Abstract
Differential mobility spectrometry (DMS) separates ions on the basis of the difference in their migration rates under high versus low electric fields. Several models describing the physical nature of this field mobility dependence have been proposed but emerging as a dominant effect is the clusterization model sometimes referred to as the dynamic cluster-decluster model. DMS resolution and peak capacity is strongly influenced by the addition of modifiers which results in the formation and dissociation of clusters. This process increases selectivity due to the unique chemical interactions that occur between an ion and neutral gas phase molecules. It is thus imperative to bring the parameters influencing the chemical interactions under control and find ways to exploit them in order to improve the analytical utility of the device. In this paper we describe three important areas that need consideration in order to stabilize and capitalize on the chemical processes that dominate a DMS separation. The first involves means of controlling the dynamic equilibrium of the clustering reactions with high concentrations of specific reagents. The second area involves a means to deal with the unwanted heterogeneous cluster ion populations emitted from the electrospray ionization process that degrade resolution and sensitivity. The third involves fine control of parameters that affect the fundamental collision processes, temperature and pressure.
Introduction
The central thesis of this paper is a study of the parameters that control, optimize, and stabilize the chemical interactions that drive the separation process of a differential mobility/mass spectrometer system (DMS/MS). It is the second in a series of three papers describing the optimization and control of three principle aspects of DMS/MS instrumentation. In a previous paper we described the control and optimization of the important physical parameters of such a system including analyzer geometry, transport gas flow control, and ion transmission efficiency.1 In the third paper we describe novel electronics optimized to provide the asymmetric radiofrequency (RF) fields at the high voltages required for these devices and the effects of different waveforms on resolution.2
DMS separates ions on the basis of the difference of their migration rates under high versus low field.3,4,5 This distinguishes DMS from ion mobility spectrometry (IMS) which separates ions solely on the basis of their low field mobility. With DMS ions are subjected to alternating high and low electric fields at high frequency. Since the mobility of an ion is different in high and low fields, the ion will drift in one or the other direction depending on the normalized ratio of the mobility constants in the high and low fields, referred to as the alpha parameter. The deviation of the drift trajectory is corrected by a DC offset potential referred to as the compensation voltage (CV) that is specific to a given ion. The physics governing the motion of an ion and its clusters through high pressure gases under the influence of low electric fields is well understood.6 Less well understood is the reasoning behind the difference in the mobility characteristics of an ion under high field conditions versus low fields. In other words what is the physical nature of the dependence of ion mobility under different field strengths, otherwise referred to as the field mobility dependence? Several models have been proposed to rationalize this dependence but one that shows particular promise is the clusterization model.7 Other mechanisms likely contribute but the clusterization model, sometimes referred to as the dynamic cluster-decluster model, correlates well with the strong chemistry dependence seen in the empirical data.8,9,10
Gas phase ion-neutral interactions are the basis of classical ion mobility spectrometry whereby an ion or ion clustered with neutral molecules drifts through a background gas under the influence of a static electric field. These interactions, which determine the mobility and thus flight time of the ion, are determined by collision dynamic parameters such as the size and shape of the collision partners and any electrostatic interactions that occur between them. These interactions remain constant during the transit of the ion through the analyzer and they are frequently chemical in nature. With DMS these collision dynamics are constantly changing between two different states as the ion transits the analyzer. The clusterization model depicts the ion to be in a clustered state while migrating through the low electric field portion of the RF field and to be in a declustered or less clustered state as it migrates through the high field portion of the waveform. This amplifies the difference in the mobility characteristics of an ion in these two fields often resulting in improved resolution and peak capacity. The energy involved in the formation and dissociation of the clusters is highly specific to the chemical interactions between an ion and a neutral gas phase molecule. It is thus imperative to bring the parameters influencing the chemical interactions under control and find ways to exploit them in order to improve the analytical utility of the device.
In this paper we describe three important areas and present experimental results that need consideration in order to stabilize and capitalize on the chemical processes that dominate a DMS separation. The first involves means of controlling the dynamic equilibrium of the clustering reactions with high concentrations of specific reagents. The second area involves a means to deal with unwanted heterogeneous cluster ion populations that degrade resolution and sensitivity. The third involves fine control of the temperature and pressure which influence the fundamental collision processes.
Experimental
All samples were purchased from Sigma Chemical Co (St. Louis, MO) unless otherwise stated. For infusion experiments, samples were diluted in solvent comprising 50/50 methanol/water with 0.1% formic acid (Fischer Scientific, Nepean, ON). The structures of all compounds utilized in this work can be found under the names used in this paper in the following on-line data bases: http://www.ncbi.nlm.nih.gov/sites/entrez?db=pcsubstance&term= and/or http://www.chemblink.com/
The details of the mass spectrometer, DMS, and the means utilized to interface them have been described previously.1 Briefly, a planar DMS was interfaced to an API 5000™ instrument by sealing the DMS cell to the inlet orifice of the mass spectrometer. The dimensions of the DMS cell were 30mm long × 10 mm wide with a 1 mm gap between the electrodes which were chosen to provide high transmission efficiency with the gas flow provided by the mass spectrometer. The transport gas was nitrogen provided by the curtain gas supply which could be modified with volatile liquids. An asymmetric RF field was generated using a bisinusoidal waveform from a custom tuned-harmonic generator2. The generator was operated at 3 MHz, providing separation voltage (SV) with peak magnitude from 0 – 3333 V (5000 V peak-to-peak). The DC compensation voltages (CV) could be scanned ±200 volts or set to a particular value within that range. For some experiments, a high precision barometer was used to track the barometric pressure (Setra Model 276 with 0.1% accuracy, Setra, Boxborough, MA) and feedback this information to the DMS power supply controller.
The standard TurboV™ ion source was used on the mass spectrometer with an extension flange to adjust the position of the source relative to the extended protrusion of the curtain plate when the DMS was installed. A modified curtain plate was built in-house that incorporated a ceramic heater (Kyocera, JP) and a ceramic bead network to form a heat exchanger for the curtain gas prior to its release into the curtain chamber. Volatile liquids were added in this region to modify the curtain/transport gas, introduced with either a bubbler or a Shimadzu model 10A LC pump. A custom heater controller was designed in-house to heat the ceramic heater to variable temperatures up to a maximum of approximately 450° C. The temperature of the transport gas was determined at each heater setting using a thermocouple.
