Abstract
Fibrosis is a major clinical problem associated with as many as 45% of all natural deaths in developed nations. It can affect all organs and accumulating evidence indicates that fibrogenesis is not merely a bystander product of injury, but is a central pathological problem directly contributing to loss of organ function. In the majority of clinical cases, fibrogenesis is strongly associated with the recruitment of leukocytes, even in the absence of infection. Although chronic infections are a significant cause of fibrogenesis, in most cases fibrotic disease occurs in the context of sterile injury, such as microvascular disease, toxic epithelial injury or diabetes mellitus. Fibrogenesis is a direct consequence of the activation of extensive, and previously poorly appreciated, populations of mesenchymal cells in our organs which are either wrapped around capillaries and known as ‘pericytes’, or embedded in interstitial spaces between cell structures and known as resident ‘fibroblasts’. Recent fate-mapping and complementary studies in several organs indicate that these cells are the precursors of the scar-forming myofibroblasts that appear in our organs in response to injury. Here we will review the literature supporting a central role for these cells in fibrogenesis, and highlight some of the critical cell to cell interactions that are necessary for the initiation and continuation of the fibrogenic process. This article is part of a Special Issue entitled: Fibrosis: Translation of basic research to human disease.
Keywords: Fibrillar extracellular matrix, Fibrosis, Resident mesenchymal cell, Pericyte, Myofibroblast, Anti-fibrotic therapeutics
1. Introduction
Repair of damaged tissues after chronic or repetitive injury is of critical importance to survival. The repair process typically involves two distinct steps: a regenerative phase, where injured cells are replaced by cells of the same type, leaving no lasting evidence of damage; and a phase of fibrosis (also known as scarring) where connective tissue replaces normal, parenchymal tissue [1]. Although initially beneficial, a failure of the normal wound healing response to terminate results in chronic disease, contributing to morbidity and mortality for millions of people worldwide which, in early stages, may be asymptomatic. In the USA, it has been estimated that organ fibrosis is a contributory factor in as many as 45% of all natural deaths [1].
Despite having distinct etiological and clinical manifestations depending on the injured tissue, fibrosis is a common pathological response: in all cases it is characterized by the sustained production of growth factors, proteolytic enzymes, angiogenic factors, and fibrogenic cytokines, leading to the progressive and excessive production, deposition, and contraction of fibrillar extracellular matrix (ECM) components, a process of matrix remodeling. This production of fibrillar ECM may be a tissue response to limit organ damage, or to maintain tissue architecture. However, in cases where fibrosis does not cease and resolve, the accumulation and contraction of fibrillar ECM results in the distortion of tissue architecture. This is a major problem not only for organs that move (e.g. heart and lungs) but also for relatively static organs (e.g. kidney and liver). An expansion and stiffening of the interstitium that surrounds parenchymal units (for example, the nephron) disrupts physiological function and capillary microperfusion. In addition, the cells responsible for fibrillar ECM deposition, known as myofibroblasts, and the associated inflammatory leukocytes, directly damage tissues including capillaries and thereby promote organ failure.
Fibroproliferative disease (i.e. fibrosis and proliferation of fibrogenic cells) may affect tissues in any solid organ, including skin, kidneys, muscle, lungs, cardiac and vascular system, central nervous system, eyes, liver, pancreas and intestine. Many of the common diseases in which fibrogenesis is implicated have few, or no, current therapies and are managed largely by supportive measures. At this time, there are no therapeutics targeting the fibrogenic process approved for use by the US Food and Drug Administration and only a few are licensed in Europe and Japan. Despite this, the prospects for effective anti-fibrotic therapies based on targeting key participants in fibrogenic cascades are excellent, and urgently required.
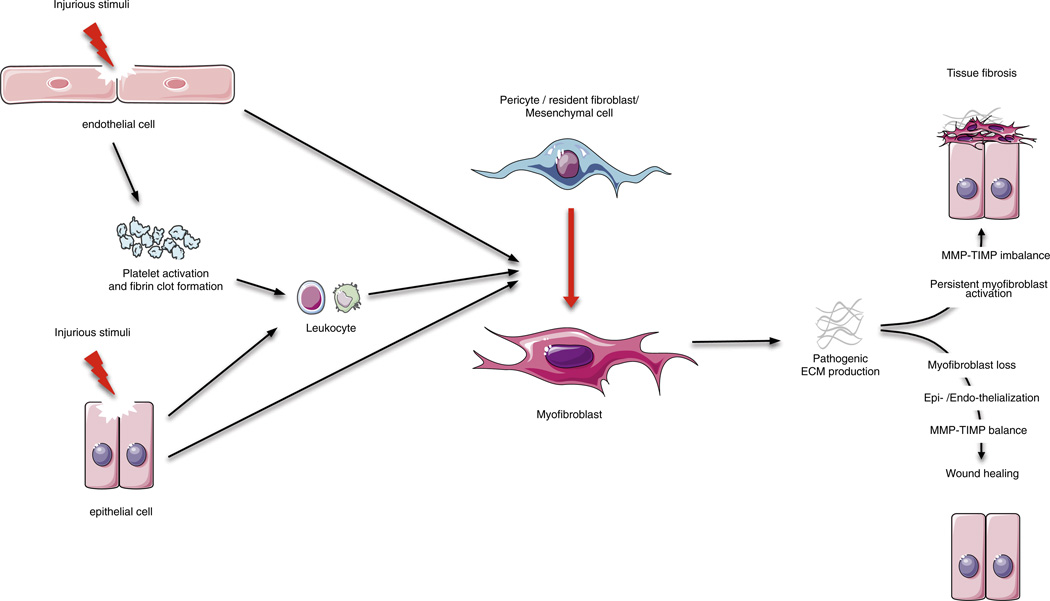
The focus of this review is the scar-forming cell observed in fibrogenesis, known as the myofibroblast (or sometimes disease-associated fibroblast) and its precursors (Fig. 1). We will first describe how inflammatory factors, and damage to local parenchymal cells, potentiate its activation. Then, we will discuss research from several distinct experimental models that have provided evidence on the origin of myofibroblasts during fibrogenesis. Finally, we will discuss potential avenues of therapy arising from this research.
Fig. 1.
Overview of wound healing: tissue regeneration or fibrosis. Epithelial and/or endothelial damage caused by various insults release inflammatory mediators that initiate an anti-fibrinolytic-coagulation cascade, which triggers blood clot formation. This is followed by an inflammatory phase, during which leukocytes are recruited at the site of injury. Damaged epithelial and/or endothelial can also directly activate the pericyte–myofibroblast transition. Then, myofibroblasts derived from pericytes/resident fibroblasts/mesenchymal cells produce fibrillar ECM components. Collagen fibers become organized, blood vessels are restored to normal, scar tissue is eliminated, and epithelial and/or endothelial cells divide and migrate to regenerate the damaged tissue. However, persistent inflammation can lead to chronic myofibroblast activation and excessive accumulation of ECM components ultimately resulting in fibrosis.
