
The CPC mixtures were formed into cylinders 3mm in diameter and 6mm in height using stainless steel molds for compression testing or into bars 1.5mm in height, 2mm in width and 25mm in length using PTFE molds for 3-point bending tests. The mixtures were molded by hand with the aid of the blunt end of a 7/64” drill bit. The ends of the molds were covered with glass slides then placed into 37°C, 100% humidity chambers for 20 hours. The specimens were removed from their molds after 20 hours and immersed in the simulated physiological solution at 37°C for four hours prior to mechanical testing. Testing was performed on an Instron model 5544 mechanical testing machine. Samples dimensions were measured by digital calipers. All tests were performed immediately after being removed from solution. For compression tests, a crosshead speed of 1mm/min was used. For 3-point bending tests, a 20mm span and 0.5mm/min crosshead speed were used. Work-of-fracture for 3-point bending samples was calculated as the area under the load-displacement curve divided by the specimen’s cross-sectional area.20 Six or more, and five or more samples of each ELP-CPC mixture were tested for compression and bending tests, respectively. Statistical analysis was performed using one-way ANOVA and Tukey’s Honestly Significant Difference Test.
Microstructural Analysis
Immediately after mechanical testing, the samples were immersed in ethanol to halt the setting reaction then dried in a 50°C oven. The fracture surfaces of selected samples were sputter coated with gold and visualized using a scanning electron microscope (Hitachi S5000).
3. Results & Discussion
ELP Protein Design
We synthesized a set of elastin-like polypeptides (Table 1) to emulate the non-covalent interactions available to natural biocomposite proteins. Specifically, the goals of our protein design were, first, to generate ELPs which would vary in the possible backbone-backbone and backbone-ion interactions and, second, to create ELPs which would have interfacial interactions by binding to HAP. These interactions are depicted in Figure 1. We believe that satisfying these goals establishes a suitable starting library for studying sequence property relationships. To generate synthetic proteins with the desired interactions, a series of ELP genes were synthesized via a seamless, iterative cloning method which is akin to previously implemented techniques (Table 1, Figure 2A-B).28,44 The backbone sequences were transferred to expression vectors containing the desired N-terminal and C-terminal sequences in-frame. The proteins were expressed, purified via the ITC method41, and characterized for purity and monodispersity by MALDI-TOF MS (Figure 2C). Each protein was verified to be within 0.5% of its expected molecular weight. As shown in Fig 2C, the proteins span a 2kDa range from ~51-53 kDa. The resulting ELPs include uniformly neutrally (V125), negatively (E125), and positively (K125) charged backbones. The ELP length of 125 pentapeptides was chosen such that the neutral ELP would transition in the temperature range of biological interest (between room temperature and physiological temperature). The neutral backbone could then be used to investigate the effects of the temperature transition on our composites. The negatively and positively charged backbones were then designed with the same length as this would allow us to exclude polymer molecular weight as a factor in our experiments 28,44. To create the HAP binding ELPs we linked an octaglutamic acid (E8) motif to either the C or the N and C-terminal of V125 to generate V125-E8 & V125-E8E8. High concentrations of negatively charged sidechains are common in several non-collagenous proteins in bones and teeth including osteopontin, dentin matrix protein, and osteocalcin.45-47 In fact, the E8 motif is found in the sequence of bone sialoprotein. The negatively charged groups are believed to play a role in HAP binding, calcium ion binding, and mineralization regulation.46 Previous work has shown that bone binding can be conferred to short peptides or small molecule drugs by conjugating them with oligoglutamic or oligoaspartic acid sequences.40,48 In addition, previous incorporation of short binding motifs into ELPs without loss of stimuli-responsiveness suggests that functional sequences such as E8 can be modularly added into ELP sequences.49 The charged backbone ELPs, K125 and E125, were designed as starting points to investigate the effects of polymer backbones with dispersed charges. These backbones might interact ionically with each other or with solution ions. Previously, a silk-elastin polymer with similarly dispersed charged residues was shown to influence the disperson of nanoparticles in clay-protein nanocomposites.50The variety of polymer architectures and uniformity in backbone length and charge distribution demonstrates the power of using PBPs in endeavors such as this; it would be difficult to create a similar library using conventional polymer chemistry. Furthermore, the nature of the cloning method lends itself to the simple formation of different molecular weight libraries or to the formation of block-copolymer architectures in the future.
Table 1.