Results and Discussion
1. Controlling the Dynamic Equilibrium of the Clustering Process
The addition of neutral gas phase molecules to the transport or background gas of IMS and DMS has been observed as a means to improve separations.6,10,11,12,13 The formation of ion-molecule clusters is central to separation in ion mobility in general and DMS in particular. This is an equilibrium process driven by the gas phase concentration of polar neutral molecules in the transport gas resulting in cluster ion populations that are bound by non-covalent forces. The balance of clustered ion sizes depends on pressure, effective temperature, and concentrations. In this section, we provide a schematic overview of the thermodynamics of this type of cluster formation, and its influence on DMS spectra.
The (SV) field used in DMS varies between high field and low field regimes at a rate in the MHz range. This variation can be modeled as a field-dependent effective temperature synchronous with the SV field because of the high collision frequency at atmospheric pressure.14 When ion-neutral clustering is occurring to a significant extent, the time-varying effective temperature, Teff, can cause a time-varying change in ion size and, therefore, a synchronous change in ion-mobility cross-section. This reversible cluster formation provides a method for the amplification of differential mobility effects in DMS. Because the change in cluster number occurs between the low and high field regimes during the SV waveform in DMS, the differential mobility can be greatly enhanced.
In the following discussion, we represent an ion A, which may be of either polarity and any charge state, when it is clustered with n neutrals of type C, as An. The bare ion is then written as A0 or simply, as A. There will be an equilibrium between An and An-1 determined by the Gibbs free energy at the current pressure and temperature for the association reaction. We can write this process, and the relationship between concentrations as shown below.
| (1) |
For simplicity, we have used An to represent both the clustered species CnA, and to represent the concentration of such a species.
With the assumption of local thermal equilibrium (LTE) during the SV waveform at the changing effective temperature, [T=Teff(E/N], the cluster-modified ion mobility coefficient depends on the time-varying SV field, and on the Gibbs free energies as a function of cluster size. If the Gibbs free energy is independent of cluster size, the distribution of cluster number is of the Poisson or exponential type, with the concentration of species An, the total amount of A (Atotal) and average cluster number nave, given by
| (2) |
These equations are approximate since the equilibrium constant and free energy are dependent on cluster size, with free energy change decreasing for larger cluster sizes. If the free energy values are known, explicit sums can be used to determine fractional population, and average cluster number.
One example of the dependence of free energy on cluster size is given in Lukyanov et. al. for alkali cation and halogen anion water clusters for n=1 to 6 (Table 3 in that publication).15 The experimental free energy values for each of the eight alkali or halogen ions decrease irregularly with cluster size, but are generally reduced by about 30% per additional water. This decrease in cluster affinity with cluster size reduces the mean cluster size from that given by equation (2). For many cluster types, structures and energies can be calculated by molecular mechanics or other methods to determine the balance between entropy and enthalpy effects in larger systems.
The effective ion mobility coefficient is a function of SV field, and therefore of time. It can be written in terms of a dynamic cluster number which depends on the time-varying effective temperature, Teff.7
| (3) |
where pn is the fraction of cluster size n, M is the neutral mass, and m is the ion mass. The DMS differential mobility in the presence of a modifier is then the difference between mobility coefficients for different cluster sizes. A formula for Teff is given in Mason and McDaniel16 that is appropriate for atomic systems, but for molecular ions, the effective temperature is somewhat more complicated, and depends on SV field, bulk gas temperature, and the nature of ion and neutral as described previously.14 As a crude approximation, simple empirical approximations for mobility as a function of mass can be used to estimate the effect of modifiers on a specific ion-neutral system. One such empirical formula for the dependence of mobility coefficient on mass is given below.
| (4) |
From equations 3 and 4, it is apparent that there are two effects of dynamic ion-neutral cluster formation on the DMS spectrum.
Variation of peak position in CV space. A dynamic change in the average cluster number (nav) between high and low field parts of the DMS waveform changes differential mobility leading to shifts in CV for peaks that may be quite dramatic. These CV shifts can improve DMS resolution and peak capacity.
Modified peak width. When cluster formation is important, the low field regime will have a higher average cluster number, and a wider cluster distribution than in the high field regime. This wider cluster distribution results in some dispersion in the differential mobility, and an increase in peak width. This effect is not large because of the weak dependence of mobility coefficient on mass for higher masses, as in the empirical formula given above.
Equations 1–4 are written for homogeneous clusters, with one ion type and one type of clustering neutral. Under conditions of incomplete electrospray desolvation, heterogeneous clusters of different sizes and compositions may be present in addition to small droplets. These clusters can be understood in terms of dynamic behavior in the same way, but will show a much greater range of differential mobility values and a correspondingly greater peak width. They are discussed in more depth in the next section of this paper.
We have just presented a thermodynamic model of the clustering/declustering process. Direct experimental measurements of the physical characteristics of the clusters that influence DMS separations are difficult to perform. The size or degree of clustering and the molecular weight distribution of the cluster ion populations that are subjected to the separation process occurring at atmospheric pressure in a DMS are difficult to characterize largely because they do not survive the transport process into a mass spectrometer for mass analysis. The bond energies of typical clusters with non-polar neutral species are so low that they do not survive intact during the transport from atmosphere to the vacuum system of a mass spectrometer equipped with an atmospheric pressure ion source. Collisions in the free jet expansion are sufficiently energetic to disrupt these clusters to the bare molecular ion.
The molecular size of the cluster ion population increases with increasing modifier concentration in the transport gas and varies with modifier species. Different modifiers will produce different CV values that are not necessarily predictable but are reproducible. Also, and in accordance with the clusterization model, increasing the modifier concentration may result in an increase in the alpha parameter, increased compensation voltages, and improved peak capacity as a consequence of the difference in mobility between larger and smaller cluster species. High concentrations of modifiers added to the transport gas are desirable and more important for DMS than for IMS. Concentrations as high as several percent show improved separations with DMS whereas typical modifier concentrations for IMS are in the low ppm range.