2. Relationship between inflammation and fibrosis
Acute and chronic inflammatory reactions play an important part in triggering the activation of myofibroblasts in many different organs (Fig. 1). Acute inflammation is a rapid response to injurious agents while chronic inflammation is a process of prolonged duration in which the inflammatory cascade lasts for weeks or months. In acute inflammation, the exposure of epithelial and/or endothelial cells to injurious stimuli such as bacteria, toxins, or therapeutically administered drugs, activates an inflammatory wound-healing response that can lead to a temporarily excessive deposition of fibrillar ECM components in the affected tissues. This excess ECM is thought to largely resolve, although contemporary literature suggests even this resolution may be incomplete in many instances [2,3]. In contrast, pathogenic fibrosis results from persistent inflammatory triggers, and is a pathological mechanism during which inflammation, tissue destruction and repair processes occur simultaneously.
2.1. Activation of the coagulation cascade
Wound-healing responses start directly after injury with the formation of a blood clot that initiates a cascade of events. It involves complex interactions between coagulation, inflammation, angiogenesis, and cellular migration and proliferation [4]. When epithelial and/or endothelial cells of an organ are damaged, they release inflammatory mediators that initiate an anti-fibrinolytic coagulation cascade within the vasculature, resulting in the formation of both blood clots and a provisional ECM. Blood-clots release thrombin, a potent inducer of platelet degranulation. Platelet degranulation promotes vasodilation, an increase in blood vessel permeability, and the production of ECM-degrading enzymes (known as matrix metalloproteinases, MMPs); all of these events promote the inflammatory responses by allowing the recruitment of immune and non-immune cells.
2.2. Recruitment of the innate myeloid cells
Neutrophils and macrophages are the first cell types recruited at the site of injury. Activated macrophages and neutrophils phagocytize foreign material and bacteria, also removing damaged cells and stromal debris. They also release cytokines and chemokines, which are mitogenic and chemotactic for adaptative immune cells, thus amplifying the wound-healing response. As a combined consequence of all these factors, wound healing can only occur after infiltrating inflammatory cells are brought under physiological control.
2.3. Activation of the adaptative immune cells
Concomitantly, lymphocytes become activated and secrete various wound-healing/pro-fibrotic cytokines and growth factors, including platelet-derived growth factor (PDGF), transforming growth factor 1 β (TGFβ-1) and interleukin (IL)-13. These mediators allow local mesenchymal cells to transform into α-smooth muscle actin (α-SMA)-expressing and ECM-producing myofibroblasts.
2.4. Activation of myofibroblasts and wound-healing
Once activated, myofibroblasts move into the wound-bed using the initial fibrin as a scaffold, and begin to proliferate. This promotes wound contraction, the process in which the edges of the wound migrate towards the center. Myofibroblasts also secrete factors that are mitogenic and chemotactic for epithelial and/or endothelial cells, allowing their proliferation and migration and regeneration of the site of injury.
2.5. Persistent activation of myofibroblasts and fibrosis
When inflammation becomes chronic, myofibroblasts continuously synthesize ECM, causing the formation of a fibrotic scar. Consequently, removal of the inflammatory trigger is mandatory to stop the progression of tissue remodeling and allow the normal tissue architecture to be restored after injury. Treatment with drugs (antivirals, antibiotics, antifungals) may be effective where infectious agents are implicated, but knowledge of the specific inflammatory stimulus is a prerequisite [5]. In many fibrotic diseases, the tissue-damaging signal is either unknown or cannot be easily eliminated. In this case, identifying the key mediators of the innate and adaptive immunity as potential targets is one method for stopping the progression of fibrosis.
Disease-initiating inflammatory and ischemic events converge on the appearance and activation of myofibroblasts; because of this, fibrosis research over the past 15 years has focused on the question of their cellular origin, the mechanism of their activation and the molecular/cellular pathways by which they can be deactivated.
3. Myofibroblasts produce the pathogenic fibrillar ECM in fibrosis
Although a number of cell types have the potential to produce and secrete ECM, including epithelial cells, endothelial cells and leukocytes, it is myofibroblasts that produce the pathogenic, fibrillar collagenous matrix observed in all forms of fibrosis. Especially, myofibroblasts deposit collagen types I and III, found in fibrotic organs, but also collagen type IV, glycosaminoglycans, heparan sulfate proteoglycans, and glycoproteins such as laminins and fibronectin.
Myofibroblasts, originally termed “wound fibroblasts,” were first defined by electron microscopy studies of skin wounding in 1972, and characterized by abundant rough endoplasmic reticulum, elliptical and speckled nuclei, a spindle-shaped cell body, and the absence of lysosomes [6,7]. They are defined biochemically by their expression of the intermediate filaments desmin, vimentin, and the contractile protein αSMA (in humans, one of six currently identified proteins in the actin family). αSMA is also found in vascular smooth muscle cells (vSMCs) and other cells of mesenchymal origin [8]. Contemporary studies distinguish ‘disease-associated fibroblasts’ from myofibroblasts by the expression of αSMA, but depending on the species, the organ studied, and the degree of cellular activation, these overlapping names represent the same type of cell. Myofibroblasts are also characterized morphologically by the formation of stress fibers, and physiologically, by their ability to contract tissues. Indeed, αSMA incorporation into the actin–myosin machinery is frequently observed as a cell becomes more contractile [9,10]. This is an important characteristic for a cell that undergoes contraction, increasing isometric tension within the tissue [10–13].
In liver and kidney in particular, over-activity of the interstitial myofibroblast is the convergent point for almost all chronic diseases. Other pathologies in which myofibroblast activity is a significant factor include myocardial infarction, chronic obstructive pulmonary disease, diabetes mellitus, chronic colitis, atherosclerosis, stroke, and skin diseases such as scleroderma.
4. Resident mesenchymal precursors are the major source of myofibroblasts across multiple organs
4.1. Resident mesenchymal cells
It is well-established that interstitial fibroblasts can proliferate in response to injury, and can activate to become myofibroblasts [14–17]. As part of this process, interstitial fibroblasts reduce their expression of 5’-nucleotidase and increase expression of αSMA [14]. Interstitial fibroblasts, perivascular fibroblasts, pericytes and hepatic stellate cells (HSCs) are all resident mesenchymal precursors of the myofibroblast: they are distinguishable from each other only by their tissue location, a variance in their anatomical connections with endothelial cells, and a limited specialization of function, such as vitamin A storage in the case of HSCs [18–26]. When localized to arterioles, the resident mesenchymal cells are known as perivascular fibroblasts, and when localized to capillaries they are known as pericytes. HSCs may be considered a specialized form of pericyte, whereas interstitial fibroblasts are those resident mesenchymal cells identified in the stroma [18,27,28]. Once pericytes and HSCs have activated and detached from the capillary bed, they then become indistinguishable from interstitial fibroblasts that have concomitantly transformed into myofibroblasts [14,21,29,30].
During development, mesenchyme-derived cells express αSMA but, unlike myofibroblasts in adult tissue, they are not responsible for scar formation. Rather, they form the loose connective tissue of the stroma, regulate angiogenesis, and induce branching morphogenesis in the developing epithelium [31]. In some cases, during development, mesenchymal to epithelial transdifferentiation (MET) occurs; the most extensively studied example is the formation of tubular epithelium in the developing kidney from metanephric mesenchyme [32,33]. Although the reverse process (EMT) is an important pathological indicator in cancer, and may readily occur in vitro, fate-tracing studies have demonstrated it is not a contributory factor to the appearance of myofibroblasts in either liver, lung or renal fibrosis [19,34–36]. However, as stated below, studies unequivocally show that the injured epithelium alone can directly trigger fibrogenesis in many organs, including liver, lung and kidney.
