Abstract
Prior evidence has suggested a link between caudate dopaminergic functioning and cognition in Parkinson’s disease (PD). In this dual tracer study we analyzed the relationship between nigrostriatal dopaminergic dysfunction and the expression of the previously validated PD cognition-related metabolic pattern (PDCP). In this study, 17 non-demented PD patients underwent positron emission tomography (PET) imaging with [18F]-fluorodeoxyglucose to measure PDCP expression, and [18F]-fluoropropyl-β-CIT (FPCIT) to measure dopamine transporter (DAT) binding. Automated voxel-by-voxel searches of the FPCIT PET volumes were performed to identify regions in which DAT binding significantly correlated with PDCP expression values. The findings were validated using prespecified anatomical regions-of-interest (ROIs). Voxel-wise interrogation of the FPCIT PET scans revealed a single significant cluster in which DAT binding correlated with PDCP expression (p<0.05, corrected). This cluster was localized to the left caudate nucleus; an analogous correlation (r=−0.63, p<0.01) was also present in the “mirror” region of the right hemisphere. These findings were confirmed by the presence of a significant correlation (r=−0.67, p<0.005) between PDCP expression and DAT binding in caudate ROIs, which survived adjustment for age, disease duration, and clinical severity ratings. Correlation between caudate DAT binding and subject expression of the PD motor-related metabolic pattern was not significant (p>0.21). In summary, this study demonstrates a significant relationship between loss of dopaminergic input to the caudate nucleus and the expression of a cognition-related disease network in unmedicated PD patients. These baseline measures likely function in concert to determine the cognitive effects of dopaminergic therapy in PD.
Keywords: Parkinson’s disease, cognition, caudate, dopamine
Introduction
The clinical definition of Parkinson’s disease (PD) traditionally rests on the motor manifestations. Nevertheless, a substantial number of non-motor symptoms can be prominent, including cognitive deficits and behavioral abnormalities. The point prevalence of dementia in PD is as high as 31%, and disturbances of executive, memory, and visuospatial functioning are commonly encountered in non-demented patients (Aarsland and Kurz, 2010; Alves et al., 2006; Green et al., 2002; Janvin et al., 2005).
Functional imaging studies have implicated multiple mechanisms for cognitive dysfunction in PD, including alterations in nigrostriatal, mesocortical and mesolimbic dopamine neurotransmission, changes in the activity of ascending cholinergic pathways, and deposition of abnormal protein aggregates in cortical association regions (Hirano et al., 2012). The pathological hallmark of PD is the progressive loss of nigral dopaminergic neurons. Presynaptic nigrostriatal dysfunction can be evaluated in vivo using a variety of dopaminergic imaging approaches. Among these, radiotracers that bind to the striatal dopamine transporter (DAT) have been extensively utilized as markers of dopaminergic attrition in PD (Hirano et al., 2010; Thobois et al., 2004), largely with regard to motor function. Within the striatum, dopaminergic loss is most pronounced in the putamen, particularly in the posterior motor-related portion of this structure (Bruck et al., 2005; Hilker et al., 2005; Ma et al., 2002; O'Brien et al., 2004). By contrast, a number of studies have linked the cognitive manifestations of PD to dopaminergic dysfunction in the caudate nucleus (Carbon et al., 2004; Ekman et al., 2012; Ito et al., 2002; Jokinen et al., 2009; O'Brien et al., 2004; Polito et al., 2012; van Beilen et al., 2008).
While the measurement of caudate/putamen DAT binding and other markers of presynaptic nigrostriatal dopamine dysfunction provide useful in vivo descriptors of PD pathology, metabolic imaging has been used to delineate the more widespread functional consequences of the neurodegenerative process (Eidelberg, 2009; Niethammer and Eidelberg, 2012). Varying degrees of cortical hypometabolism have been discerned in PD patients, with circumscribed frontal and parieto-occipital deficits in individuals without cognitive dysfunction, and more extensive decrements in those with greater impairment on neuropsychological testing (Hosokai et al., 2009; Huang et al., 2008; Pappata et al., 2011). Indeed, spatial covariance mapping has disclosed specific metabolic brain networks related to the motor as well as the cognitive manifestations of the disorder (Eidelberg, 2009). The PD motor-related pattern (PDRP) is characterized by increased pallido-thalamic and pontine metabolic activity, associated with relatively reduced activity in the premotor cortex, supplementary motor area, and parietal association regions (Ma et al., 2007). By contrast, the PD cognition-related pattern (PDCP; Fig. 1) is topographically distinct and is characterized by metabolic reductions in frontal and parietal association areas and relative increases in the cerebellar vermis and dentate nuclei (Huang et al., 2007a). PDRP and PDCP expression values have been found to correlate with clinical ratings of motor and cognitive disability in multiple patient populations (Eidelberg, 2009). Moreover, in longitudinal studies, subject expression of the two patterns has been observed to increase with disease progression, albeit at significantly different rates (Huang et al., 2007b; Tang et al., 2010).
Figure 1. Parkinson’s disease cognition-related metabolic pattern.