An example of liquid modifier-assisted DMS separation is illustrated in Figure 1 showing the separation of 6 isobaric compounds of m/z 316. Figure 1A shows the separation that is achieved with no modifier present in the nitrogen transport gas. The peaks are spread over approximately a 12 V window under these conditions. Figures 1B–D demonstrate the improvement in peak capacity that is achieved by adding 2-propanol modifier into the curtain gas, and thus the transport gas, at various concentrations. Increasing the modifier concentration spreads the peaks over a wide CV window, providing an improvement in the peak capacity on the order of approximately 5 fold. With 3.1% modifier (1C), the 6 isobars are sufficiently resolved that each of them can be selected with a discrete CV potential. While the peak capacity improves by increasing the concentration of clustering modifier, ion losses may also increase depending upon the ratio of proton affinities for the modifier and the analyte of interest. For instance, addition of 1.5% of the modifier 2-propanol to a carrier gas stream comprised of nitrogen will completely eliminate the signal for low proton affinity analytes such as bentazon and naproxen. However, addition of 1.5% methylene chloride to the same system leaves the signals for bentazon and naproxen unchanged. This simple example highlights the control over the separation process afforded by the use of chemical modifiers and the importance of controlling the gas phase concentration. In addition to peak capacity improvement, isobaric interferences can be eliminated by choosing modifiers with proton affinities lower than the analyte of interest, but higher than the interfering species. In such a situation the modifier acts similar to a classical dopant whose primary purpose is for the control and the alteration of the ionization process. One difference between dopants, used to control the ionization process, and modifiers, used to control the separation process, is that dopant concentrations are typically in the low ppm range while modifiers are found to be in excess of 10,000 ppm.
Figure 1.
Plot of CV versus MRM normalized signal intensity during infusion of six compounds at 10 μL/min. The compounds were 1) oxfendazole, 2) clonazepam, 3) flusilazole, 4) bromazepam, 5) chlorprothixene, 6) pamaquin. The modifier was 2-propanol in all cases metered at different flows into the nitrogen transport gas to give final modifier concentrations of A) 0%, B) 1.6%, C) 3.1%, and D) 6.2%. The (M+H)+ ions of the 4 compounds had the same nominal mass but different product ions were monitored for each. The separation voltage was 4200 V p-p and the carrier gas was maintained at approximately 42°C. The 2-propanol modifier was delivered with an LC pump to accurately control the gas phase modifier concentration.
The extent of the peak capacity improvements observed with clustering modifiers depends on the chemical interactions that occur between the modifier and analytes. Because the effect is chemical in nature different modifiers will produce different results providing the opportunity to alter the selectivity of the DMS separation by changing this parameter. An example of the differences in DMS separations that occur with different modifiers is presented in Figure 2 for 4 isobaric compounds of m/z 301. Figures 2A–C demonstrate the separation capability for the 4 compounds in the absence of modifiers (2A), in the presence of nitrogen transport gas saturated with water (2B), and 2-propanol (2C) after passing through a bubbler. Both modifiers improve peak capacity, similar to the data presented in Figure 1, and the optimal CV values for the compounds that undergo clustering are shifted to more negative values for both. Correlating the extent of the negative CV shift to the degree of clustering, it is apparent that different compounds aggregate to different degrees with water than with the alcohol. The elution order in CV space is inverted for the 2 data sets. This opens up the opportunity for the application of different types of modifiers to achieve selectivity enhancement.
Figure 2.
Plot of CV versus MRM normalized signal intensity during infusion of four compounds at 10 μL/min. The four compounds were 1) desmethylclomipramine, 2) dianabol, 3) clobazam, 4) temazepam. The data were generated (A) with no modifier, (B) water added as the modifier, and (C) 2-propanol added as the modifier to transport gas. The (M+H)+ ions of the 4 compounds had the same nominal mass but different product ions were monitored for each. The separation voltage was 4500 V p-p and the carrier gas was maintained at approximately 42°C. The modifiers were introduced at saturating concentrations by bubbling the transport gas (curtain gas) through a reservoir of each liquid. This technique is simple but does not accurately control the concentration of the modifier.
Two designs of differential mobility ion filters are in common use, cylindrical and planar. Originally referred to as a Field Ion Spectrometer by Mine Safey Associates Inc., the cylindrical design, using curved electrodes, has become commonly described as FAIMS (field-asymmetric waveform ion mobility spectrometry)17. The term DMS has maintained its association with the planar designs since its earliest conception18. Differences beween them have been described1 but with both DMS and FAIMS configurations it has been observed that the use of mixed non-polar gases for the transport gas can increase CV peak positions, possibly increasing instrument selectivity.19 The use of He/N2 transport gas mixtures is especially common in FAIMS analyses.
We have made an experimental comparison of the effect on DMS resolution of modifier addition and of the use of He/N2 gas mixtures in the transport gas. The results show that modifiers and non-polar gas mixtures have quite different effects on DMS peak positions and selectivity, as shown in Figure 3 for a mixture of 5 compounds. The figure shows that the addition of 2-propanol to the DMS drift gas at a 2.5% level increases the CV range from 4 V to about 25 V with a modest increase in peak width, for an overall increase in selectivity of about 5×. The small increase in peak width is due to the distribution of DMS low-field cluster sizes, as discussed previously. At this high SV value, all the peaks are shifted to more negative CV values to a greater or lesser extent by the 2-propanol modifier. In contrast, the use of 45.6% He in the transport gas causes peaks to shift together to more positive CV values with no significant change in the CV range occupied by the compounds, but a modest decrease in peak width, for a small increase in selectivity of about 1.2×. The decrease in peak width occurs because DMS widths are inversely proportional to low field mobility, and ion mobility is higher in He mixtures than in air or N2. Thus, we find that the use of non-polar gas mixtures for transport gas does not act as a chemical modifier that can significantly affect peak capacity. However, gas mixtures can provide a small increase in DMS resolution due to reduced peak width.
Figure 3.
Plot of CV versus MRM normalized signal intensity during infusion of 5 compounds. The compounds were 1) morphine, 2) haloperidol, 3) succinyl choline, 4) clenbuterol, and 5) verapamil. The data were generated with no modifier added to the nitrogen transport gas (A), no modifier added to a transport gas mixture comprised of 54% nitrogen and 46% helium (B), and 2-propanol added to the nitrogen transport gas at a concentration of 2.5% (C). The (M+H)+ ions of the 5 compounds had different nominal masses. The separation voltage was 3500 V p-p. As in Figure 1 an HPLC pump was used to dispense the modifier.
The signal for compound 3 disappeared with the addition of He at this level. Compound 3 is succinyl choline, the highest mobility compound in this experiment. It exists in the gas phase as a doubly charged ion, the charge coming from the two quaternary amine moieties which are permanent charges unaffected by changes in the proton affinities of the transport gas. Its disappearance cannot be due to a loss of charge to the transport gas for this reason. Also an explanation of the disappearance as a result of fragmentation by helium collisions is unlikely. A more plausible explanation is that the ions struck the walls of the analyzer because of the high mobility of a doubly-charged ion of low mass in a helium transport gas1.