Whereas, during development, mesenchymal cells are metabolically active and migratory, in the adult organ they down-regulate expression of many genes, including αSMA, and enter into a relative quiescence. In this state, they occupy various niches across the entire body where they may perform a variety of physiological functions, including maintenance of the connective tissue, regulation of immune responses and stabilization of the microvasculature [30,37,38]. However, following acute or chronic injury, mesenchymal cells are reactivated in mature tissue and transform to myofibroblasts.
Although the origin of the myofibroblast in fibrosis has been known to be local to the site of initial injury since at least 1963, it is only recently with genetic fate-mapping that the precursor to the myofibroblast can be conclusively demonstrated to be the resident mesenchymal cell [7,18–20,39,40]. Strong evidence for this has arisen commensurately from independent studies on the kidney, skin, skeletal muscle, lung, liver, and brain.
4.2. Myofibroblast progenitors in the kidney
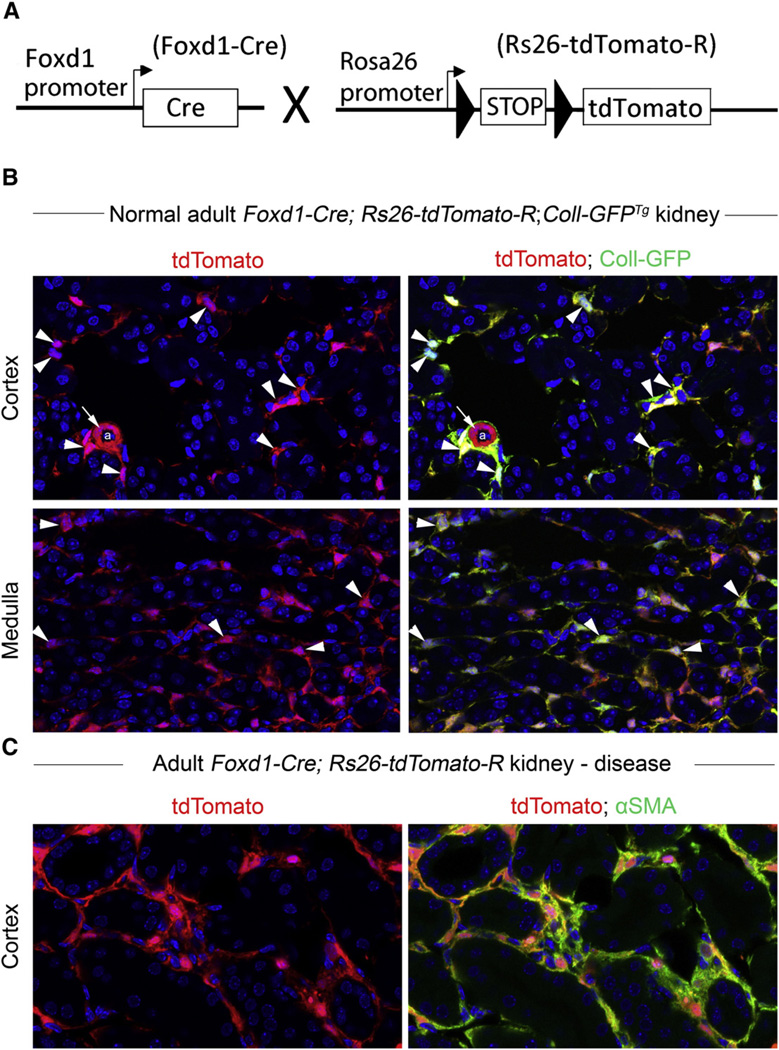
The fate of pericytes after renal injury was initially determined by the careful analysis of collagen Iα1 expression patterns and genetic fate-mapping based on the transcription factor forkhead box D1 (Foxd1) in mice (Figs. 2–3) [19,21]. First, Lin et al. analyzed transgenic mice in which the promoter for CollIα1 drove expression of GFP (CollIα1-GFPTg mice) [21]. Pericytes were observed to detach from capillaries, migrate into the interstitium, markedly up-regulate collagen Iα1 expression, and re-express the chondroitin sulfate proteoglycan marker NG2 and αSMA in response to injury: morphologically and biochemically, these pericytes were now indistinguishable from myofibroblasts. Kinetic modeling highlighted that pericyte proliferation and cell-cycle dynamics were consistent with the total number of myofibroblasts observed during fibrosis. Humphreys et al. generated Foxd1-Cre;Rosa26 transgenic mice in which mesenchymal cells were permanently labeled during embryonic development [19] (Fig. 3). Foxd1 is known to regulate the commitment of undifferentiated metanephric mesenchyme to become mesenchymal cells early in renal development, and can thus be used as a marker of mesenchymal origin. In Foxd1-GCE mice (GCE: GFP-Cre-Estrogen receptor), the GFPCreERT2 fusion protein is only able to recombine genomic DNA at LoxP sites when in the nucleus; and this can only occur when the endogenous Foxd1 gene is active (during early embryonic kidney development) or the estrogen receptor (ER) agonist, tamoxifen, is applied exogenously. In non-fibrotic kidneys, strong LacZ (galactosidase) expression was detected in interstitial cells of both the medulla and cortex. These cells did not express αSMA or endothelial markers, but they were positive for CD73 (ecto-5′-nucleotidase; marker of mesenchymal lineage) and PDGFRβ, a pericyte marker. The authors concluded that these cells were the same as those detected by Lin et al. in CollIα1-GFPTg mice [19]. These cells increased in number after acute kidney injury and expressed αSMA. Indeed, almost 100% of αSMA cells in these mice were also positive for LacZ, strongly suggesting that the majority, if not all, of the myofibroblasts in the fibrotic kidneys were derived from Foxd1 progenitors. To increase experimental stringency, another fate-tracing method was utilized. Tamoxifen (estrogen receptor ligand) was used to induce activation of LacZ in FoxD1-CreERT2 mice. Applied on embryonic day 10.5, tamoxifen induced 20% of stromal cells to become LacZ positive. These cells were then traced as becoming 20% of Foxd1-derived pericytes in the adult kidney. After kidney injury these pericytes expanded 15-fold and began to strongly express αSMA; none of the cells expressed αSMA before injury. Strikingly, this population of LacZ+ cells represented 20% of the total myofibroblast population, in accordance with the original proportion of tamoxifen-induced cells.
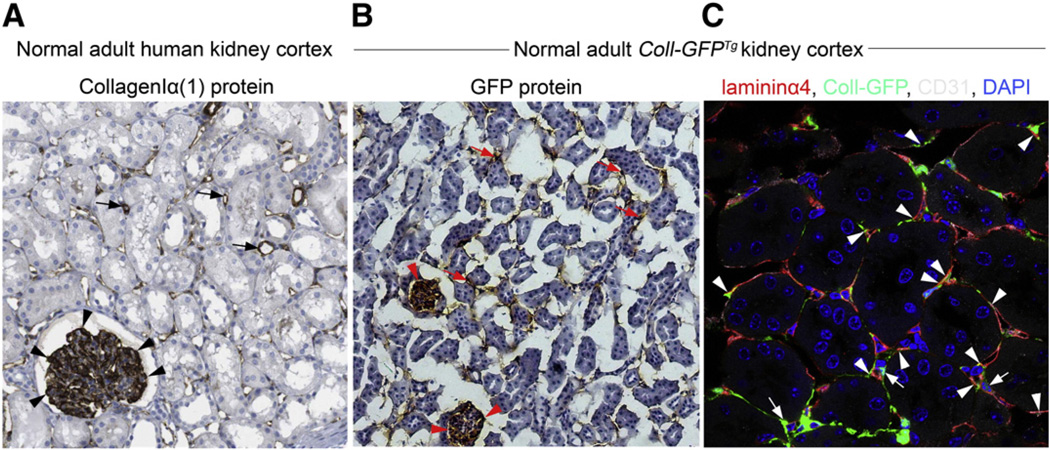
Fig. 2.