List of synthesized ELPs
| Name | N-terminus | Backbone | C-terminus |
|---|---|---|---|
| V125 | MSGVG | (VPGVG)125 | VPG |
| V125-E8 | MSGVG | (VPGVG)125 | VPGSEEEEEEEE |
| V125-E8E8 | MSEEEEEEEEGPG | (VPGVG)125 | VPGSEEEEEEEE |
| K125 | MSGVG | [(VPGVG)2VPGKG(VPGVG)2]25 | VPG |
| E125 | MSGVG | [(VPGVG)2VPGEG(VPGVG)2]25 | VPG |
ELP-ELP and ELP-ion interactions
We characterized protein-protein or protein-ion interactions by measuring transition temperatures (Tts) as determined by solution turbidity as seen in Table 2. Two solutions with equal ionic strengths were used. One contained 10mM Ca+2 ions while the other did not. As expected, the most hydrophobic ELPs, V125 and V125-E8, transitioned at 27°C and 33°C, respectively. V125-E8 showed a small (2 °C) response to calcium ion concentration. Under physiological conditions these proteins are above their Tts and therefore should engage in intermolecular and intramolecular hydrophobic interactions. Interestingly, in the calcium free V125-E8E8 solution, no strong turbidity change was seen up to 95°C. We believe that the charged end groups may be suppressing the transition or that its bola-amphiphilic nature may result in the formation of soluble, charge-stabilized complexes. In contrast, V125-E8E8 in the presence of calcium ions had a 40°C Tt. This suggests that if the E8 motifs are sufficiently neutralized by calcium, as may occur during CPC setting or by mineral binding, V125-E8E8 might also be near its Tt at physiological temperatures. Both E125 and K125 had Tts out of physiological temperature ranges. Unlike K125 and V125, E125 showed a calcium ion dependent shift in Tt to a lower temperature. This indicates that E125’s negatively charged side chains are specifically interacting with calcium ions and may form the type of ion-mediated sacrificial bonds depicted in Figure 1D. These types of bonds have been shown to be active in the energy dissipating mechanisms of osteopontin and in mussel byssal fibers.18,51 Finally, we created 50:50 mixtures of K125 and E125 and demonstrated that backbone-backbone charge-charge interactions such as those depicted in Figure 1C are occurring as indicated by the lower temperature transition of the mixtures compared to solutions with either protein alone.
Table 2.
List of ELP transition temperatures in °C in 20mM Tris-Cl, pH 7.4
| Name | 150mM NaCl | 120mM NaCl 10mM CaCl2 |
|---|---|---|
| V125 | 27 | 27 |
| V125-E8 | 33 | 31 |
| V125-E8E8 | No Transition | 40 |
| K125 | 51 | 51 |
| E125 | 71 | 62 |
| K125/E125 50:50 | 43 | 44 |
Interactions with HAP
We characterized sequence-dependent protein-mineral interactions using ELP-HAP binding assays (Figure 3A). In short, ELPs and HAP crystals were mixed in solution to allow binding. Afterwards, HAP and any bound ELP were separated by centrifugation and the remaining ELP concentration in solution was quantified. The binding assays confirmed that, despite the small size of the E8 motif relative to the full protein (1.25% or 2.5% of the length in amino acids), the ELPs are imparted with an ability to bind HAP. Interestingly, at the same HAP:ELP mass ratio, E125, which has 25 glutamates does not appear to bind as well as the E8 containing elastins. This may be due to greater competition for binding sites. The distributed glutamates likely lead to a more extended bound conformation for E125 compared to the E8 ELPs. In addition E8 likely has a higher affinity because binding of glutamate sidechains decreases charge-charge repulsion in the highly charged motif and places the other sidechains in close proximity to the mineral surface for cooperative binding. These results are consistent with previous studies highlighting the importance of negative charge clustering over total negative charge when determining HAP affinity.52 Although there are reports regarding the importance of cationic residues for HAP binding52,53, K125 showed little to no binding activity. Even when the ratio of HAP to ELP was increased, no increase in percent binding was observed (Results not shown). Therefore, K125 likely does not present its basic residues in a manner suitable for binding. Finally, the neutral ELP, V125, showed no binding activity to HAP. These results demonstrate that ELPs can be used to investigate sequence dependent protein binding to HAP and other materials. Future modification by genetic engineering or chemical modification will allow us to test the role of binding motif length or to perform testing on other synthetic54,55 and naturally-derived56,57 binding motifs. Engineereed HAP-binding ELPs might also be used as therapeutic agents to target HAP while presenting bioactive ligands or drug molecules.26,29,56
Figure 3. Binding assay and dispersion assay.