Both the results for modifier addition and for non-polar mixtures as transport gas can be understood qualitatively in terms of the generic behavior of the ion mobility coefficient, K(E/N), with electric field or with effective temperature (see Fig 7-5-2 in reference 16, and Fig. 3.25 in reference 5). For all types of differential mobility curves, types A, B, and C, the ion mobility coefficient decreases smoothly at high E/N due to the onset of behavior indicative of the hard sphere collision model. In that regime, the collision energies become so high, and collision durations so short that the weak, long-range, ion-neutral polarizability energies are unimportant. As first shown by Wannier,20 ion mobility in the hard sphere collision limit decreases approximately as 1/sqrt (E/N).
As many authors have noted, the addition of He to the transport gas moves the onset of hard sphere collision behavior to lower E/N values, so that the high field portion of the DMS waveform samples the hard sphere collision part of the mobility curve. Because behavior in the very-high field regime is common to all systems, CV curves for all compounds as a function of SV (as seen in dispersion plots) move similarly. As a result, the use of a He gas mixture causes CV values for different compounds to shift as a group to more positive CV, not necessarily improving the resolving power.
In contrast, the addition of a modifier capable of reversible ion-neutral clustering during the SV waveform modifies the E/N dependence of the ion mobility coefficient in a much different way. The high field, hard-collision, limiting dependence of the ion mobility coefficient will not be affected because the ion will be declustered in very high field. At low field, the ion is clustered as determined by the Gibbs free energy for the process. Low field mobility, K(E/N), is then significantly reduced from that of the unclustered ion. As the field increases, K(E/N) tends to approach the unclustered value because the effective temperature is increased, and cluster size decreases. This effect can be quite significant, as we have shown, and causes CV values for the majority of ion species to move to more negative values. These CV changes are highly dependent on clustering free energies, and thus are highly dependent on chemical properties, unlike the He mixture effect which is independent of chemical characteristics.
2. Heterogeneous Clusters from Electrospray Ionization
Clustering of ions and neutral gas phase molecules is a consequence of ionization at atmospheric pressure. Ions generated during the electrospray process are a combination of bare molecular ions and ions clustered or contained in small droplets of the electrospray solvent. The relative proportion of ions, ion-clusters, and charged droplets is highly dependent on the degree to which the charged nebulized liquid is desolvated. When mobility devices in general, and DMS in particular, are used as a component of a liquid chromatography electrospray ionization mass spectrometry system the extent of the production of these heterogeneous cluster ion populations is related to mobile phase introduction flow rate. When the mobile phase flow rates extend into the hundreds of micolitres per minute range a large proportion of the ions produced by the ion evaporation process are created as clusters and small droplets of widely varying composition. Cluster ion populations formed in this way are highly heterogeneous and different from the relatively homogeneous cluster ion populations formed in the gas phase during the interaction of an ion with the background transport gas. A particular ion will exist in a wide variety of different clustered states covering a broad distribution of molecular weights and chemical compositions. This occurs whether or not high desolvation temperatures are used to evaporate the pneumatically nebulized electrospray, although the problem is exacerbated at low temperatures. A mobility device at atmospheric pressure will separate the components of the distribution and in so doing sensitivity for the targeted analyte, as detected by the mass spectrometer, will be reduced in addition to the mobility resolution and peak capacity. Without the mobility separation all components of the cluster ion distribution are drawn into the mass spectrometer and declustered to the bare molecular ion in the initial vacuum stages. With the DMS filtering at atmospheric pressure, set to pass a targeted analyte at a specific compensation voltage, only a portion of the cluster ion population enters the mass spectrometer.
Electrospray sources operating at liquid flows in the nanolitre to low microlitre per minute range produce fewer clusters and, depending on the analyte and solvent chemistry, will often produce unclustered molecular ions prior to the vacuum inlet. This is apparent when CV scans of an electrosprayed solution of a standard compound are done at high and low liquid flow rates. The data in Figure 4 show such a comparison with the compound pamaquin under fixed inlet conditions. The apparent loss of resolution as the flow rate is raised is due to the formation of increasingly heterogeneous analyte/cluster ion populations and possibly the persistence of small droplets within the mobility analyzer. Similar to the situation described above where modifiers were added to cluster with analytes in the gas phase, there is no indication in the mass spectra acquired at the various CV voltages of the identity of these clusters, only the molecular ion survives the free jet expansion. At mL/min solvent flows, the clustering may still persist, as indicated by broad CV scans, when ion source temperatures are raised as high as 600°C using the standard TurboIonSpray™ desolvation gases. Under these conditions, additional heating is required to drive the equilibrium towards the dry ion.
Figure 4.
Plot of CV scans versus MRM signal for pamaquin ions at various solvent flow rates, 1) 1 μL/min, 2) 5 μL/min, 3) 10 μL/min, 4) 25 μL/min, and 5) 125 μL/min. The apparent loss of resolution at high flows is due to the generation of a heterogeneous cluster ion population when ions are desorbed from the charged droplets. The desolvation gas temperature was maintained constant throughout. The carrier gas flow was heated to ≈ 42°C, and the SV set to 3000 V p-p in all cases.
RF heating effects in DMS
As mentioned briefly above, declustering by means of energetic collisions is possible under vacuum but is exceedingly difficult at atmospheric pressure because the gas density is too great to allow for sufficient ion acceleration to occur. Declustering or fragmentation have been observed for a few cases with high RF amplitudes.21,22 This suggests that one possible approach to remove the heterogeneous clusters formed from the electrospray ionization process at high sample flows is to make use of RF heating.
Movement of ions in gases under the influence of an electric field includes a sequence of steps. Ions are accelerated between collisions and gain energy from the applied electric field through increasing the kinetic energy as demonstrated in Equation 5,
| (5) |
where q is ion charge, λ is the distance which ions pass between two collisions (the mean free path), and E is the electric field strength. The mean free path depends on the collision cross section Ω of the ion-molecular pairs and molecular density N of transport gases as shown in Equation 6.
| (6) |
Therefore, the energy gained between collisions is directly proportional to the strength of the electric field, inversely proportional to gas density, and depends on parameters of the ion-molecule interaction. Energy is gained and lost in subsequent collisions, with steady-state reached after 10–15 effective momentum-transfer collisions.23
After that, velocity of ion movement can be characterized by an ion mobility coefficient, K, as described in Equation 7,
| (7) |
which balances the effects of friction (collisions) and electric field forces on ions.