Characterization of pericytes in normal human kidney biopsy sample and in Coll-GFPTg mouse kidney cortex. (A) Normal adult human kidney cortex, immunostained for CollagenIα(1) protein. Note that CollagenIα(1) protein is strongly expressed in glomerular podocytes (arrowheads), and in perivascular cells from capillaries (arrows). (B) Normal adult Coll-GFPTg kidney cortex, immunostained for GFP. Coll-GFPTg mice express GFP under regulation by the Coll1α1 promoter, so GFP protein expression indicates Coll1α1 transcription. Note that GFP-expressing cells (glomerular podocytes (arrowheads), perivascular cells (arrows)) in Coll-GFPTg mice are the same as Coll1α1-expressing cells in human kidney cortex. Therefore, Coll-GFPTg mice are faithful reporters of all cells that produce CollagenIα(1) protein in human kidney. (C) Four color-confocal image of the cortex of the Coll-GFPTg mouse kidney stained for the capillary basement membrane-specific protein Lamininα4 (red) and the endothelial marker CD31 (white). Note numerous GFP+ cells that lack endothelial, epithelial or leukocyte markers (not shown) that form extensive processes along capillaries. Note direct interactions with endothelial cell bodies (arrows) and note Coll-GFP+ processes passing through splits in capillary basement membrane (arrowheads).
Fig. 3.
Results of fate mapping of Foxd1 progenitors in normal adult and injured kidney using the Foxd1-Cre;Rosa26-tdTomatoR mouse. (A) Schema showing the cross of Foxd1-Cre recombinase allele with TdTomato reporter allele driven by the universal promoters at the Rosa26 locus. Bigenic mice recombine genomic DNA at the Rosa locus only in cells that have activated the Foxd1 transcription factor gene in nephrogenesis. (B) Combining the Foxd1-Cre and Rosa26-tdTomatoR alleles with Coll-GFPTg in a single mouse indicates that perivascular cells of the Foxd1 lineage in kidney overlap almost completely with Coll-GFP+ pericytes (arrowheads). Image shows kidney cortex and kidney medulla. Note that vascular smooth muscle cells in arteriole do not express Coll1α1 (arrows). (C) In kidney injury in Foxd1-Cre;Rosa26-tdTomatoR mice (shown here is UUO d7), perivascular fibroblast and pericyte populations expand. However, now all of the expanded population of interstitial Foxd1-progenitor derived cells co-express αSMA, the marker which defines these cells as myofibroblasts.
More recently, Asada et al. have studied renal pericytes from an earlier developmental time-point [41]. They fate-mapped myelin protein zero (P0 cells) from the neural crest to metanephric mesenchyme during embryogenesis. P0 is expressed in migrating neural crest cells in the early embryonic stages as well as in Schwann cells, which also originate from neural crest at later stages. The authors demonstrated that the majority of erythropoietin-producing fibroblasts in the adult healthy murine kidney derived from P0+ cells that infiltrate into the kidney during embryonic development (from E13.5 onwards). They also demonstrated, in three different models of renal fibrosis that, in response to injury, the P0+ fibroblasts lose their ability to produce erythropoietin and become myofibroblasts. Moreover, administration of neuroprotective agents restored the ability of these cells to produce erythropoietin and attenuated fibrosis. Given that P0-Cre fate-mapped cells populate around Six2+ progenitor cells in the developing kidney, and that these cells overlap with the Foxd1-PDGFR+ cells that have previously been identified to be important in the development of renal fibrosis, there is a strong indication that the Foxd1-derived cells described by Humphreys et al. are the same cells as those described by Asada et al.
4.3. Myofibroblast progenitors in dermis and skeletal muscle
It is in the skin that the myofibroblast was first described: as a “contractile cellular element” contributing to scar formation following inflammation in dermal wounds [42]. However, it was in later experiments that the origin of myofibroblasts was demonstrated to be local to damaged tissue [7,39]. As in the kidney, this has recently been confirmed by genetic fate-mapping in skin and skeletal muscle. The enzyme A disintegrin and metalloprotease 12 (ADAM12) was used by Dulauroy et al. to trace cells of the neural crest-derived embryonic mesenchyme in murine models [40]. By crossing Adam12-Cre mice to Cre-dependent Rosa26floxSTOP-RFP reporter mice, they were able to label ADAM12+ cells with both red fluorescent protein (RFP) and green fluorescent protein (GFP), whereas all ADAM12+ progeny cells were labeled with RFP only. ADAM12+ cells migrated in early embryogenesis to somites, skin, gut and brain, developing into several different cellular populations. One specific ADAM12+ group was characterized by the additional expression of the pericyte marker PDGFRβ on their cell surface. Through careful observation, these cells were found to localize within the capillary basement membranes of skeletal muscle and dermis, wrapping their plasma membrane processes around endothelial cells. Upon injury, RFP+ cells expanded in number such that by day 20, post-lesion, the vast majority of αSMA+ myofibroblasts were progeny of ADAM12+ cells. By specifically ablating the ADAM12+ population of cells, fibrosis was attenuated in both skeletal muscle and skin. Moreover, in an updated version of the classic para-biosis study by Ross et al., Adam12-Cre;RFP reporter mice were surgically sutured together with wild-type partners. By tracking ADAM12 with confocal microscopy, it was confirmed that all αSMA+ myofibroblasts in acutely injured muscle and dermis are derived from tissue-resident mesenchymal cells, and not recruited from the bloodstream.
In patients with muscular dystrophies there is an accumulation of adipocytes and collagen Iα1-producing cells in skeletal muscle; this prompted two independent groups to address the origin of cells responsible for fibro/adipogenic infiltration into skeletal muscle after injury. Uezumi et al. and Joe et al. identified a population of either SM/C2.6-, PDGFRα + or Lin-, Sca1 +, α7integrin-, PDGFRα+ cells respectively, that are progenitors of both adipocytes and ‘fibrocytes’ in undamaged and damaged skeletal muscle; they were distinct from muscle progenitors [43–45]. In addition, using constitutively active PDGFRα knock-in mice, Olson and Soriano demonstrated that forced activation of PDGFRα signaling induced systemic fibrosis, including that of skeletal muscle [46]. Given that PDGFRα is also commonly used as a pericyte marker and pericytes have been shown to be progenitor cells for white adipocytes [47], this suggests that fibro/ adipogenic progenitor cells are pericytes that have the capacity to become adipocytes and vice versa.
4.4. Myofibroblast progenitors in the liver
The resident mesenchymal cell of the liver is the HSC; it is analogous to the pericyte and perivascular fibroblast in other organs. The HSC was first characterized in 1985, and has also been called the Ito cell and the lipocyte [48]. HSCs are identifiable in the healthy organ as vitamin A storing cells resident in the sub-endothelial space of Disse between endothelial cells and hepatocytes [49]. In accordance with regulatory roles in microvasculature for other pericyte-like cells, HSCs have an important function in maintaining the fenestrated endothelium of the sinusoidal capillary [28]. In the first instance by electron microscopy, and later through sophisticated genetic studies, the HSC has been confirmed as the main progenitor pool for myofibroblasts during liver fibrosis [36,48,50].