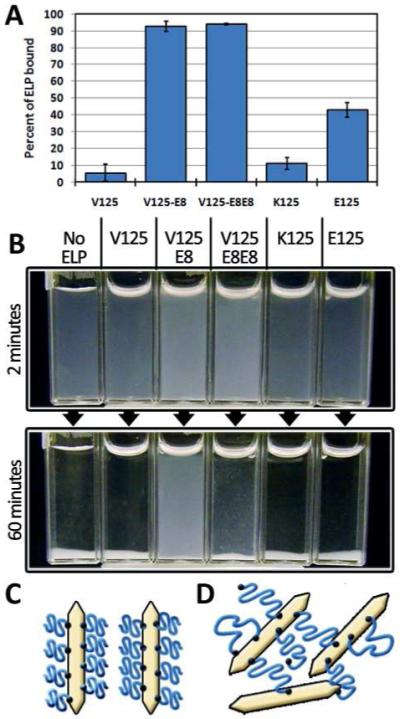
A) The octaglutamic acid terminated elastins, V125-E8 and V125-E8E8 bound well to hydroxyapatite crystals while the negatively charged ELP backbone, E125, bound significantly less. The non-negatively charged ELP, V125 and K125, showed negligible binding. B) When ELPs were mixed with nHAP in solution, only the octaglutamic acid terminated ELPs were able to noticeably inhibit sedimentation with V125-E8 having a much more pronounced dispersing effect. C) Schematic of V125-E8 binding causing steric inhibition of aggregation. D) Schematic of V125-E8E8 particle bridging.
We investigated the solution behavior of ELP-nanohydroxyapatite (nHAP) mixtures by visually observing their time dependent sedimentation behavior. Complete sedimentation occurred within 30 minutes when hydrothermally synthesized nHAP was dispersed in V125, K125, or E125 solutions. Consistent with the binding results V125-E8 and V125-E8E8 containing solutions decreased the rate of sedimentation. (Figure 3B). In contrast to a previous study using clay nanoparticle sheets, the ELPs with periodic charge (E125 and K125) had no effect on particle dispersion.50 In V125-E8 solutions there was no visible sedimentation for several hours. This can be explained by the formation of a brush layer after V125-E8 binds which sterically hinders aggregation of nHAP particles (Figure 3C). V125-E8E8 solutions also sedimented at a slower rate. We attribute this to initial binding and steric stabilization followed by polymer bridging leading to particle aggregation and sedimentation (Figure 3D).58 Like many nanoparticles, nHAP is prone to aggregation in the absence of stabilizers, limiting its effectiveness as a material for composite reinforcement.59,60 These results demonstrate that ELPs can be used as thermoresponsive colloidal dispersants for nHAP as has been shown previously for ELP-bound gold nanoparticles.61 ELP dispersed nHAP might therefore be used in nHAP reinforced composite materials.60 Meanwhile, telechelic ELPs such as V125-E8E8 may be used to bridge similar or dissimilar materials depending on the terminal amino acid sequences. This property may be useful for the development of composite hydrogel networks.62
ELP-CPC composite materials
In order to investigate the effects of our ELP constructs on the mechanical properties of ELP-HAP composites, we synthesized and characterized ELP-containing calcium phosphate cements. The HAP forming CPC formulation composed of dicalcium phosphate anhydrous (DCPA) and tetracalcium phosphate (TTCP) was used.63 In the absence of ELPs, the maximum powder to liquid (P/L) mass ratio which allowed for suitable mixing and handling was 3:1. 10 wt% solutions of ELPs were used for fabricating CPC samples because higher percentages became difficult to handle due to their viscosity. When 10 wt% solutions of V125-E8 and V125-E8E8 were mixed with the CPC powders there were significant changes to the cements’ rheological and mechanical properties. CPC powders mixed with water at a 3:1 P/L ratio formed moldable putties, while, at the same ratio, E8 containing ELPs formed highly fluid mixtures. The effect was more pronounced for V125-E8 containing cements compared to V125-E8E8. This behavior is consistent with the HAP dispersion assay and suggests that the E8 ELPs are acting as plasticizers by binding to the CPC particles and promoting particle dispersion. A similar effect has been demonstrated with sodium citrate and industrial superplasticizer modified CPCs although dispersion in those cases is promoted by charge repulsion.64,65 The increased fluidity of E8 ELP containing cements allows for increases in the P/L ratio because less water is required to solvate the cement particles. Increased P/L ratios have been shown to result in less porous, mechanically stronger cements. Therefore, samples were tested at P/L ratios of 4.25 to 1 for V125-E8 and 4 to 1 for V125-E8E8. Both ELPs resulted in cements with statistically significant increases in compressive strength going from ~36MPa for control cements to ~57MPa for both V125E8 and V125E8E8 containing cements (P<0.001) Interestingly, although increasing P/L ratios can increase strength by reducing porosity64, the E8 ELPs appeared to have an intrinsic strengthening effect. As evidence, surprisingly, when 5 wt% V125-E8 solutions were used at a P/L ratio of 4, the compressive strength (~37MPa) was not significantly increased compared to control. If the increased strength was due to purely to decreased porosity then similar strength improvements should have been found for 5% and 10% formulations. In addition, by SEM analysis, the E8 elastins appeared to have qualitatively larger pores compared to the control cement as has been seen previously in plasticized CPCs (Figure 5A-C).65 These pores may be the result of air entrainment due to the ELPs’ surfactant-like structures although we did not investigate this possibility further.66 Regardless, this porosity should decrease strength, once again pointing to the presence of an intrinsic strengthening mechanism. At higher magnifications in the SEM, it was apparent that the ELPs altered the crystal growth process (Figure 5D-F). V125-E8 containing cements appeared to have larger crystallites with more rounded edges compared to the sharply faceted crystallites seen in