This general overview demonstrates the important role that the nature of the collision process plays in determining the coefficient of mobility. The generalized dependence of the ion mobility coefficient on effective temperature is shown in Mason and McDaniel Figure 7-5-2.16 There are at least three regions where different collision processes are dominant. When ions move (with lowest velocity) at low field conditions, the cross section for elastic scattering between every collision is significant due to polarization interaction of ions with drift gas molecules. Cross sections in this area are highest and the effective coefficient of mobility smallest. With increasing electric field, although the velocity of ion movement is increased, the collision cross section is decreased due to the decreasing role of polarization forces, and the effective coefficient of mobility K(E) is increased. When contact interactions dominate the polarization interaction, the collision cross section is smallest and is determined by geometric sizes of ions and molecules. Further increase of the kinetic energy of interaction leads to the hard sphere collision model. In that case, as we have noted, the ion mobility coefficient decreases inversely proportional to the square root of electric field and effective coefficient of mobility is again decreased with effective temperature.
RF heating and bulk heating effects in DMS are closely related. Both result in increased ion internal energy and ion-neutral collision energy and change the ion mobility coefficient, which is the basic physical parameter measured in DMS. The analysis of Krylov et al.14 uses three simple principles to model the RF heating and bulk heating effects:
Mobility of any single ion species as a function of electric field and bulk temperature can be represented by the ratio of electric field to number density (E/N), commonly expressed in Townsend units, as a function of a single variable, effective temperature. This approximation is generally quite accurate, and is discussed in detail elsewhere.16 This unifying variable, effective temperature, is defined in terms of the mean ion-neutral collision energy at the particular bulk temperature and RF magnitude.
When the electric field is zero, the effective temperature is equal to the bulk temperature. This observation allows the simultaneous analysis of DMS data obtained with controlled bulk temperature, and varied RF magnitude.
Molecular ions behave differently than atomic ions for collisions in electric fields, so an analyte-dependent correction factor is necessary in the standard relationship between bulk temperature and ion mobility, and the unifying coordinate, effective temperature. This correction factor can be determined from experimental data at different bulk temperatures.
The physical basis for the difference between the behavior of molecular ions and atomic ions can be traced to the additional rotational and vibrational degrees of freedom in molecular ions. Decades of research have shown that the cross-sections for the exchange of rotational energy, and to a lesser degree, for exchange of vibrational energy, are much larger than the gas-kinetic cross-sections that change translational energy and determine ion mobility coefficients. Molecular ions accelerated in a field lose rotational and vibrational energy before they undergo a hard sphere collision that thermalizes rotational, vibrational, and translational degrees of freedom in the center of mass frame. As a result, effective temperatures are lower for molecular ions than they would be for atomic ions under the same conditions.
A strong SV field increases the effective temperature for a given ion above the bulk gas temperature, potentially leading to declustering. Under some conditions, SV heating is capable of causing ion fragmentation like that used routinely in MS/MS. For instance, fragmentation of the oxygen anion adduct of methyl salicylate (MO −2) ions to (M-H)− ion has been observed,21 as well as the fragmentation of diphenylmethane and other species.22 A detailed description of RF heating has also been given by Robinson et al.24 Finally, Nazarov et al. demonstrated that RF heating effects can lead to increased monomer/dimer ratios with increasing SV in DMS.25 As a striking example, we show DMS-MS results for fluorine loss from SF6 anion. Figure 5A shows a dispersion plot for the analysis of SF6 with various SV settings. The dispersion plots were generated by synchronizing stepping the separation voltage (SV) as compensation voltage (CV) was rapidly scanned. The range of SV was nominally 500–1500V in Δ10V scan steps which synchronized with CV scan every second. The discontinuity in the dispersion plot illustrates the dissociation of the SF6 anions with formation of SF5 anions. Inserted mass spectra (Figures 5B and 5C) show the presence of SF6 anions with m/z =146 at the low SV branch of the dispersion plot and fragmented SF5 anions m/z= 127 on the high SV branch, when RF heating causes fragmentation within the 1 ms timescale of transit through the DMS analytical region. This kinetic rate for unimolecular dissociation is reached at a specific effective temperature, which corresponds to a particular mean collision energy, as discussed for molecular ions by Krylov et. al.14 There are two ways to reach the required effective temperature or collision energy: bulk heating of the gas or increase in the SV voltage. To determine the balance between these factors, we performed additional experiments which vary transport gas temperature and measure RF voltage to fragment SF6 anions. Experimental measurements show that increasing the transport gas temperature from 100° C to 150° C lowers the onset for fragmentation from 1350V to 1100V SV, and these data give an approximate equivalence for this system of 1° C per 5V change in SV amplitude near 1200V (E= 24 KV/cm).
Figure 5.
Fragmentation of SF6 anions due to RF heating in the DMS analytical gap. A) For a bulk gas temperature of 150°C fragmentation occurs at approximately SV = 1100 V in the dispersion plot; B) mass spectrum before fragmentation shows only m/z=146 (SF6−); C) mass spectrum after fragmentation (SF6− → SF5− + F) shows SF5−.(m/z 127).
High temperature wall-less reactor
As mentioned above, RF ion heating and bulk gas heating effects in DMS are closely related. We describe here in more detail the use of bulk heating to reduce the heterogeneous cluster ion population. The goal is to desolvate/decluster electrospray generated clusters then recluster with a desired gas-phase reaction forming a homogeneous population in the DMS cell. Heat transfer is highly efficient at atmospheric pressure due to the high frequency of molecular collisions and radiative heat transfer. Various means for heating the cluster ions in the gas prior to the entrance of a DMS cell can be envisioned in addition to RF heating just described. One approach, diagrammatically shown in Figure 6, uses a wall-less mixing region with counter-current gas flows (Curtain GasTM) to accomplish this. Hot desolvation gas containing a mixture of the inert nitrogen curtain/transport gas with the modifier flows counter to the incoming ion clusters and source gas in a wall-less area. Flow is non-laminar in this region which maximizes the residence time of the cluster ion species in the heated region to drive desolvation to the extent possible. The background gas has a high concentration of modifier driving the equilibrium toward the desired homogeneous cluster ion population. The outflow of drying gas in front of the DMS analyzer region also helps to prevent neutral solvents and very large droplets from entering and contaminating the mobility analyzer region.
Figure 6.
Diagram of a DMS/MS interface showing the heated desolvation region. For more details regarding this interface design see reference (1).