During the switch from their resident functions (storing vitamin A, and regulating vascular integrity), HSCs detach from their resident tissue-beds, and acquire all the hallmarks of myofibroblasts: increased proliferation, cessation of communication with their cognate cellular partners (hepatocytes and endothelial cells), single-cell migration, the release of fibrillar collagenous matrix, and the release of proinflammatory and fibrogenic cytokines such as TGFβ1 and PDGF [51,52]. HSC activation may also involve the production of endothelin (ET)-1, nitrous-oxide signaling, and activation of the renin–angiotensin system [53–56].
When first characterized by Friedman et al. the cultured HSC, or lipocyte, was calculated as producing over 20 times the mass of collagen (types I, III and IV) than cultured endothelial cells, and over 10 times the mass of collagen produced by hepatocytes. Although, the observation by Friedman et al. that “phenotypic resemblance of [lipocytes] to fibroblasts supports the hypothesis that lipocytes may be precursors of the fibroblast-like cells observed in liver injury” [48] was a qualitative one, a very recent study has shown this to indeed be the case. In carbon tetrachloride (CCl4) and alcoholic models of liver fibrosis, Kisseleva et al. fate-mapped HSCs with Coll-Cre− and Coll-CreER mice, demonstrating that these cells go on to become the collagen-producing myofibroblasts seen in liver fibrosis [18].
4.5. Myofibroblast progenitors in the central nervous system
It was in the brain that the pericyte was first identified [57]. As with other tissues, pericytes in the central nervous system (CNS) are necessary for the integrity of microvasculature, namely the blood–brain barrier (BBB) [37,58]. A recent paper has demonstrated that the pericyte is the major source for myofibroblasts in CNS scar-formation (fibrosis) [20]. The authors utilized a new marker for CNS pericytes, Glast, in order to trace the fate of these cells in transgenic mice. It was found that pericytes form the core of scars in injured CNS tissue, and that they give rise to stromal cells that produce collagenous fibrils. These stromal cells still displayed PDGFRβ on their surface, in accordance with a pericyte origin. The matrix-producing stromal cells were not derived from astrocytes, a cell type previously held to be responsible for scar-formation. Moreover, this is one of the first studies (along with, for example, Lin et al. [21]) to depict in high-resolution confocal-microscopy pericytes in the act of detaching from the capillary bed.
5. Pericyte structure and identification
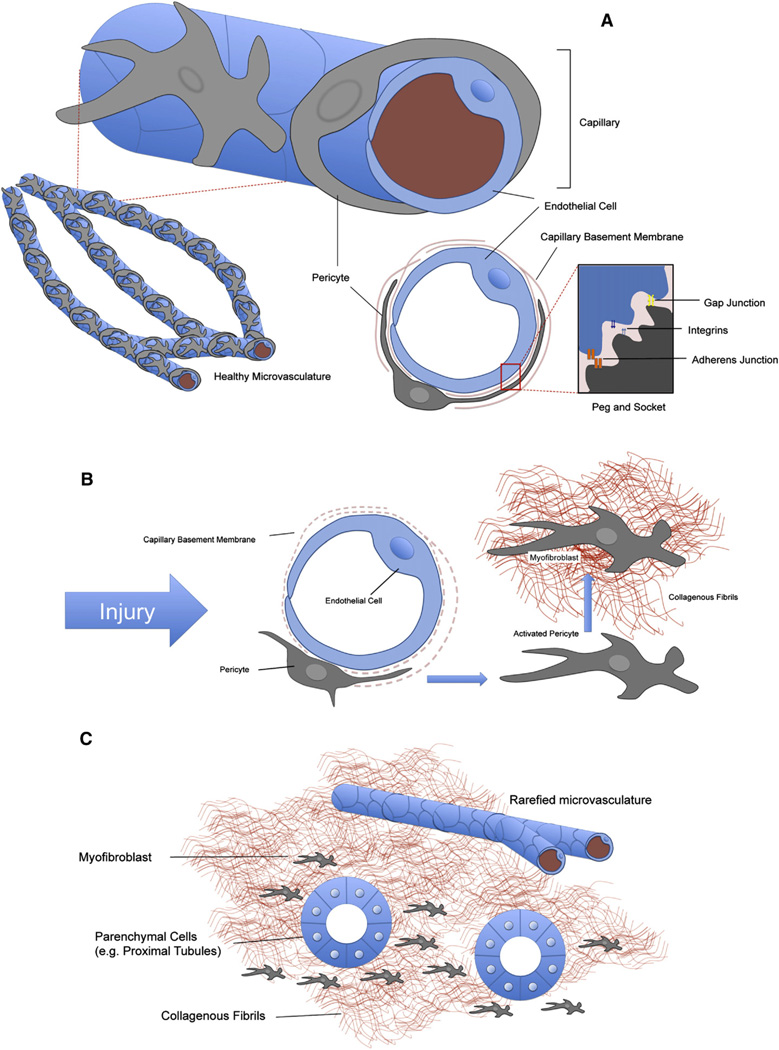
As well as being defined by their Foxd1-associated mesenchymal origins in several organs including skin and kidney, pericytes are a type of mural cell: they are defined as providing trophic and mechanical support to blood vessels [59]. Pericytes, in contrast to vSMCs, share a basement membrane with endothelial cells and can be in direct contact via extended cell-processes: these are a key morphological characteristic of the pericyte. Endothelial cells and pericytes make contact through interruptions in the capillary basement membrane known as “peg and socket” junctions [60] (Fig. 4). These are cytoplasmic invaginations that may contain tight, gap and adherens junctions as well as integrins [60,61]. It is the discontinuous nature of the basement membrane, and the peg and socket that allows an intimate signaling relationship to exist between endothelial cells and pericytes; one which includes electrical signaling as well as receptor-ligand signaling [62].
Fig. 4.
Pericyte localization in the microvasculature. (A) Schematic depicting pericyte–endothelial interaction in healthy tissue. Pericytes are mesenchymal cells that are found in the microvasculature where they partially cover capillary endothelial cells. Pericytes have long membranous processes that are partially or completely embedded in capillary basement membrane (CBM), which they share with endothelial cells. The CBM is frequently incomplete, allowing direct cell to cell contact with the endothelial cell. Here a number of connections can form, including cytoplasmic invaginations known as peg & socket processes. It is thought that the peg and sockets can help facilitate bilateral communication between endothelial cells and pericytes, including PDGF–VEGF signaling. (B) In response to injury or wounding, pericytes detach from capillaries, proliferate and begin to deposit pathological fibrillar matrix. In the process, CBM is degraded and endothelial cells lose the support of pericytes (both their mechanical support, and pericyte VEGF secretion and other trophic signals). (C) Pericyte detachment has two major consequences. Firstly, the injured organ is left with a rarefied microvasculature, with wide-spread capillary drop-out (indicated schematically by a reduction in their number). The resulting hypoxia in the organ can feed-back to produce more fibrogenic signals. Secondly, the deposition of collagens and laminins by pericytes/myofibroblasts distorts parenchymal tissue architecture (for example, nephrons in the kidney) and hardens the interstitial spaces between these functional tissues, impeding their normal functions.