control cements. V125-E8E8 containing cements appeared to also have more rounded crystallites in addition to a number of wide and flat crystallites. The exact effect of these morphological changes is unknown. In addition to binding the surfaces of the cement crystallites, the E8 elastins might also become physically occluded into the crystal lattices as the crystals precipitated. There is some evidence that this may occur in natural biomineralized systems67 and it has been shown to occur in synthetic systems.68,69 In fact, occlusion of macromolecules within mineral crystals can increase the mechanical properties of the crystals.69 The promising improvements in compressive strength led us to further characterize the flexural strength and work of facture of E8 ELP containing cements using three-point bending tests (Figure 4B). No significant difference was found when V125 was used as the additive compared to control samples. However, the flexural strength was found to improve from ~9.4MPa for control samples to ~14.6 MPa or ~14.1 MPa for V125-E8 or V125-E8E8 containing samples, respectively. In addition, the work-of-fracture increased from ~13.1 J/m2 to ~21.2 J/m2 or 20.9 J/m2 for V125-E8 or V125-E8E8 containing samples, respectively.
Figure 5. SEM analysis of ELP and CPC composites.
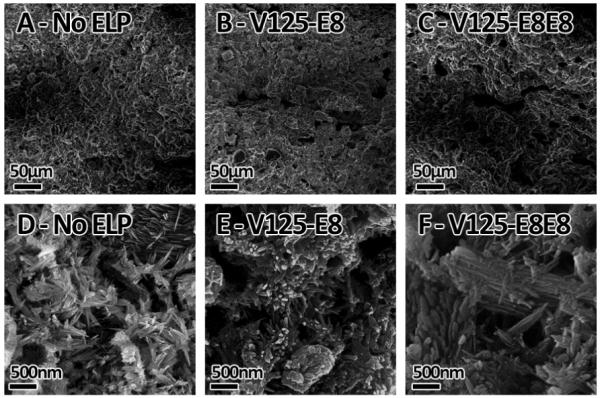
A & D. Control, no-ELP samples. B & E V125-E8 containing cements. C & F. V125-E8E8 containing cements. A-C) Low magnification view of cement surfaces showing different porosities. C-F) High magnification view of cement fracture surfaces showing different crystal morphologies.
Figure 4. Mechanical characterization of ELP-CPC composites.

A) Results of compressive strength tests shows enhanced strength for V125-E8 and V125-E8E8 containing cements. E/K125 refers to a liquid phase containing 10wt% ELP composed of a 50:50 mixture of E125 and K125. (B) 3-point bending tests showed improvements in both flexural strength (left columns) and work of fracture (right columns) for cements incorporating E8 elastins.
The uniformly charged ELPs were also tested as CPC additives. In addition, a 50:50 mixture of K125 and E125 was tested in order to investigate the effect of ionic backbone-backbone interactions (Fig. 1C). Using 10 wt% solutions, the use of V125, K125, E125, and 50:50 K125/E125 solutions did not change the suitable P/L ratio nor did they have statistically significant effects on the compressive strength of the resulting materials (Figure 4A). For V125 and K125 we can attribute this to the lack of significant interactions with the inorganic phase. Previously, polyanionic polymers such as alginate70 and polyacrylic acid23 were shown to alter CPC mechanical properties, presumably due to interactions between the carboxylic acid groups and calcium ions released during cement setting.71,72 The lack of an effect from addition of E125 may be due to its lower charge density or weaker HAP binding ability compared to the previously used anionic polymers. In addition, 50:50 mixtures of E125 and K125 showed no effects. Despite the protein-protein and protein-ion interactions available to these proteins suggested by the transition temperature experiments, the lack of strong interactions with HAP may be preventing those energy dissipating bonds from actively playing a role in altering the mechanical properties. For each of these ELP-CPC composites, we cannot rule out that the mineral to protein ratio was not optimal. There should be an ideal quantity of polymeric additive to yield the best properties.73 For example, previous work showed that only certain inorganic to organic ratios yielded strength improvements, after which, there were decreases in performance.24 These results show that ELP incorporation provides sequence-dependent mechanical property improvements to CPCs under multiple loading modes. Based on these results, future work can combine ELPs with other additives to further improve the mechanical properties.74
Injectability and Washout Resistance of ELP-CPC Composite
We demonstrated that incorporation of V125-E8 allows for formation of an injectable CPC-based bone composite with increased stability in fluids. Because of the aforementioned fluidity of CPCs containing E8 elastins, CPC-V125-E8 mixtures could be injected through a 16 gauge needle even at a P/L ratio of 4:1 (Supporting Movie 1). The injectability of CPCs is important for minimally invasive techniques such as vertebroplasty and for implantation sites that are poorly accessible or narrow.75 Washout tests qualitatively determine the stability of cements by immersion of cements in solution and visualization of the loss of material to a surrounding solution. The loss of material known as washout can be medically hazardous and limits the utility of injectable formulas.76 When E8 containing cements reached physiological temperatures, they became cohesive putties due to the ELPs’ inverse temperature transition. The effect of this cohesiveness was readily apparent in washout tests. The E8 containing cements remained as cohesive masses when placed into physiological buffer whereas control cements disintegrated (Figure 6). Therefore, the thermoresponsive cohesiveness observed in our cements is promising for surgical applications as it allows for highly injectable, yet washout resistant, cements.