Heterogeneous clusters can be reduced using this approach. A good test compound is 5-fluorouracil which, because of its high polarity, clusters extensively during electrospray ionization at virtually any liquid flow rate. Figure 7 shows two CV scans both at the relatively low flow rate of 10 μL/min. When no heat is used, the presence of heterogeneous clusters can be inferred by the broad CV trace. Heating the transport gas flow in the turbulent region at the entrance to the DMS to ≈ 160°C shows the conversion to a dry ion, the majority of which can be transmitted at a single CV value. The peak intensity is approximately 70% of that to be expected from the mass spectrometer without any mobility cell installed. Losses of ≈30% are what would be expected from a DMS cell of this design due to normal diffusion of ions to the walls during their transit of the cell.1
Figure 7.
Plot of CV versus MRM signal intensity during infusion of 5-fluorouracil (mw 129) in 50/50 methanol/water solvent at 10 μL/min. A) Performance in the absence of the heat exchanger, where the transport gas temperature was ≈ 42° C. B) Improved peak shape and intensity when the transport gas was heated to ≈ 165° C. The baseline for 5-FU without the DMS installed under these inlet flow conditions was 1.2 × 105 cps.
There is a high degree of compound dependency observed when measuring the DMS losses due to the formation of heterogeneous clusters by ESI. This is to be expected as the phenomena are chemical in nature. Table 1 shows the results for the transmission efficiencies to be expected from 6 diverse chemical species under low flow conditions, where the ionization process favors molecular ion production, and high flow conditions where heterogeneous clusters predominate. The use of the heated reaction region brings the transmission efficiency measurement for the two flow rates to within a factor of approximately 3 of each other on the average. Without this region losses can exceed an order of magnitude at the highest solvent flows.
Table 1.
Coefficient of transmission for various compounds analyzed at low flow rate (10 μL/min) and at high flow rate (200 μL/min) on a system equipped with a 1×10×30 mm DMS electrode set. The heat exchanger temperature was optimized for each of the compounds. Coefficient of transmission was determined by the ratio of signal with the DMS installed to the signal obtained with the DMS not installed on the instrument.
| Sample | m/z | Low Flow Rate Coefficient of Transmission | High Flow Rate Coefficient of Transmission |
|---|---|---|---|
| Reserpine | 609 | 0.77 | 0.28 |
| Taurocholic Acid | −514 | 0.79 | 0.25 |
| 5-Fluorouracil | −129 | 0.89 | 0.13 |
| Phenylbutazone | 309 | 0.74 | 0.34 |
| Safranin Orange | 315 | 0.82 | 0.27 |
| Furosemide | −329 | 0.75 | 0.25 |
| Average | 0.72 | 0.25 |
Data verifying the different origins of the two cluster ion populations is provided in Figure 8. The CV scans shown in Figure 8A show the effect of increasing solvent flow into the pneumatically assisted electrospray inlet. As the flow is increased clusters and small droplets of increasingly heterogeneous nature are produced from the ionization process. The data in Figure 8B shows the effect of adding a similar load of the same solvent as in Figure 8A but this time directly to the transport gas in the form of vaporized gas phase neutral molecules, keeping the electrospray flow rate low. Despite the high concentration of background neutral molecules of the same composition as the mobile phase, the ions do not form heterogeneous cluster populations as shown in the CV traces with and without modifier added to the transport gas. Under all conditions the ion source desolvation temperatures were kept at 300° C and the DMS inlet temperatures at 100° C providing equal opportunity for desolvation of clusters with each experiment. Clusters formed from the ionization process are different and more heterogeneous from those formed from pure gas phase ion molecule reactions.
Figure 8.
Heterogeneous versus homogeneous clustering processes for samples of minoxidil. A) CV scan data acquired for minoxidil under fixed source and interface temperature conditions, where the total solvent load from the source was adjusted. A tee was installed in the source so that a constant flow of 10 μL/min minoxidil could be mixed with a variable pump flow comprising 50/50 methanol/water. The total solvent flow presented to the source was 1) 10 μL/min, 2) 50 μL/min, 3) 100 μL/min, 4) 250 μL/min, 5) 500 μL/min, and 6) 750 μL/min. B) CV scan data for samples of minxodil introduced at 10 μL/min under two different transport gas conditions. (I). No modifier added to the nitrogen transport gas. (II). Modifier of the same composition as the electrospray solvent was vaporized in the transport gas using a liquid flow of 700 μL/min. The total transport gas flow was 3.7 L/min in both cases.
3. Control of the collision parameters: temperature and pressure
The number of collisions of ions with gas molecules and the chemical interactions that occur during these collisions affect the stability of CV values. Temperature14 and pressure21 both play a role.
Temperature
The temperature of the transport gas is inversely proportional to the gas density thereby affecting the number of collisions of the ions with the gas. It also influences the chemical interactions that occur, shifting clustering equilibrium in one direction or the other. The importance of controlling this parameter is illustrated in Figure 9. The transport gas temperature was controlled and regulated using the ceramic heat exchangers shown in Figure 6. The effect of temperature changes on both the CV peak position and shape are dramatic, because of changes in both E/N ratio and cluster ion state. However, in this case it appears that changes in the cluster ion state are most important since the observed shift is toward positive CVs. The higher the cluster number the more negative the CV value. The differential mobility of some clusters do not alter extensively under conditions of changing temperature, for example the clusters of compounds 2 and 3. Other compounds exhibit radical alterations of clustering state, such as compound 1. The data illustrates the opportunities available for controlling the selectivity of a separation with temperature alone.
Figure 9.
Effect of transport gas temperature on the separation of 6 different isobaric compounds of nominal m/z 309. The transport gas temperatures were A) 47°C; B) 98°C; C) 122°C; D) 146°C; E) 179°C. The separation voltage was 3500 V p-p and 2-propanol modifier was provided to the curtain gas at a concentration of ≈ 5%. The compounds were isobaric with nominal m/z 309 Daltons. The numbers in the figure refer to the following compounds: 1) nifenazone; 2) bestatin; 3) warfarin, 4) quinoxifen, and 5) benoxinate.
It is apparent from the data in Figure 9 that for this parameter to be analytically useful it is critical to maintain constant temperature conditions within a DMS analyzer. A 24°C increase in temperature provides compensation voltage shifts ranging from ≈ 1–9 V. The temperature precision required to maintain CV positions to within ± 0.3 V for these compounds can then be estimated to be ≈ 1°C (worst case scenario) from these data points. For a compensation voltage peak half width of approximately 2.5 V, shifting of the peak maximum by ≈ 0.3 V results in less than 5% reduction in analytical signal quantifying a reasonable CV stability to target.