This network, known as PDCP, is characterized by metabolic reductions (blue) involving the premotor cortex (PMC), rostral supplementary motor area (preSMA) and the precuneus, associated with covarying increases (red) in the cerebellum and dentate nucleus (DN) (Huang et al., 2007a). [The display was superimposed onto a single-subject MRI brain template and thresholded at z=2.44, p<0.01.]
While the clinical correlates of PDRP and PDCP expression have been studied extensively, the relationship between individual patient differences in network activity and nigrostriatal dopaminergic functioning is not well understood. In this dual tracer positron emission tomography (PET) study, non-demented PD patients were scanned with [18F]-fluorodeoxyglucose (FDG) PET to quantify PDRP and PDCP expression. The same subjects were additionally scanned with [18F]-fluoropropyl-β-CIT (FPCIT) PET to quantify caudate and putamen DAT binding. We then used correlation analysis to study the relationship between these descriptors of the disease process.
Methods
Subjects
We studied 17 right-handed PD patients (men/women: 11/6, age: 63.2±9.0 years (mean±SD); disease duration: 5.3±4.8 years) with mild to moderate motor symptoms and without dementia (Hoehn and Yahr score: 2.0±0.9; off-state Unified Parkinson’s Disease Rating Scale (UPDRS) motor rating: 17.6±9.9). A diagnosis of idiopathic PD was made by a trained movement disorders specialist according to the UK Brain Bank criteria (Hughes et al., 1992). None of the subjects had known causative factors or a family history of parkinsonism and none exhibited dementia, gaze abnormalities, or ataxia. Of the subjects, 10 had more severe motor involvement on the right body side, and seven had more severe motor signs on the left. At the time of imaging, five of the subjects were drug-naïve; the remainder had been treated for at least six months with levodopa and/or dopamine agonist medication.
Ethical permission for the procedures was obtained from the Institutional Review Board of North Shore University Hospital. Written consent was obtained from each subject following detailed explanation of the scanning procedures.
PET Imaging
All subjects underwent PET imaging with FDG and FPCIT in separate scanning sessions as described elsewhere (Huang et al., 2007b). The two scans were separated by an average of 22.3±7.3 days. For both scans, subjects fasted overnight and antiparkinsonian medications were discontinued at least 12 hours before the start of the imaging procedure. PET imaging was performed in 3-D mode using the GE Advance tomograph at North Shore University Hospital in a dimly lit room with minimal auditory stimulation.
The FDG PET scans were used to measure pattern expression in each subject. To do so, the images were spatially normalized to a Talairach-based template and smoothed using a Gaussian kernel at FWHM=10 mm. All image processing was performed using Statistical Parametric Mapping (SPM) software (Wellcome Department of Cognitive Neurology, London, UK) running in MATLAB (Mathworks, Natick, MA). The disease-specific patterns assayed in this study have been previously characterized and extensively validated in independent populations (Eidelberg, 2009; Niethammer and Eidelberg, 2012). PDCP and PDRP subject scores, which quantify pattern expression in each FDG PET scan, were computed for each subject using a fully-automated voxel-based algorithm (software available at http://www.fil.ion.ucl.ac.uk/spm/ext/#SSM). For comparison with previously published data, network expression was standardized with respect to reference values from healthy volunteer subjects (Spetsieris and Eidelberg, 2011). For the PDCP, subject scores were z-scored with respect to values from a fixed reference group comprised of 15 healthy volunteer subjects as described elsewhere (Huang et al., 2008; Huang et al., 2007a; Mattis et al., 2011). Analogously, the PDRP subject scores were standardized with respect to values from the 33 normal subjects whose scans were used originally to derive the pattern (Eidelberg, 2009; Ma et al., 2007; Spetsieris and Eidelberg, 2011).
The FPCIT PET scans were used to measure DAT binding as a quantitative index of presynaptic dopaminergic function in each subject. This was accomplished by aligning each scan to the image frame acquired at 40 minutes post-injection so as to correct for motion artifacts. All dynamic frames were averaged over 0–27 minutes to obtain a high-count mean image resembling the normal distribution of cerebral blood flow (Ma et al., 2002). Regions-of-interest (ROIs) were defined for the caudate, putamen, and occipital cortex of each hemisphere; this was performed manually in native space of each subject. The same set of ROIs was placed on a single slice summed over the striatal sections of the mean image and were individually adjusted for each subject. We estimated caudate and putamen DAT binding separately for each hemisphere by the striatal-to-occipital ratio, defined as (striatum – occipital)/occipital counts in a single 10-minute frame, beginning 90 minutes after tracer injection (Huang et al., 2007b; Ma et al., 2002). For each subject, right and left DAT binding values were separately averaged for each region. The resulting mean caudate and putamen DAT binding values were compared to the analogous ROI measurements from FPCIT PET scans acquired in 10 age-matched healthy subjects (men/women: 4/6; age: 60.0±9.9 years). For the volume-of-interest (VOI) analysis (see below), image maps of DAT binding index were also generated over the same 10-minute frame by calculating (voxel – occipital)/occipital ratios and spatially normalizing to the same anatomic space as the FDG PET scans described above (Ma et al., 2002).