Although no truly specific biomarkers for pericytes have been detected, they do express the vascular growth factors angiopoietin-1 and vascular growth factor (VEGF), and the developmentally important PDGF receptors α and β [30,63]. They also express a number of markers associated with mesenchymal stem cells, including CD44, CD73, CD105 and CD146 [64]. Of note is that cells continue to express PDGFRβ after they become myofibroblasts, and that, conversely, pericytes may express collagen I transcript even in healthy tissue [21]. In addition, pericytes variably express a number of neurological markers including NG2, synaptopodin, GFAP, and p75 nerve growth factor receptor (NFGR).
A recent study in the kidney has identified two novel pericyte markers, ADAMTS1 and TIMP3 (respectively: a disintegrin and metalloprotease with thrombospondin motifs-1; and tissue inhibitor of metalloproteinase 3), that may have functional implications in the transition of pericytes to myofibroblasts. In ex vivo experiments whereby cultured pericytes stabilized endothelial tubular networks (essentially, a reconstituted capillary), TIMP3 was found to promote stabilization, whereas ADAMTS1 impeded it. As well as validating two potential pericyte markers, this study elucidates the importance of the pericyte to vascular stability [65].
6. Pericyte interactions with the microvasculature
As aforementioned, confocal microscopy has allowed high-resolution images of the pericyte detaching from the capillary bed [20]. What is clear from a recent study is that this detachment is a function of deregulated pericyte–endothelial cell signaling [30]. Pericytes express PDGFR-β on their cell-surface and secrete VEGF; endothelial cells, in reciprocation, express VEGF receptor 2 on their cell-surface, and secrete PDGF. When either of these receptors is blocked, both fibrosis and capillary rarefaction are ameliorated during kidney injury. Interestingly, during disease progression, pericytes switch from producing angiogenic isoform VEGF164, and begin secreting dys-angiogenic VEGF120 and VEGF188 isoforms. Moreover, blockade of either the VEGF or PDGF receptor pathway results in a commensurate break on pericyte differentiation and proliferation, but VEGFR2 blockade also results in attenuated macrophage recruitment [30]. This result tantalizingly hints at a new functional nexus between inflammation, capillary and fibrotic injury: a paradigm that is likely to grow with our understanding of pericyte biology. In a further example of cross-talk, endothelial cells recruit pericytes during angiogenesis through paracrine release of PDGF-BB and heparin binding epidermal growth factor (HB-EGF) [66].
The biomechanics, and the signal transduction pathways, involved in pericyte detachment are yet to be fully elucidated. It is likely to involve a complex interplay between integrins, focal adhesion kinase (FAK), p21 protein activated kinase (PAK) and the actin–myosin cytoskeleton. Unsurprisingly, given its well-established effects on the cytoskeleton, the Rho-family of GTPase proteins has been shown to play a role in (retinal) pericyte contractibility, as well as paracrine effects upon endothelial cells [67]. However, this study relied on indirect delineation of the Rho pathway by inhibiting downstream Rho-kinase (ROCK1/2), and overexpressing Rho-mutants (which do not discriminate between, for example, RhoA, RhoB and RhoC). The Rho family is large, with at least twenty mammalian isoforms, and a fine-interplay between them determines cell behavior [68].
The exact nature of pericyte-CBM, and pericyte–endothelial cell co-ordinations requires further study (Fig. 4). However, it is known that pericyte recruitment by endothelial cells is accompanied by increased expression of integrin types α5β1, α3β1, α6β1, and α1β1, which bind fibronectin, nidogens, laminin isoforms, and collagen type IV [69].
7. Epithelial cell signaling in fibrogenesis
Recent fate-mapping studies indicate that whereas epithelial-to-mesenchymal transition (EMT) can occur in vitro, it does not contribute to the appearance of myofibroblasts in vivo [19,20,34]. However, studies unequivocally show that the injured epithelium alone can directly trigger fibrogenesis in many organs. For example in adult kidney injury, in addition to endothelial cells, injured epithelial cells produce PDGF and TGFβ1, and the TGFβ1 activator integrin αvβ6 is restricted to kidney epithelium [70–74]. Increased epithelial expression of TGFβ1 is accompanied by activation of intracellular canonical (Smad) and non-canonical (PAK2, c-Abl, Akt, tuberin and mTorC1) signaling pathways in the epithelium itself [75,76]. Blocking TGFβ1 and its downstream effectors can attenuate kidney injury and fibrosis [75–78], whereas transgenic overexpression of TGFβ1 in kidney epithelial cells is sufficient to trigger interstitial kidney fibrosis in the absence of any evidence of EMT[35, 79]. Moreover, a recent study demonstrated that TGFβ1 released from injured renal epithelium can activate the canonical Smad pathway in pericytes, leading to their activation to myofibroblasts [80]. Therefore, injured epithelium is a source of ligands that are important factors in driving fibrogenesis. In addition, epithelial cells are significant sources of proinflammatory cytokines and chemokines, which contribute directly to fibrogenesis in several organs [81].
8. Therapeutic approaches to the treatment of fibrosis: targeting pericytes and myofibroblasts
Although fibrosis was at one time considered a progressive and irreversible disease, data from animal models and human studies have provided clear evidence that fibrosis can be at least partially reversible. There is now some clinical evidence that fibrosis can regress in a variety of chronic liver diseases, observed either on cessation of the cause of liver injury or treatment of the underlying disease; but the evidence is limited or debated, frequently being based on small, retrospective cohort studies [82]. Regression of fibrosis in chronic kidney disease has also been achieved in experimental models following therapy with renin–angiotensin system blockers [83]. These results, however, contrast with large-scale human studies that observed at best, renin angiotensin system blockade slows the rate of loss of renal function [84]. The terms “reversal or reversibility” are often used to imply a complete restoration of normal architecture after the establishment of fibrosis. A better term instead would be “regression” of fibrosis, indicating that the fibrosis content is less than earlier, without quantifying the extent of regression or suggesting that the histology has returned completely to normal.
To date, despite the enormous unmet medical need for an effective antifibrotic agent, the majority of the potential drug products are still undergoing clinical trials to prove their efficacy and safety. Among the potential drugs that target receptors expressed by myofibroblasts or growth factors released by them, pirfenidone is an orally active p38 kinase inhibitor that reduces the activity of TGF-β1 and TNF-α in vitro. It is the first anti-fibrotic drug to be approved for the treatment of idiopathic pulmonary fibrosis, both in Europe and Japan (but not in the USA) [85]. Therapeutic TGF-β1-neutralizing antibodies have also been developed and tested on a mouse model of lung fibrosis [86]. Fresolimumab (GC1008), a human monoclonal antibody that inactivates all forms of TGF-β1 is undergoing clinical trials by Genzyme as a treatment for patients with focal segmental glomerulosclerosis [87]; its application is also explored in systemic sclerosis and idiopathic pulmonary fibrosis.