Figure 6. Washout studies of ELP and CPC composites.

Cements were immediately immersed in 37°C HEPES buffer after mixing. The ELP free mixtures were unstable and disintegrated while the ELP containing mixtures remained cohesive.
4. Conclusion
We designed biomimetic ELP matrices to fabricate novel bionanocomposite materials and characterized their protein-protein interactions, protein-ion interactions, HAP binding ability and mechanical properties in the form of ELP-CPC composites. This study demonstrates the flexibility and power of using genetically engineered proteins as tools for studying sequence-property relationships of bone nanocomposite materials. By imparting ELPs with varying modes of inter/intra molecular bonding and interfacial bonding we were able to demonstrate sequence specific property changes in ELP-HAP mixtures. Our results showed that interfacial binding to HAP through octaglutamic acid motifs was critical for imparting functionalities such as nanoparticle dispersion and mechanical property improvements in ELP-CPC composites. In the absence of binding, the physical properties of the protein had no effects. Using our genetic engineering method we can further explore the relationship between polymer structure and composite properties by changing and combining factors such as binding motif length, the motif itself, and molecular weight. New backbone-backbone interactions can be explored using other repeat-sequence motifs28,50 or through the incorporation of known protein-based associative or folded domains.27,77 Meanwhile, genetic insertion of bioactive motifs,38 and incorporation into other bone-composite fabrication techniques78 will expand ELP functionality. In addition ELPs may be utilized to determine sequence-property relationships in more fundamental studies on biomineralization or for single-molecule force spectroscopy.18,79 We believe that our results are not limited to applications involving HAP, but can be expanded to explore composites with other metals and minerals. Through further iterative, rational design and combination with methods for synthesizing organized architectures8,80, our approach may yield strong and tough materials for future load-bearing applications such as hard-tissue replacements.
Supplementary Material
Acknowledgement
This work was supported by an NIH R-21 award (DE 018360-02) and a National Science Foundation Early Career Development Award (DMR-0747713).