Correlation Analysis
Two approaches were used to assess the relationship between regional DAT binding and metabolic network activity in the standard anatomic space. Firstly, without hypothesis, we explored the entire FPCIT PET image volume for discrete regions (i.e., clusters of voxels) in which local DAT binding correlated with metabolic network activity (PDCP and PDRP) measured in FDG PET scans from the same subjects. In separate voxel-wise searches, PDCP subject scores were entered as covariates in which positive and negative contrasts were defined as “1, 0” and “−1, 0” in SPM. Moreover, to account for the potential influence of individual differences in subject age and in the duration and severity of motor symptoms, the correlation analysis was repeated with these descriptors as “nuisance variables.” In these analyses, voxel-wise correlations were thresholded at p=0.001, uncorrected at peak voxel and were considered to be significant for p<0.05, corrected. For each significant cluster, the individual data were scrutinized graphically for potential bimodality and/or outlier effects. This was accomplished by measuring DAT binding in each spatially-normalized scan within a spherical VOI (radius=5 mm) centered on the peak voxel of the cluster. Correlations were additionally assessed post-hoc in a “mirror” VOI centered at the same coordinates on the opposite cerebral hemisphere.
The magnitude of correlations identified by voxel-wise interrogation of the whole brain volume can be exaggerated through selection bias (Kriegeskorte et al., 2009; Vul et al., 2009). To validate the observed correlations, we followed-up using an anatomical ROI approach (Kriegeskorte et al., 2010). Here, confirmatory searches for correlations between network activity and DAT binding were restricted to the anatomically defined ROIs described above. Relationships between caudate and putamen DAT binding and pattern expression were assessed by computing Pearson’s correlation coefficients for each pair of variables. Multiple regression analysis was used to reassess these relationships after accounting for age, disease duration, and severity of motor symptoms. These analyses were performed using JMP5.0 (SAS Institute, Cary, NC) and were considered significant at p<0.05.
Results
Compared to the healthy controls, DAT binding was reduced in both the caudate and putamen in the PD subjects (percentage of normal±SEM: left caudate 82% ± 6.2, right caudate 80% ± 5.7, average caudate 81% ± 5.8; left putamen 43.6% ± 5.5, right putamen 44.3% ± 4.5, average putamen 44% ± 4.3). Average PDCP score was 1.04 ± 0.87, and average PDRP score was 2.45 ± 1.37.
Voxel-wise searches across the entire FPCIT PET brain volume revealed only one significant region in which local DAT binding correlated with PDCP expression. This cluster (Fig. 2A) was localized to the left caudate region (coordinates: x=−14, y=20, z=−2 mm; Zmax=4.06, p<0.05, corrected). The highly significant correlation between DAT binding in this region and PDCP expression was substantiated by graphical review of the individual VOI data (Fig. 2B, top). Post-hoc analysis revealed an analogous relationship with DAT binding (r=−0.63, p<0.01) in the right caudate region (Fig. 2B, bottom), which was discerned when the “mirror” VOI coordinates were placed in the same position on the opposite cerebral hemisphere (see Methods). On both sides, correlations between the caudate DAT binding and PDCP values remained significant after adjusting for age, UPDRS motor ratings, and disease duration (left: p<0.001; right: p<0.05). No significant region was identified in which DAT binding correlated with PDRP expression.
Figure 2. PDCP expression correlated with caudate DAT binding.
A. A single cluster was identified in a voxel-wise whole brain search of the FPCIT PET images for regions with significant correlations between DAT binding and PDCP activity (see text). This region was localized to the left caudate nucleus (x=−14, y=20, z=−2 mm; Zmax=4.06, p<0.05, corrected). A smaller cluster (arrow) was identified in the right caudate nucleus at the less stringent hypothesis-testing threshold of p=0.005, uncorrected. [The display was superimposed onto a single-subject MRI brain template and thresholded at t=2.95, p<0.005). L indicates the left cerebral hemisphere.]
B. Display of individual data from a spherical volume-of-interest centered at the peak voxel of the left caudate cluster (p<0.001, top) and from a mirror volume placed at the same coordinates on the right cerebral hemisphere (p<0.01, bottom).
C. The results were validated using an independent correlation analysis that employed anatomically defined regions-of-interest to define the boundaries of the left and right caudate nucleus (see text). DAT binding measurements in these hypothesis-testing regions (presented as percent of the normal mean) were found to correlate significantly with PDCP expression values (left caudate: p<0.004; right caudate: p<0.02). [The best fitting regression line for each correlation is depicted by a solid line; the 95% confidence intervals by broken curves.]