Ongoing trials are investigating the potential of imatinib mesylate, a small-molecule tyrosine kinase inhibitor marketed by Novartis, which inhibits signaling of PDGFRs (and also c-Abl and c-kit), as a treatment for nephrogenic systemic fibrosis [88], and newer generation of tyrosine kinase inhibitors in the treatment of pulmonary fibrosis. A fully humanized neutralizing monoclonal antibody, targeting the CTGF protein which is known to be an important pro-fibrotic factor both as a downstream effector and cooperative mediator of TGFβ1 action, is used in clinical trials for the treatment of diabetic nephropathy [89] as well as idiopathic pulmonary fibrosis by Fibrogen. However, there is evidence that other molecules targeting more recently identified pathways are making progress in the clinic. Readers are directed to a recent excellent review discussing extensively these drugs [90].
9. Conclusion
In all cases of fibrosis, pathogenic myofibroblasts are derived primarily from resident mesenchymal cells. These cells are found in almost all tissues, with a varied nomenclature accordingly. When localized to the microvasculature, they are known as pericytes. All resident mesenchymal cells respond to injury by activating dormant genes from their development, such as αSMA and NG2, and migrating into the site of injury. Here they begin depositing the collagenous fibers and laminins, which characterize fibrosis. Such a view – with one cell-type differentiating into myofibroblasts in response to injury – presents us with the prospect of a unified model of fibrogenesis across the entire body.
Acknowledgements
We thank Drs Benjamin D. Humphreys, Colin Ip, and Christine Abrass for assistance with images. The Duffield Lab is funded by NIH grants DK93493, DK84077, and DK87389, University of Washington, Genzyme research in progress grant, Nephcure Foundation, and a research agreement from Regulus Therapeutics. NH is a Fulbright Scholar funded by Diabetes UK. CF is funded by a Sanofi Renal Fellowship Award.
Footnotes
This article is part of a Special Issue entitled: Fibrosis: Translation of basic research to human disease.
Conflict of interest
JSD is on the Scientific Advisory Board of Promedior Inc., and Regulus Therapeutics. He is the founder of Muregen LLC. He has consulted with Takeda, Boehringer Ingelheim, Bristol Mayer Squibb, GlaxoSmithKline, Biogen Idec and Gilead Pharmaceutical Companies.
References
- 1.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 3.Selman M, Rojas M, Mora A, Pardo A. Aging and interstitial lung diseases: unraveling an old forgotten player in the pathogenesis of lung fibrosis. Semin. Respir. Crit. Care Med. 2010;31:607–617. doi: 10.1055/s-0030-1265901. [DOI] [PubMed] [Google Scholar]
- 4.Monroe DM, Hoffman M. The clotting system – a major player in wound healing. Haemophilia. 2012;18:11–16. doi: 10.1111/j.1365-2516.2012.02889.x. [DOI] [PubMed] [Google Scholar]
- 5.Salloum S, Tai AW. Treating hepatitis C infection by targeting the host. Transl. Res. 2012;159:421–429. doi: 10.1016/j.trsl.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabbiani G, Majno G. Dupuytren's contracture: fibroblast contraction? An ultrastructural study. Am. J. Pathol. 1972;66:131–146. [PMC free article] [PubMed] [Google Scholar]
- 7.Ross R, Everett NB, Tyler R. Wound healing and collagen formation. VI. The origin of the wound fibroblast studied in parabiosis. J. Cell Biol. 1970;44:645–654. doi: 10.1083/jcb.44.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skalli O, Schürch W, Seemayer T, Lagacé R, Montandon D, Pittet B, Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab. Invest. 1989;60:275–285. [PubMed] [Google Scholar]
- 9.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab. Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- 10.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am. J. Pathol. 2001;159:1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase-mediated contraction of isolated stress fibers. J. Cell Biol. 2001;153:569–584. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogatkevich GS, Tourkina E, Abrams CS, Harley RA, Silver RM, Ludwicka-Bradley A. Contractile activity and smooth muscle alpha-actin organization in thrombin-induced human lung myofibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;285:L334–L343. doi: 10.1152/ajplung.00417.2002. [DOI] [PubMed] [Google Scholar]
- 14.Picard N, Baum O, Vogetseder A, Kaissling B, Le Hir M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat, Histochem. Cell Biol. 2008;130:141–155. doi: 10.1007/s00418-008-0433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desmoulière A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast, Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 16.Strutz F, Zeisberg M, Hemmerlein B, Sattler B, Hummel K, Becker V, Müller GA. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int. 2000;57:1521–1538. doi: 10.1046/j.1523-1755.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharpe CC, Dockrell ME, Noor MI, Monia BP, Hendry BM. Role of Ras isoforms in the stimulated proliferation of human renal fibroblasts in primary culture. J. Am. Soc. Nephrol. 2000;11:1600–1606. doi: 10.1681/ASN.V1191600. [DOI] [PubMed] [Google Scholar]
- 18.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 21.Lin S-L, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BLM. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138:371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sponheim J, Pollheimer J, Olsen T, Balogh J, Hammarström C, Loos T, Kasprzycka M, Sørensen DR, Nilsen HR, Küchler AM, Vatn MH, Haraldsen G. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am. J. Pathol. 2010;177:2804–2815. doi: 10.2353/ajpath.2010.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development. 2012;139:2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype, Cell Struct. Funct. 2003;28:105–112. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–825. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- 29.Kaissling B, Le Hir M. The renal cortical interstitium: morphological and functional aspects, Histochem. Cell Biol. 2008;130:247–262. doi: 10.1007/s00418-008-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am. J. Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao J, Sakurai H, Nigam SK. Branching morphogenesis independent of mesenchymal–epithelial contact in the developing kidney. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7330–7335. doi: 10.1073/pnas.96.13.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 33.Horster M, Huber S, Tschöp J, Dittrich G, Braun G. Epithelial nephrogenesis. Pflugers Arch. 1997;434:647–660. doi: 10.1007/s004240050448. [DOI] [PubMed] [Google Scholar]
- 34.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, Brenner DA. Hepatocytes do not undergo epithelial–mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, Glick AB, Hähnel B, Hosser H, Gröne H-J, Kriz W. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am. J. Pathol. 2010;177:632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu AS, Diaz R, Hui J-J, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 38.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SHE. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Grillo HC. Origin of fibroblasts in wound healing An autoradiographic study of inhibition of cellular proliferation by local X-irradiation. Ann. Surg. 1963;157:453–467. doi: 10.1097/00000658-196303000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dulauroy S, Di Carlo SE, Langa F, Eberl GER, Peduto L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 41.Asada N, Takase M, Nakamura J, Oguchi A, Asada M, Suzuki N, Yamamura K-I, Nagoshi N, Shibata S, Rao TN, Fehling HJ, Fukatsu A, Minegishi N, Kita T, Kimura T, Okano H, Yamamoto M, Yanagita M. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J. Clin. Invest. 2011;121:3981–3990. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohnheim JF. Ueber entzundung und eiterung. Path. Anat. Physiol. Klin. Med. 1867;40:1–79. [Google Scholar]