Footnotes
Supporting Information Available
Detailed ELP cloning and purification methods, description of oligonucleotides and plasmids used for cloning and video of cement injectability. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Li X, Chang W-C, Chao YJ, Wang R, Chang M. Nano Lett. 2004;4:613. [Google Scholar]
- (2).Chai H, Lee JJ-W, Constantino PJ, Lucas PW, Lawn BR. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7289. doi: 10.1073/pnas.0902466106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GAG, Stucky GD, Morse DE, Hansma PK. Nat. Mater. 2005;4:612. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- (4).Mayer G. Science. 2005;310:1144. doi: 10.1126/science.1116994. [DOI] [PubMed] [Google Scholar]
- (5).Aizenberg J, Weaver JC, Thanawala MS, Sundar VC, Morse DE, Fratzl P. Science. 2005;309:275. doi: 10.1126/science.1112255. [DOI] [PubMed] [Google Scholar]
- (6).Rho J-Y, Kuhn-Spearing L, Zioupos P. Medical Engineering & Physics. 1998;20:92. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- (7).Smith BL, Schaffer TE, Viani M, Thompson JB, Frederick NA, Kindt J, Belcher A, Stucky GD, Morse DE, Hansma PK. Nature. 1999;399:761. [Google Scholar]
- (8).Munch E, Launey ME, Alsem DH, Saiz E, Tomsia AP, Ritchie RO. Science. 2008;322:1516. doi: 10.1126/science.1164865. [DOI] [PubMed] [Google Scholar]
- (9).Dean MN, Swanson BO, Summers AP. Integr. Comp. Biol. 2009;49:15. doi: 10.1093/icb/icp012. [DOI] [PubMed] [Google Scholar]
- (10).Deshpande AS, Beniash E. Cryst. Growth Des. 2008;8:3084. doi: 10.1021/cg800252f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Price PA, Toroian D, Lim JE. J. Biol. Chem. 2009;284:17092. doi: 10.1074/jbc.M109.007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Nudelman F, Pieterse K, George A, Bomans PHH, Friedrich H, Brylka LJ, Hilbers PAJ, de With G, Sommerdijk NAJM. Nat Mater. 9:1004. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Olszta MJ, Cheng X, Jee SS, Kumar R, Kim Y-Y, Kaufman MJ, Douglas EP, Gower LB. Mater. Sci. Eng., R. 2007;58:77. [Google Scholar]
- (14).Nudelman F, Pieterse K, George A, Bomans PHH, Friedrich H, Brylka LJ, Hilbers PAJ, de With G, Sommerdijk N. Nature Materials. 2010;9:1004. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Weiner S, Wagner HD. Annu Rev Mater Sci. 1998;28:271. [Google Scholar]
- (16).Giachelli CM, Steitz S. Matrix Biol. 2000;19:615. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- (17).Hartmann MA, Fratzl P. Nano Lett. 2009;9:3603. doi: 10.1021/nl901816s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Fantner GE, Adams J, Turner P, Thurner PJ, Fisher LW, Hansma PK. Nano Lett. 2007;7:2491. doi: 10.1021/nl0712769. [DOI] [PubMed] [Google Scholar]
- (19).Dorozhkin SJ. Mater. Sci. 2008;43:3028. [Google Scholar]
- (20).Xu HHK, Quinn JB, Takagi S, Chow LC. J. Dent. Res. 2002;81:219. [PubMed] [Google Scholar]
- (21).Friedman CD, Costantino PD, Takagi S, Chow LC. J. Biomed. Mater. Res. 1998;43:428. doi: 10.1002/(sici)1097-4636(199824)43:4<428::aid-jbm10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- (22).Stevens B, Yang Y, Mohandas A, Stucker B, Nguyen KT. J. Biomed. Mater. Res., Part B. 2008;85B:573. doi: 10.1002/jbm.b.30962. [DOI] [PubMed] [Google Scholar]
- (23).Miyazaki K, Horibe T, Antonucci JM, Takagi S, Chow LC. Dent. Mater. 1993;9:41. doi: 10.1016/0109-5641(93)90104-x. [DOI] [PubMed] [Google Scholar]
- (24).Mickiewicz RA, Mayes AM, Knaack D. J. Biomed. Mater. Res. 2002;61:581. doi: 10.1002/jbm.10222. [DOI] [PubMed] [Google Scholar]
- (25).Pan ZH, Jiang PP, Fan QY, Ma B, Cai HP. J. Biomed. Mater. Res., Part B. 2007;82B:246. doi: 10.1002/jbm.b.30727. [DOI] [PubMed] [Google Scholar]
- (26).Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoti A. J. Am. Chem. Soc. 2007;130:687. doi: 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Shen W, Kornfield JA, Tirrell DA. Macromolecules. 2007;40:689. [Google Scholar]