Because of the possibility of selection bias in these data-driven searches (see Methods), we confirmed the findings by directly correlating pattern expression with independent DAT binding measurements performed in the native image space using prespecified anatomically defined ROIs. Pairwise correlations between PDRP/PDCP subject expression and caudate and putamen DAT binding are presented in Table 1. PDCP subject scores exhibited an inverse relationship with DAT binding in both striatal regions (caudate: r=−0.67, p<0.005; putamen: r=−0.51, p<0.05). However, after accounting for individual differences in age, UPDRS motor ratings, and disease duration, the correlation between PDCP expression and caudate DAT binding remained significant (p=0.01, multiple regression), whereas the correlation with putamen DAT binding did not (p>0.20). As in the voxel-wise analysis, the correlation between caudate DAT binding and PDCP expression was greater in magnitude on the left side (left: r=−0.69, p<0.004; right: r=−0.60, p<0.02; Fig. 2C). Similar to the voxel-wise analysis, the correlations with PDRP expression and striatal DAT binding were not significant.
Table 1.
Pairwise correlations between network activity and striatal DAT binding
| Pattern Expression | DAT Bindinga | |||
|---|---|---|---|---|
| PDCP | PDRP | Caudate | Putamen | |
| PDCP | r=0.43 | r=−0.67** | r=−0.51* | |
| PDRP | r=−0.32 | r=−0.22 | ||
| Caudate DAT Binding | r=0.84*** | |||
p<0.05,
p<0.01,
p<0.001
Left/right average of caudate and putamen ROI values (see Methods)
Discussion
PD leads to motor and cognitive dysfunction. Pathologically, the most salient feature of this disease is loss of dopaminergic input to the striatum. In this study we set out to investigate potential relationships between localized nigrostriatal abnormalities and the more widespread metabolic network changes that have been observed in this disorder. Using entirely data-driven voxel searches of the FPCIT PET scans without hypothesis, as well as more restricted searches using anatomically defined ROIs, we demonstrated a specific relationship between caudate DAT binding and PDCP expression, which survived adjustment for individual differences in age, disease severity and duration. These findings are consistent with prior studies suggesting a role for the caudate nucleus in cognition, as well as a link between dopaminergic input to this region and impaired performance on tests of executive functioning in PD patients (Carbon et al., 2004; Cheesman et al., 2005; Muller et al., 2000; van Beilen et al., 2008). In this vein, dopaminergic imaging of PD patients with mild cognitive impairment or dementia indicates greater loss of radiotracer uptake in the caudate than their non-demented counterparts (Ekman et al., 2012; Ito et al., 2002; O'Brien et al., 2004). Significant correlation has been reported between verbal and visual memory functioning and caudate fluoro-dopa uptake, along with associations between task performance and measures of hippocampal and prefrontal atrophy (Jokinen et al., 2009). Similarly, the level of dopaminergic dysfunction in the caudate nucleus modulates metabolic activity in associative frontostriatal circuitry (Polito et al., 2012).
The voxel-wise search revealed a significant cluster in the left caudate, with a non-significant cluster in the right caudate. Asymmetry in caudate volume has been reported specifically for progressive non-fluent aphasia (Looi et al., 2008), but is not known if dopaminergic dysfunction exhibits any systematic asymmetry in PD. Moreover, we found in post-hoc analysis that both DAT binding in both the right and left caudate correlated with PDCP expression values. Thus, a more likely explanation is the fact that PDCP itself is not a symmetrical pattern (Fig. 1), and its expression in a given subject is more closely associated with metabolic activity in the left, relative to the right, cerebral hemisphere.
The observed correlation between caudate DAT binding and expression of the broadly distributed PDCP network highlights the relevance of nigral dopaminergic input to this structure and cognitive functioning in early PD patients. Nonetheless, it is important to note that individual differences in caudate DAT binding account for under 50% of the variation in PDCP expression observed across the subjects. In other words, cognition-related metabolic network activity is not determined solely by loss or dysfunction of nigral dopaminergic projections to the caudate. Rather, extra-striatal dopaminergic and other neurotransmitter systems and/or pathological processes are likely to be involved in this aspect of the disease process. For instance, evolving deficits in cholinergic neurotransmission are common in PD (Bohnen et al., 2003; Hilker et al., 2005), and several of the cortical PDCP nodes correspond closely in position with areas found independently to exhibit reduced acetylcholinesterase activity in PD patients (Huang et al., 2007a). It is also possible that the evolving network-related metabolic reductions seen with advancing cognitive symptoms reflect the development of inherent cortical pathology (Braak et al., 2003; Niethammer and Eidelberg, 2012). In all likelihood, the cognitive manifestations of PD are derived from an interplay of all these mechanisms, with one or the other dominating at different stages of the disease.