- 43.Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FMV. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uezumi A, Fukada S-I, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 45.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada SI. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 46.Olson LE, Soriano P. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev. Cell. 2009;16:303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson LE, Soriano P. PDGFRβ signaling regulates mural cell plasticity and inhibits fat development. Dev. Cell. 2011;20:815–826. doi: 10.1016/j.devcel.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc. Natl. Acad. Sci. U.S.A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kisseleva T, Brenner DA. The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis. J. Hepatol. 2012;56:965–972. doi: 10.1016/j.jhep.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman SL. Evolving challenges in hepatic fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 52.Reynaert H, Thompson MG, Thomas T, Geerts A. Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut. 2002;50:571–581. doi: 10.1136/gut.50.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Housset C, Rockey DC, Bissell DM. Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin 1. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9266–9270. doi: 10.1073/pnas.90.20.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Görbig MN, Ginès P, Bataller R, Nicolás JM, Garcia-Ramallo E, Tobías E, Titos E, Rey MJ, Clària J, Arroyo V, Rodés J. Atrial natriuretic peptide antagonizes endothelin-induced calcium increase and cell contraction in cultured human hepatic stellate cells. Hepatology. 1999;30:501–509. doi: 10.1002/hep.510300201. [DOI] [PubMed] [Google Scholar]
- 55.Loureiro-Silva MR, Cadelina GW, Groszmann RJ. Deficit in nitric oxide production in cirrhotic rat livers is located in the sinusoidal and postsinusoidal areas. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G567–G574. doi: 10.1152/ajpgi.00452.2002. [DOI] [PubMed] [Google Scholar]
- 56.Bataller R, Sancho-Bru P, Ginès P, Brenner DA. Liver fibrogenesis: a new role for the renin–angiotensin system. Antioxid. Redox Signal. 2005;7:1346–1355. doi: 10.1089/ars.2005.7.1346. [DOI] [PubMed] [Google Scholar]
- 57.Rouget C. Mémoire sur le dévelopement, la structure et les propriétés physiologiques des capillaires sanguins et lymphatiques. Arch. Physiol. Norm. Pathol. 1873;5:603–633. [Google Scholar]
- 58.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood– brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- 60.Armulik A. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 61.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp. Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 62.Mogensen C, Bergner B, Wallner S, Ritter A, d’Avis S, Ninichuk V, Kameritsch P, Gloe T, Nagel W, Pohl U. Isolation and functional characterization of pericytes derived from hamster skeletal muscle. Acta Physiol (Oxf.) 2011;201:413–426. doi: 10.1111/j.1748-1716.2010.02206.x. [DOI] [PubMed] [Google Scholar]
- 63.Sundberg C, Kowanetz M, Brown LF, Detmar M, Dvorak HF. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab. Invest. 2002;82:387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 64.Gerlach JC, Over P, Turner ME, Thompson RL, Foka HG, Chen WCW, Péault B, Gridelli B, Schmelzer E. Perivascular mesenchymal progenitors in human fetal and adult liver. Stem Cells Dev. 2012 doi: 10.1089/scd.2012.0296. (121016061216008) [DOI] [PubMed] [Google Scholar]
- 65.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin S-L, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J. Am. Soc. Nephrol. 2012;23:868–883. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kutcher ME, Kolyada AY, Surks HK, Herman IM. Pericyte Rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am. J. Pathol. 2007;171:693–701. doi: 10.2353/ajpath.2007.070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ridley AJ. Historical overview of Rho GTPases. Methods Mol. Biol. 2012;827:3–12. doi: 10.1007/978-1-61779-442-1_1. [DOI] [PubMed] [Google Scholar]
- 69.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukuda K, Yoshitomi K, Yanagida T, Tokumoto M, Hirakata H. Quantification of TGF-beta1 mRNA along rat nephron in obstructive nephropathy. Am. J. Physiol. Renal. Physiol. 2001;281:F513–F521. doi: 10.1152/ajprenal.2001.281.3.F513. [DOI] [PubMed] [Google Scholar]
- 71.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J. Am. Soc. Nephrol. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 72.Ma L-J, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(−/−) mice. Am. J. Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moyano JV, Greciano PG, Buschmann MM, Koch M, Matlin KS. Autocrine transforming growth factor-{beta}1 activation mediated by integrin {alpha}V {beta}3 regulates transcriptional expression of laminin-332 in Madin–Darby canine kidney epithelial cells. Mol. Biol. Cell. 2010;21:3654–3668. doi: 10.1091/mbc.E10-06-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF, Sheppard D, Gardner HA, Violette SM. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am. J. Pathol. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang S, Wilkes MC, Leof EB, Hirschberg R. Noncanonical TGF-beta pathways, mTORC1 and Abl, in renal interstitial fibrogenesis. Am. J. Physiol. Renal. Physiol. 2010;298:F142–F149. doi: 10.1152/ajprenal.00320.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El Chaar M, Chen J, Seshan SV, Jha S, Richardson I, Ledbetter SR, Vaughan ED, Poppas DP, Felsen D. Effect of combination therapy with enalapril and the TGF-beta antagonist 1D11 in unilateral ureteral obstruction. Am. J. Physiol. Renal. Physiol. 2007;292:F1291–F1301. doi: 10.1152/ajprenal.00327.2005. [DOI] [PubMed] [Google Scholar]
- 78.Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- 79.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M, Niggli FK, Kriz W, Gröne H-J, Koesters R. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat. Med. 2008;14:979–984. doi: 10.1038/nm.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu C-F, Chiang W-C, Lai C-F, Chang F-C, Chen Y-T, Chou Y-H, Wu T-H, Linn GR, Ling H, Wu K-D, Tsai T-J, Chen Y-M, Duffield JS, Lin S-L. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte–myofibroblast transition in obstructive kidney fibrosis. Am. J. Pathol. 2012:1–15. doi: 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seki E, De Minicis S, Gwak G-Y, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuppan D, Pinzani M. Anti-fibrotic therapy: lost in translation? J. Hepatol. 2012;56:S66–S74. doi: 10.1016/S0168-8278(12)60008-7. [DOI] [PubMed] [Google Scholar]
- 83.Dussaule J-C, Guerrot D, Huby A-C, Chadjichristos C, Shweke N, Boffa J-J, Chatziantoniou C. The role of cell plasticity in progression and reversal of renal fibrosis. Int. J. Exp. Pathol. 2011;92:151–157. doi: 10.1111/j.1365-2613.2011.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ronco P, Chatziantoniou C. Matrix metalloproteinases and matrix receptors in progression and reversal of kidney disease: therapeutic perspectives. Kidney Int. 2008;74:873–878. doi: 10.1038/ki.2008.349. [DOI] [PubMed] [Google Scholar]
- 85.Bouros D. Pirfenidone for idiopathic pulmonary fibrosis. Lancet. 2011;377:1727–1729. doi: 10.1016/S0140-6736(11)60546-1. [DOI] [PubMed] [Google Scholar]
- 86.Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD. TGF-beta-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L151–L161. doi: 10.1152/ajplung.00389.2006. [DOI] [PubMed] [Google Scholar]
- 87.Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DRW, Peters H, Rota S, Remuzzi G, Rump LC, Sellin LK, Heaton JPW, Streisand JB, Hard ML, Ledbetter SR, Vincenti F. A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kay J, High WA. Imatinib mesylate treatment of nephrogenic systemic fibrosis. Arthritis Rheum. 2008;58:2543–2548. doi: 10.1002/art.23696. [DOI] [PubMed] [Google Scholar]
- 89.Adler SG, Schwartz S, Williams ME, Arauz-Pacheco C, Bolton WK, Lee T, Li D, Neff TB, Urquilla PR, Sewell KL. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin. J. Am. Soc. Nephrol. 2010;5:1420–1428. doi: 10.2215/CJN.09321209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu. Rev. Pathol. Mech. Dis. 2012;8 doi: 10.1146/annurev-pathol-020712-163930. (121023133009008) [DOI] [PMC free article] [PubMed] [Google Scholar]