- (28).Wu X, Sallach R, Haller CA, Caves JA, Nagapudi K, Conticello VP, Levenston ME, Chaikof EL. Biomacromolecules. 2005;6:3037. doi: 10.1021/bm0503468. [DOI] [PubMed] [Google Scholar]
- (29).Girotti A, Reguera J, Rodríguez-Cabello J, Arias F, Alonso M, Testera A. J. Mater. Sci.: Mater. Med. 2004;15:479. doi: 10.1023/b:jmsm.0000021124.58688.7a. [DOI] [PubMed] [Google Scholar]
- (30).Huang J, Wong C, George A, Kaplan DL. Biomaterials. 2007;28:2358. doi: 10.1016/j.biomaterials.2006.11.021. [DOI] [PubMed] [Google Scholar]
- (31).Yu SM, Conticello VP, Zhang G, Kayser C, Fournier MJ, Mason TL, Tirrell DA. Nature. 1997;389:167. doi: 10.1038/38254. [DOI] [PubMed] [Google Scholar]
- (32).Arias F, Reboto V, Martín S, López I, Rodríguez-Cabello J. Biotechnol. Lett. 2006;28:687. doi: 10.1007/s10529-006-9045-3. [DOI] [PubMed] [Google Scholar]
- (33).Urry DW, Hugel T, Seitz M, Gaub HE, Sheiba L, Dea J, Xu J, Parker T. Philos. Trans. R. Soc., B. 2002;357:169. doi: 10.1098/rstb.2001.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Trabbic-Carlson K, Setton LA, Chilkoti A. Biomacromolecules. 2003;4:572. doi: 10.1021/bm025671z. [DOI] [PubMed] [Google Scholar]
- (35).Urry DW, Parker TM, Reid MC, Gowda DC. J. Bioact. Compat. Polym. 1991;6:263. [Google Scholar]
- (36).Chow DC, Dreher MR, Trabbic-Carlson K, Chilkoti A. Biotechnol. Prog. 2006;22:638. doi: 10.1021/bp0503742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Fong BA, Wu W-Y, Wood DW. Protein Expression Purif. 2009;66:198. doi: 10.1016/j.pep.2009.03.009. [DOI] [PubMed] [Google Scholar]
- (38).Straley KS, Heilshorn SC. Adv. Mater. 2009;21:4148. [Google Scholar]
- (39).Panitch A, Yamaoka T, Fournier MJ, Mason TL, Tirrell DA. Macromolecules. 1999;32:1701. [Google Scholar]
- (40).Murphy MB, Hartgerink JD, Goepferich A, Mikos AG. Biomacromolecules. 2007;8:2237. doi: 10.1021/bm070121s. [DOI] [PubMed] [Google Scholar]
- (41).Meyer DE, Chilkoti A. Nat. Biotechnol. 1999;17:1112. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- (42).Wang X, Zhuang J, Peng Q, Li Y. Nature. 2005;437:121. doi: 10.1038/nature03968. [DOI] [PubMed] [Google Scholar]
- (43).Tas AC. J. Am. Ceram. Soc. 2009;92:2907. [Google Scholar]
- (44).McDaniel JR, MacKay JA, Quiroz F. G. a., Chilkoti A. Biomacromolecules. 2010;11:944. doi: 10.1021/bm901387t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Denhardt D, Guo X. FASEB J. 1993;7:1475. [PubMed] [Google Scholar]
- (46).Benesch J, Mano J. o. F., Reis RL. Tissue Eng., Part B. 2008;14:433. doi: 10.1089/ten.teb.2008.0121. [DOI] [PubMed] [Google Scholar]
- (47).Papagerakis P, Berdal A, Mesbah M, Peuchmaur M, Malaval L, Nydegger J, Simmer J, Macdougall M. Bone. 2002;30:377. doi: 10.1016/s8756-3282(01)00683-4. [DOI] [PubMed] [Google Scholar]
- (48).Yokogawa K, Miya K, Sekido T, Higashi Y, Nomura M, Fujisawa R, Morito K, Masamune Y, Waki Y, Kasugai S, Miyamoto K.-i. Endocrinology. 2001;142:1228. doi: 10.1210/endo.142.3.8024. [DOI] [PubMed] [Google Scholar]
- (49).Lao UL, Sun M, Matsumoto M, Mulchandani A, Chen W. Biomacromolecules. 2007;8:3736. doi: 10.1021/bm700662n. [DOI] [PubMed] [Google Scholar]
- (50).Drummy LF, Koerner H, Phillips DM, McAuliffe JC, Kumar M, Farmer BL, Vaia RA, Naik RR. Mater. Sci. Eng., C. 2009;29:1266. [Google Scholar]
- (51).Harrington MJ, Masic A, Holten-Andersen N, Waite JH, Fratzl P. Science. 2010;328:216. doi: 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Gorbunoff MJ. Anal. Biochem. 1984;136:433. doi: 10.1016/0003-2697(84)90240-9. [DOI] [PubMed] [Google Scholar]
- (53).Goobes R, Goobes G, Shaw WJ, Drobny GP, Campbell CT, Stayton PS. Biochemistry. 2007;46:4725. doi: 10.1021/bi602345a. [DOI] [PubMed] [Google Scholar]
- (54).Roy MD, Stanley SK, Amis EJ, Becker ML. Adv. Mater. 2008;20:1830. [Google Scholar]