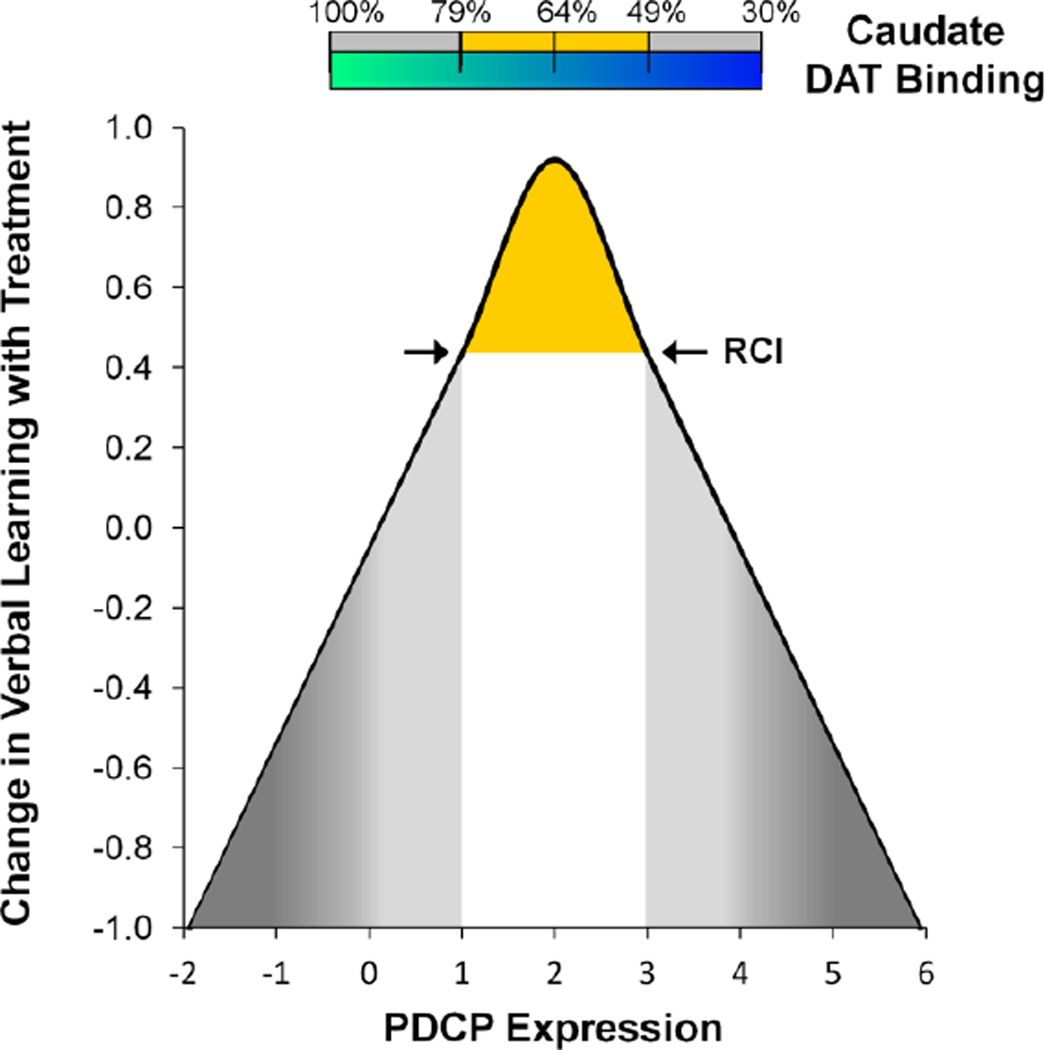
The current findings may also shed light on some of the apparent inconsistencies in the effects of dopaminergic therapy on cognitive functioning that have been observed in non-demented PD patients (Fig. 3). We have recently reported that improvement in verbal learning performance during levodopa treatment correlates with baseline PDCP expression (Mattis et al., 2011). Meaningful treatment-mediated cognitive improvement in these individuals (defined independently according to the Reliable Change Index) (Jacobson and Truax, 1991; Maassen, 2004) was associated with baseline PDCP expression exceeding 1.0 (Mattis et al., 2011). Using the linear equation that best related off-state PDCP values to mean caudate DAT binding based on the current data, we estimated the threshold (i.e., PDCP=1.0) for dopaminergically mediated cognitive improvement to correspond to an approximately 21% reduction in caudate DAT binding. By contrast, intact caudate dopaminergic input is associated with subthreshold network activity at baseline, and no cognitive change during levodopa treatment. In this regard, treatment-mediated decrements in task performance have been noted in PD patients with low baseline PDCP expression (subject scores < −1.0), which in accordance with the “dopamine overdose” hypothesis (Cools, 2006; Gotham et al., 1988), is associated with supernormal caudate DAT binding levels. We further note that reductions in caudate DAT binding in the 35–50% range are associated with moderately high baseline PDCP expression (subject scores between 2.0 and 3.0), and concomitant treatment-mediated cognitive benefit. That said, further loss of caudate dopaminergic afferents accompanied by PDCP elevations ≥ 3.0 may not necessarily promote a positive cognitive response to medication. Indeed, we have found that PDCP values in this range are consistent with substantial cognitive impairment at baseline, or even frank dementia (Eidelberg, 2009; Huang et al., 2008). In such individuals, network modulation during levodopa treatment, and the associated cognitive response, may be restricted by the deposition of abnormal protein aggregates at key PDCP nodes (Niethammer and Eidelberg, 2012). It is likely that interventions for the cognitive side effects of dopaminergic treatment in PD patients will involve other neurotransmitter systems. For example, one may envision using acetylcholinesterase inhibitors (Emre et al., 2004). This may be relevant in individuals at the low end of the PDCP spectrum, who may be particularly susceptible to dopaminergic overdose effects involving caudate nucleus and its associated prefrontal pathways (Carbon et al., 2004).