Pressure
Since the ion source and DMS cell are operating at atmospheric pressure the gas density in this region is determined by the atmospheric pressure in the environment as well as the local temperature. The barometric pressure can not be controlled in atmospheric pressure ion sources which operate under ambient conditions. Variations in weather conditions are sufficient to affect DMS separations for reasons to be described and quantified in more detail below.
Simple physical considerations of the processes involved in ion-molecule collisions show that the parameter determining the ion field energy, thus influencing the compensation voltage value, is E/N, where E is the field strength and N is the gas density16. Since the gas density is proportional to the gas pressure, pressure related variations apply.
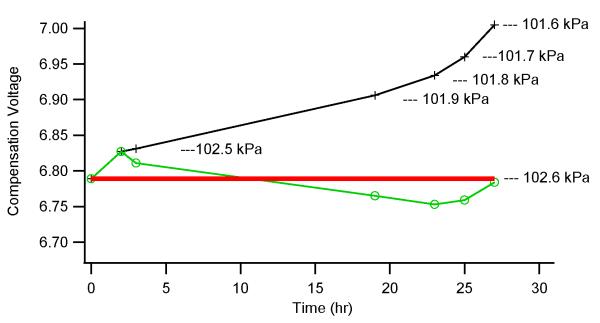
The effect of macroscopic changes in the barometric pressure on peak location in CV space has been reported previously.21 Those results demonstrated that it is critical to correct the separation voltage and compensation voltage for barometric pressure differences by reporting these values in Townsend units to enable comparison of DMS spectra generated in different locations of the world. However, a DMS/MS system designed for quantitative applications places even more stringent requirements on the reproducibility of the compensation voltage for a given analyte in an assay. For these applications, the requirement is not to compare data from various places in the world, but to maintain very high compensation voltage precision at a single location despite the typical fluctuations in barometric pressure that occur daily. Experiments with a high precision barometer were conducted to evaluate the effects of microscopic variations in the barometric pressure. Figure 10 shows a comparison of data acquired for a sample of reserpine with and without barometric pressure compensation. The data were acquired with repeated CV scans during a 27 hour time period where the barometric pressure decreased from 102.6 kPa to 101.6 kPa. As a result of the steady increase in E/N, the reserpine peak continuously drifted towards a higher positive CV value, ending up shifted by 0.2 V from its starting location when the separation voltage was not corrected to maintain a constant E/N ratio. As demonstrated in Figure 10, the systematic drift of the CV with changing barometric pressure can be eliminated, giving a maximum deviation from the initial CV value of 0.04 V when the barometric drift was monitored and compensated for by adjusting the separation voltage periodically to maintain a constant E/N.
Figure 10.
Compensation for Pressure Variation. DMS peak drift with barometric pressure was tracked over a 27 hour window where the barometric pressure decreased from 102.6 kPa to 101.6 kPa. The data show the CV drift that occurs when the separation voltage is not corrected to maintain a constant E/N ratio (trace marked with +), and the elimination of systematic drifting when the separation voltage is corrected to maintain a constant E/N ratio (trace marked with o).
4. Concluding Data
Control of the chemical interactions that play a large role in the separation process of a DMS/MS system is critical to maintain reproducibility, stability, and to maximize the separation power of the device. In previous publications we described the importance of gas flow control and the stability of the electric fields required for differential mobility separation1,2 to the same end. In this study the importance of controlling temperature, pressure, and aspects of the clustering phenomena have been considered. Experiments were conducted to determine the CV reproducibility for an entire DMS/MS system employing the controls described here as well as the previous two publications. Table 2 shows the results of a reproducibility trial conducted by repeating CV scans for 3 compounds over a 5 hour time period with and without modifiers. The 3 compounds selected for this experiment include a compound that clusters heavily with the modifier (quinoxifen), a compound that clusters moderately with the modifier (bestatin), and a compound that does not significantly cluster with the modifier (nifenazone) under these conditions. The greatest variability is shown by the compound that clusters to the greatest degree. Never-the-less the maximum CV voltage shift observed would produce a change in the intensity of an ion being measured by SIM or MRM of only 5%, acceptable for most quantitative analyses. The error observed is likely due to imprecision in the liquid delivery to the transport gas which can be improved upon. An HPLC pump was used in this case which gives on the order of 1% flow precision and greatest flow stability when operated under several tens of bar backpressure. No backpressure was introduced in the system for these experiments.
Table 2.
Stability data
| Quinoxifen | Bestatin | Nifenazone | |
|---|---|---|---|
| Ave Postion (V) | −7.93 | 2.93 | 9.90 |
| St. Dev. (V) | 0.22 | 0.16 | 0.03 |
| Ave Position without Modifiers (V) | 9.80 | 11.4 | 10.80 |
| CV Shift Due to Modifier (V) | 17.73 | 8.47 | 0.9 |
Conclusions
The difference between low and high field ion mobility coefficients is used in DMS / MS interfaces to suppress chemical noise, and to separate target ions that are close in m/z and difficult to separate by mass spectrometry. In this paper, we have discussed chemical effects that can enhance the resolution of DMS / MS while still maintaining the quantitative analytical capabilities important in mass spectrometry. Resolution is increased by the addition of polar clustering modifiers to the gas stream which increases the CV range spanned by compounds of similar m/z at the cost of a small increase in line width.
We have also examined the conditions that promote the creation of clusters with DMS characteristics that provide the greatest increase in resolution with little loss in sensitivity. Two different types of clusters are identified, homogeneous and inhomogeneous. Homogeneous clusters result from gas phase interactions of ions and neutral molecules, and have well-defined DMS characteristics. Inhomogeneous clusters result from incomplete desolvation in electrospray, and can show a much wider peak width with corresponding loss of intensity. We describe experimental methods that suppress inhomogeneous clusters, allowing DMS separation and chemical noise suppression to be effective.
The use of modifiers has been found to be more effective at increasing selectivity than the mixing of nonpolar gases such as helium in the transport gas, which results in a reduced peak width, but little increase in CV range. A study of the effect of temperature and pressure on peak stability has defined the level of temperature control required, and shown how a correction can be made for the variations in ambient pressure to prevent variation in peak intensity by more than a few percent.
This paper has described an efficient, simple, DMS / MS interface, methods of enhancing DMS resolution, and the control of system parameters for quantitative measurements in the presence of interfering species.
Acknowledgements
The authors extend their appreciation to Deolinda Fernandes for preparing all of the samples and solvents used in these experiments.