- (55).Segvich S, Biswas S, Becker U, Kohn DH. Cells Tissues Organs. 2009;189:245. doi: 10.1159/000151380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Gilbert M, Shaw WJ, Long JR, Nelson K, Drobny GP, Giachelli CM, Stayton PS. J. Biol. Chem. 2000;275:16213. doi: 10.1074/jbc.M001773200. [DOI] [PubMed] [Google Scholar]
- (57).Lee H, Scherer NF, Messersmith PB. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12999. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Courvoisier A, Isel F, François J, Maaloum M. Langmuir. 1998;14:3727. [Google Scholar]
- (59).Balazs AC, Emrick T, Russell TP. Science. 2006;314:1107. doi: 10.1126/science.1130557. [DOI] [PubMed] [Google Scholar]
- (60).Wadcharawadee N, Jack KS, Martin D, Trau M. Biomed. Lett. 2009;4:015003. doi: 10.1088/1748-6041/25/1/015003. [DOI] [PubMed] [Google Scholar]
- (61).Lemieux V, Adams PHHM, van Hest JCM. Chem. Commun. 2010;46:3071. doi: 10.1039/b923280j. [DOI] [PubMed] [Google Scholar]
- (62).Wang Q, Mynar JL, Yoshida M, Lee E, Lee M, Okuro K, Kinbara K, Aida T. Nature. 2010;463:339. doi: 10.1038/nature08693. [DOI] [PubMed] [Google Scholar]
- (63).Brown WE, Chow LC. In: Cements Research Progress 1986. Brown PW, editor. American Ceramic Society; Ohio: 1987. p. 352. [Google Scholar]
- (64).Barralet JE, Hofmann M, Grover LM, Gbureck U. Adv. Mater. 2003;15:2091. [Google Scholar]
- (65).Fernández E, Sarda S, Hamcerencu M, Vlad MD, Gel M, Valls S, Torres R, López J. Biomaterials. 2005;26:2289. doi: 10.1016/j.biomaterials.2004.07.043. [DOI] [PubMed] [Google Scholar]
- (66).Hesaraki S, Nemati R. J. Biomed. Mater. Res., Part B. 2009;89B:342. doi: 10.1002/jbm.b.31222. [DOI] [PubMed] [Google Scholar]
- (67).Pokroy B, Quintana JP, Caspi E. a. N., Berner A, Zolotoyabko E. Nat Mater. 2004;3:900. doi: 10.1038/nmat1263. [DOI] [PubMed] [Google Scholar]
- (68).Metzler RA, Tribello GA, Parrinello M, Gilbert PUPA. J. Am. Chem. Soc. 2010;132:11585. doi: 10.1021/ja103089r. [DOI] [PubMed] [Google Scholar]
- (69).Kim Y-Y, Ribeiro L, Maillot F, Ward O, Eichhorn SJ, Meldrum FC. Adv. Mater. 2010;22:2082. doi: 10.1002/adma.200903743. [DOI] [PubMed] [Google Scholar]
- (70).Dos Santos LA, De Oliveira LC, Rigo ECS, Carrodeguas RG, Boschi AO, De Arruda ACF. Bone. 1999;25:99S. doi: 10.1016/s8756-3282(99)00143-x. [DOI] [PubMed] [Google Scholar]
- (71).Kenny SM, Buggy M. J. Mater. Sci.: Mater. Med. 2003;14:923. doi: 10.1023/a:1026394530192. [DOI] [PubMed] [Google Scholar]
- (72).Matsuya Y, Antonucci JM, Matsuya S, Takagi S, Chow LC. Dent. Mater. 1996;12:2. doi: 10.1016/S0109-5641(96)80056-X. [DOI] [PubMed] [Google Scholar]
- (73).Hansma PK, Turner PJ, Ruoff RS. Nanotechnology. 2007;18:044026. [Google Scholar]
- (74).Moreau JL, Weir MD, Xu HHK. J. Biomed. Mater. Res., Part A. 2009;91A:605. doi: 10.1002/jbm.a.32248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Burguera EF, Xu HHK, Sun L. J. Biomed. Mater. Res., Part B. 2008;84B:493. doi: 10.1002/jbm.b.30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Wang X, Chen L, Xiang H, Ye J. J. Biomed. Mater. Res., Part B. 2007;81B:410. doi: 10.1002/jbm.b.30678. [DOI] [PubMed] [Google Scholar]
- (77).Lv S, Dudek DM, Cao Y, Balamurali MM, Gosline J, Li H. Nature. 2010;465:69. doi: 10.1038/nature09024. [DOI] [PubMed] [Google Scholar]
- (78).Liu G, Zhao D, Tomsia AP, Minor AM, Song X, Saiz E. J. Am. Chem. Soc. 2009;131:9937. doi: 10.1021/ja903817z. [DOI] [PubMed] [Google Scholar]
- (79).Valiaev A, Lim DW, Schmidler S, Clark RL, Chilkoti A, Zauscher S. J. Am. Chem. Soc. 2008;130:10939. doi: 10.1021/ja800502h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Podsiadlo P, Kaushik AK, Arruda EM, Waas AM, Shim BS, Xu J, Nandivada H, Pumplin BG, Lahann J, Ramamoorthy A, Kotov NA. Science. 2007;318:80. doi: 10.1126/science.1143176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