Figure 3. Hypothetical relationship between levodopa-mediated changes in cognitive performance, baseline PDCP expression, and caudate DAT binding.
Based on the current data and the findings of Mattis et al. (2011), we propose that an inverted U-shaped relationship exists between the changes in cognitive functioning observed during levodopa treatment (y-axis) and baseline measurements of caudate dopaminergic input (horizontal bar) and PDCP metabolic network activity (x-axis). See text for discussion. The numbers at the top of the horizontal bar represent the caudate DAT binding values that correspond to PDCP scores of 1.0, 2.0, and 3.0, respectively. These estimates were based upon the best fitting linear relationship of the two measures (mean caudate DAT binding = 0.94–0.15*PDCP score). The area under the curve associated with treatment-mediated cognitive benefit (yellow) was defined by an increase in individual subject test performance exceeding the independently determined Reliable Change Index (RCI) (Jacobson and Truax, 1991; Maassen, 2004) on the psychometric outcome measure. For verbal learning performance, the RCI cutoff (arrows) was determined to be 0.44, which is hypothesized to correspond to PDCP scores between +1.0 and +3.0 (Mattis et al., 2011). No cognitive benefit during treatment is expected for PDCP values on either side of this interval (gray).
Finally, we note that in PD, reductions in putaminal dopaminergic function generally relate to the degree of motor rather than cognitive manifestations of the disorder (Muller et al., 2000; Ribeiro et al., 2002; Rinne et al., 2000; van Beilen et al., 2008). In our study, we did not observe a correlation between PDRP expression and putaminal DAT binding. This can be explained to some degree by an incipient floor effect that progressively lowers the between-subject variability of putamen DAT binding as the illness progresses (Huang et al., 2007b). Longitudinal analysis revealed that as individual putamen values approach the floor (approximately 25% of the normal mean), the standard deviation of the measure declines faster than the mean. This leads to a continuous reduction in the dispersion of the putamen DAT binding measure (Huang et al., 2007b). Of note, we have found this effect to be more pronounced when the FPCIT PET data are analyzed using smaller posterior putamen ROIs designed to capture declining signal in the most dopaminergically denervated portion of the motor striatum. By contrast, there was no evidence of a floor effect in the data garnered from the relatively preserved caudate region. Along these lines, we note that the range of PDRP values in the current sample of mildly affected PD patients was smaller than for PDCP expression in the same subjects. The relatively narrow range of PDRP expression in this cohort limits the potential for a significant correlation to be realized between the activity of this network and DAT binding measured in either the caudate or putamen. This issue is less concerning, however, for longitudinal study designs, particularly when processes are sampled repeatedly over multiple time points. In this context, within-subject changes in PDRP expression have indeed been found to correlate robustly with concurrent declines in putamen DAT binding (Huang et al., 2007b; Tang et al., 2010).
Conclusions
In summary, this study demonstrates the presence of a significant correlation between reduced dopaminergic input to the caudate nucleus and an abnormal cognition-related metabolic brain network in non-demented PD patients. Both of these baseline measures likely play a role in determining the cognitive effects of dopaminergic therapy in PD.
Further studies are needed to examine the relationship between these variables and cognitive performance in the same subjects, and to determine how these baseline features influence neurobehavioral treatment outcomes. Further work is also needed to clarify the role of abnormalities in other neurotransmitter systems and the impact of pathological protein aggregates in mediating the cognitive manifestations of the disorder.
Acknowledgements
The authors wish to thank Dr. Thomas Chaly and Mr. Claude Margouleff for technical support, Ms. Patricia J. Allen for assistance with data analysis, and Ms. Yoon Young Choi and Ms. Toni Fitzpatrick for valuable assistance with manuscript preparation/copyediting.
Disclosure Statement
Dr. Niethammer has served as a paid consultant for Merz Pharmaceuticals.
Dr. Tang has received research support from Neurologix, Inc. and the NIH (NINDS).
Dr. Ma has received research support from the NIH (NINDS, NIDCD).
Dr. Mattis has served as a paid consultant for Neurologix, Inc., has provided expert testimony for medico-legal cases and receives grant funding from the NIH (NINDS).
Dr. Ko reports no disclosures.
Dr. Dhawan has received research support from Neurologix, Inc., the NIH (NIDCD, NIAID, NINDS), the Dana Foundation, and the Thomas Hartman Foundation for Parkinson’s Research, Inc.
Dr. Eidelberg serves on the scientific advisory boards for the Michael J. Fox Foundation and the Bachmann-Strauss Dystonia and Parkinson Foundation, Inc. and Merck & Co., Inc.; serves on the editorial board of Annals of Neurology and NeuroImage and as Associate Editor for the Journal of Neuroscience; and is listed as coinventor of patents re: Markers for use in screening patients for nervous system dysfunction and a method and apparatus for using same; has received research support from the NIH (NINDS, NCRR, NIDCD, NIAID), High Q Foundation, the Dana Foundation, and the Bachmann-Strauss Dystonia and Parkinson Foundation; has served as an expert in medico-legal cases; and has served as scientific advisor for the Thomas Hartman Foundation for Parkinson’s Research, Inc., and as a consultant for Neurologix, Inc.