References
- 1.Schneider BB, Covey TR, Coy SL, Krylov EV, Nazarov EG. Planar Differential Mobility Spectrometer as a Pre-Filter for Atmospheric Pressure Ionization Mass Spectrometry. Int. J. Mass Spectrom. 2009 doi: 10.1016/j.ijms.2010.01.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krylov EV, Coy SL, Vandermey J, Schneider BB, Covey TR, Nazarov EG. Effects of Different Waveforms and Methods of Generating Them for Differential Mobility Spectrometry. Rev. Sci. Instr. 2009 doi: 10.1063/1.3284507. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eiceman G, Karpas Z. Ion Mobility Spectrometry. 2nd ed. CRC Press, Taylor and Francis LLC; Boca Raton, FL: 2005. [Google Scholar]
- 4.Krylov EV, Nazarov EG, Miller RA. Differential mobility spectrometer: Model of operation. Int. J. Mass Spectrom. 2007;226:76. [Google Scholar]
- 5.Shvartsburg A. Differential Ion Mobility: Non-Linear Ion Transport and Fundamentals of FAIMS. CRC Press, Taylor and Francis LLC; Boca Raton, FL: 2008. [Google Scholar]
- 6.Revercomb HE, Mason EA. Theory of plasma chromatography/gaseous electrophoresis – A Review. Anal. Chem. 1975;47:970. [Google Scholar]
- 7.Krylov EV, Nazarov EG. Electric field dependence of the ion mobility. Int. J. Mass Spectrom. 2009 Accepted for publication. doi: 10.1016/j.ijms.2009.05.009. [Google Scholar]
- 8.Krylova N, Krylov E, Eiceman GA, Stone JA. Effect of Moisture on the Field Dependence of Mobility for Gas-Phase Ions of Organophosphorus Compounds at Atmospheric Pressure with Field Asymmetric Ion Mobility Spectrometry. Journal of Physical Chemistry A. 2003;107:3648. doi: 10.1021/jp0221136. [DOI] [PubMed] [Google Scholar]
- 9.Eiceman GA, Krylov E, Krylova N, Nazarov EG, Miller RA. Enhanced resolution of ions from explosives in Differential Mobility Spectrometry by vapor modified drift gas. Analytical Chemistry. 2004;76:4937. doi: 10.1021/ac035502k. [DOI] [PubMed] [Google Scholar]
- 10.Levin DS, Miller RA, Nazarov EG, Vouros P. Rapid separation and quantitative analysis of peptides using a new nanoelectrospray- differential mobility spectrometer-mass spectrometer system. Anal Chem. 2006;78:5443. doi: 10.1021/ac060003f. [DOI] [PubMed] [Google Scholar]
- 11.Asbury GR, Hill HH., Jr Using Different Drift Gases to Change Separation Factors in Ion Mobility Spectrometry. Anal. Chem. 2000;72:580. doi: 10.1021/ac9908952. [DOI] [PubMed] [Google Scholar]
- 12.Levin DS, Vouros P, Miller RA, Nazarov EG. Using a Nanoelectrospray-Differential Mobility Spectrometer-Mass Spectrometer System for the Analysis of Oligosaccharides with Solvent Selected Control over ESI Aggregate Ion Formation. J. Am. Soc. Mass Spectrom. 2007;18:502. doi: 10.1016/j.jasms.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin DS, Miller RA, Nazarov EG, Vouros P. Rapid separation and quantitative analysis of peptides using a new nanoelectrospray- differential mobility spectrometer-mass spectrometer system. Anal Chem. 2006;78:5443. doi: 10.1021/ac060003f. [DOI] [PubMed] [Google Scholar]
- 14.Krylov EV, Coy SL, Nazarov EG. Temperature effects in differential mobility spectrometry. Int. J. Mass Spectrom. 2009;279:119. [Google Scholar]
- 15.Lukyanov SI, Zidi ZS, Shevkunov SV. Ion water cluster free energy computer simulation using some of most popular ion-water and water-water pair interaction models. Chem. Phys. 2007;332:188. [Google Scholar]
- 16.Mason EA, McDaniel EW. Transport Properties of Ions in Gases. Wiley Publishing; New York: 1988. [Google Scholar]
- 17.Purves RW, Guevremont R. Mass-spectrometric characterization of a high-field asymmetric waveform ion mobility spectrometer. Rev. of Sci. Instrum. 1999;69:12, 4094–105. [Google Scholar]
- 18.Buryakov IA, Krylov EV, Nazarov EG, Rasulev U.Kh. A new method of separation of multi-atomic ions by mobility at atmospheric pressure using a high-frequency amplitude-asymmetric strong electric field. Int. J. Mass Spectrom. and Ion Processes. 1993;128:143. [Google Scholar]
- 19.Shvartsburg AA, Tang KQ, Smith RD. Understanding and Designing Field Asymmetric Waveform Ion Mobility Spectrometry Separations in Gas Mixtures. Anal. Chem. 2004;76:7366. doi: 10.1021/ac049299k. [DOI] [PubMed] [Google Scholar]
- 20.Wannier GH. Motion of Gaseous Ions in Strong Electric Fields. Bell Sys. Tech. Journal. 1953;22:170. [Google Scholar]
- 21.Nazarov EG, Coy SL, Krylov EV, Miller RA, Eiceman GA. Pressure Effects in Differential Mobility Spectrometry. Anal. Chem. 2006;78:7697. doi: 10.1021/ac061092z. [DOI] [PubMed] [Google Scholar]
- 22.Kendler S, Lambertus GR, Dunietz BD, Coy SL, Nazarov EG, Miller RA, Sacks RD. Fragmentation pathways and mechanisms of aromatic compounds in atmospheric pressure studied by GC–DMS and DMS–MS. Int. J. Mass Spectrom. 2007;263:137. [Google Scholar]
- 23.Viehland LA, Kabbe EA, Dixit VV. Moment theory of ion motion in traps and similar devices: II. Cylindrical apparatus. J. Phys. B: At. Mol. Opt. Phys. 2005;38:4011. doi: 10.1088/0953-4075/38/22/007. [Google Scholar]
- 24.Robinson EW, Shvartsburg AA, Tang K, Smith RD. Control of Ion Distortion in Field Asymmetric Waveform Ion Mobility Spectrometry via Variation of Dispersion Field and Gas Temperature. Anal. Chem. 2008;80:7508. doi: 10.1021/ac800655d. doi:10.1021/ac800655d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nazarov EG, Miller RA, Eiceman GA, Krylov E, Tadjikov B. Effect of the electric field strength, drift gas flow rate, and temperature on RF IMS response. Int. J. Ion Mobility Spectrom. 2001;4:43. [Google Scholar]