Funding
This work was supported in part by Grant No. P50NS071675 (Morris K. Udall Center of Excellence in Parkinson’s Disease Research at The Feinstein Institute for Medical Research) to D.E. from the National Institute of Neurological Disorders and Stroke and the Thomas Hartman Foundation for Parkinson’s Research, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. The sponsor did not play a role in study design, collection, analysis and interpretation of data, writing of the report or in the decision to submit the paper for publication.
Abbreviations
- PD
Parkinson’s disease
- PDCP
Parkinson’s disease cognition-related pattern
- PDRP
Parkinson’s disease motor-related pattern
- DAT
dopamine transporter
- FDG
[18F]-fluorodeoxyglucose
- FPCIT
[18F]-fluoropropyl-β-CIT
- UPDRS
Unified Parkinson’s Disease Rating Scale
- SPM
statistical parametric mapping
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Martin Niethammer, Email: mniethamme@nshs.edu.
Chris C. Tang, Email: ctang@nshs.edu.
Yilong Ma, Email: yma@nshs.edu.
Paul J. Mattis, Email: pmattis@nshs.edu.
Ji Hyun Ko, Email: jko1@nshs.edu.
Vijay Dhawan, Email: vdhawan@nshs.edu.
References
- Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2006;21:1123–1130. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, Mathis CA, Moore RY, DeKosky ST. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003;60:1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Bruck A, Aalto S, Nurmi E, Bergman J, Rinne JO. Cortical 6-[18F]fluoro-Ldopa uptake and frontal cognitive functions in early Parkinson's disease. Neurobiol Aging. 2005;26:891–898. doi: 10.1016/j.neurobiolaging.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, Eidelberg D. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neuroimage. 2004;21:1497–1507. doi: 10.1016/j.neuroimage.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Cheesman AL, Barker RA, Lewis SJ, Robbins TW, Owen AM, Brooks DJ. Lateralisation of striatal function: evidence from 18F-dopa PET in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 2005;76:1204–1210. doi: 10.1136/jnnp.2004.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for LDOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32:548–557. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman U, Eriksson J, Forsgren L, Mo SJ, Riklund K, Nyberg L. Functional brain activity and presynaptic dopamine uptake in patients with Parkinson's disease and mild cognitive impairment: a cross-sectional study. Lancet neurology. 2012;11:679–687. doi: 10.1016/S1474-4422(12)70138-2. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, Durif F, Kulisevsky J, van Laar T, Lees A, Poewe W, Robillard A, Rosa MM, Wolters E, Quarg P, Tekin S, Lane R. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med. 2004;351:2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. 'Frontal' cognitive function in patients with Parkinson's disease 'on' and 'off' levodopa. Brain. 1988;111(Pt 2):299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Green J, McDonald WM, Vitek JL, Evatt M, Freeman A, Haber M, Bakay RA, Triche S, Sirockman B, DeLong MR. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59:1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- Hilker R, Thomas AV, Klein JC, Weisenbach S, Kalbe E, Burghaus L, Jacobs AH, Herholz K, Heiss WD. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–1722. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- Hirano S, Dhawan V, Eidelberg D. PET Imaging in Movement Disorders. In: Kompoliti K, Verhagen L, editors. Encyclopedia of Movement Disorders. Academic Press; New York: 2010. pp. 452–461. [Google Scholar]
- Hirano S, Shinotoh H, Eidelberg D. Functional Brain Imaging of Cognitive Dysfunction in Parkinson's Disease. J Neurol Neurosurg Psychiatry. 2012;83:963–969. doi: 10.1136/jnnp-2011-301818. [DOI] [PubMed] [Google Scholar]
- Hosokai Y, Nishio Y, Hirayama K, Takeda A, Ishioka T, Sawada Y, Suzuki K, Itoyama Y, Takahashi S, Fukuda H, Mori E. Distinct patterns of regional cerebral glucose metabolism in Parkinson's disease with and without mild cognitive impairment. Mov Disord. 2009;24:854–862. doi: 10.1002/mds.22444. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurol. 2008;70:1470–1477. doi: 10.1212/01.wnl.0000304050.05332.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson's disease. Neuroimage. 2007a;34:714–723. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tang C, Feigin A, Lesser M, Ma Y, Pourfar M, Dhawan V, Eidelberg D. Changes in network activity with the progression of Parkinson's disease. Brain. 2007b;130:1834–1846. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, Daniel S, Kilford L, Lees A. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Nagano-Saito A, Kato T, Arahata Y, Nakamura A, Kawasumi Y, Hatano K, Abe Y, Yamada T, Kachi T, Brooks DJ. Striatal and extrastriatal dysfunction in Parkinson's disease with dementia: a 6-[18F]fluoro-L-dopa PET study. Brain. 2002;125:1358–1365. doi: 10.1093/brain/awf134. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson's disease: a community-based, 4-year longitudinal study. Journal of geriatric psychiatry and neurology. 2005;18:149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- Jokinen P, Bruck A, Aalto S, Forsback S, Parkkola R, Rinne JO. Impaired cognitive performance in Parkinson's disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism Relat Disord. 2009;15:88–93. doi: 10.1016/j.parkreldis.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Lindquist MA, Nichols TE, Poldrack RA, Vul E. Everything you never wanted to know about circular analysis, but were afraid to ask. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1551–1557. doi: 10.1038/jcbfm.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi JC, Lindberg O, Zandbelt BB, Ostberg P, Andersen C, Botes L, Svensson L, Wahlund LO. Caudate nucleus volumes in frontotemporal lobar degeneration: differential atrophy in subtypes. AJNR. American journal of neuroradiology. 2008;29:1537–1543. doi: 10.3174/ajnr.A1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Dhawan V, Mentis M, Chaly T, Spetsieris PG, Eidelberg D. Parametric mapping of [18F]FPCIT binding in early stage Parkinson's disease: a PET study. Synapse. 2002;45:125–133. doi: 10.1002/syn.10090. [DOI] [PubMed] [Google Scholar]
- Ma Y, Tang C, Spetsieris P, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson's disease: test-retest reproducibility. J Cereb Blood Flow & Metab. 2007;27:597–605. doi: 10.1038/sj.jcbfm.9600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen GH. The standard error in the Jacobson and Truax Reliable Change Index: the classical approach to the assessment of reliable change. J Int Neuropsychol Soc. 2004;10:888–893. doi: 10.1017/s1355617704106097. [DOI] [PubMed] [Google Scholar]
- Mattis PJ, Tang CC, Ma Y, Dhawan V, Eidelberg D. Network correlates of the cognitive response to levodopa in Parkinson disease. Neurology. 2011;77:858–865. doi: 10.1212/WNL.0b013e31822c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Wachter T, Barthel H, Reuter M, von Cramon DY. Striatal [123I]beta-CIT SPECT and prefrontal cognitive functions in Parkinson's disease. J Neural Transm. 2000;107:303–319. doi: 10.1007/s007020050025. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Eidelberg D. Metabolic brain networks in translational neurology: concepts and applications. Ann Neurol. 2012;72:635–647. doi: 10.1002/ana.23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JT, Colloby S, Fenwick J, Williams ED, Firbank M, Burn D, Aarsland D, McKeith IG. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol. 2004;61:919–925. doi: 10.1001/archneur.61.6.919. [DOI] [PubMed] [Google Scholar]
- Pappata S, Santangelo G, Aarsland D, Vicidomini C, Longo K, Bronnick K, Amboni M, Erro R, Vitale C, Caprio MG, Pellecchia MT, Brunetti A, De Michele G, Salvatore M, Barone P. Mild cognitive impairment in drug-naive patients with PD is associated with cerebral hypometabolism. Neurology. 2011;77:1357–1362. doi: 10.1212/WNL.0b013e3182315259. [DOI] [PubMed] [Google Scholar]
- Polito C, Berti V, Ramat S, Vanzi E, De Cristofaro MT, Pellicano G, Mungai F, Marini P, Formiconi AR, Sorbi S, Pupi A. Interaction of caudate dopamine depletion and brain metabolic changes with cognitive dysfunction in early Parkinson's disease. Neurobiology of aging. 2012;33:206, e229–e239. doi: 10.1016/j.neurobiolaging.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Ribeiro MJ, Vidailhet M, Loc'h C, Dupel C, Nguyen JP, Ponchant M, Dolle F, Peschanski M, Hantraye P, Cesaro P, Samson Y, Remy P. Dopaminergic function and dopamine transporter binding assessed with positron emission tomography in Parkinson disease. Arch Neurol. 2002;59:580–586. doi: 10.1001/archneur.59.4.580. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Portin R, Ruottinen H, Nurmi E, Bergman J, Haaparanta M, Solin O. Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18F]fluorodopa positron emission tomographic study. Arch Neurol. 2000;57:470–475. doi: 10.1001/archneur.57.4.470. [DOI] [PubMed] [Google Scholar]
- Spetsieris PG, Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson's disease: methodological issues. Neuroimage. 2011;54:2899–2914. doi: 10.1016/j.neuroimage.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Poston K, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson's disease. J Neurosci. 2010;30:1049–1056. doi: 10.1523/JNEUROSCI.4188-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thobois S, Jahanshahi M, Pinto S, Frackowiak R, Limousin-Dowsey P. PET and SPECT functional imaging studies in Parkinsonian syndromes: from the lesion to its consequences. Neuroimage. 2004;23:1–16. doi: 10.1016/j.neuroimage.2004.04.039. [DOI] [PubMed] [Google Scholar]
- van Beilen M, Portman AT, Kiers HA, Maguire RP, Kaasinen V, Koning M, Pruim J, Leenders KL. Striatal FDOPA uptake and cognition in advanced nondemented Parkinson's disease: a clinical and FDOPA-PET study. Parkinsonism Relat Disord. 2008;14:224–228. doi: 10.1016/j.parkreldis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition. Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]