Abstract
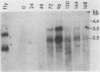
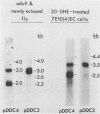
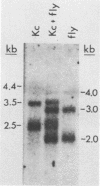
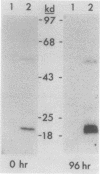
The induction of DOPA decarboxylase (DDC) activity by 20-OH-ecdysone (20-OHE) in a subline of Drosophila melanogaster Kc cells was investigated. Cells cultured in the continuous presence of the steroid hormone exhibited a 96-h temporal lag prior to a peak of DDC enzyme activity while arrested in the G2 phase of the cell cycle. The concentration of Ddc RNA increased sixfold between 72 and 96 h after initial exposure to hormone. Similarly, this increase was correlated temporally with a 26-fold increase in DDC enzyme activity. The Kc Ddc primary transcript, processing intermediate, and mature mRNA all were approximately 500 nucleotides longer than the corresponding transcripts observed for newly eclosed adult D. melanogaster. In vitro translation of poly(A)+ RNA from Kc cells resulted in an immunoprecipitable polypeptide which exhibited similar mobility on sodium dodecyl sulfate gels to that of DDC synthesized in vitro by larval epidermal poly(A)+ RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall C. J., Hirsh J. Drosophila melanogaster Ddc gene transcripts are not expressed at high levels during early embryogenesis. Dev Biol. 1986 Mar;114(1):258–259. doi: 10.1016/0012-1606(86)90401-x. [DOI] [PubMed] [Google Scholar]
- Beall C. J., Hirsh J. High levels of intron-containing RNAs are associated with expression of the Drosophila DOPA decarboxylase gene. Mol Cell Biol. 1984 Sep;4(9):1669–1674. doi: 10.1128/mcb.4.9.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cherbas P., Cherbas L., Williams C. M. Induction of acetylcholinesterase activity by beta-ecdysone in a Drosophila cell line. Science. 1977 Jul 15;197(4300):275–277. doi: 10.1126/science.877552. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark W. C., Doctor J., Fristrom J. W., Hodgetts R. B. Differential responses of the dopa decarboxylase gene to 20-OH-ecdysone in Drosophila melanogaster. Dev Biol. 1986 Mar;114(1):141–150. doi: 10.1016/0012-1606(86)90390-8. [DOI] [PubMed] [Google Scholar]
- Courgeon A. M. Action of insect hormones at the cellular level. Morphological changes of a diploid cell line of Drosophila melanogaster, treated with ecdysone and several analogues in vitro. Exp Cell Res. 1972 Oct;74(2):327–336. doi: 10.1016/0014-4827(72)90384-9. [DOI] [PubMed] [Google Scholar]
- Dewhurst S. A., Croker S. G., Ikeda K., McCaman R. E. Metabolism of biogenic amines in Drosophila nervous tissue. Comp Biochem Physiol B. 1972 Dec 15;43(4):975–981. doi: 10.1016/0305-0491(72)90241-6. [DOI] [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. In vitro culture of Drosophila melanogaster embryonic cells. In Vitro. 1970 Nov-Dec;6(3):162–172. doi: 10.1007/BF02617759. [DOI] [PubMed] [Google Scholar]
- Fain M. J., Stevens B. Alterations in the cell cycle of Drosophila imaginal disc cells precede metamorphosis. Dev Biol. 1982 Jul;92(1):247–258. doi: 10.1016/0012-1606(82)90169-5. [DOI] [PubMed] [Google Scholar]
- Falkenthal S., Parker V. P., Davidson N. Developmental variations in the splicing pattern of transcripts from the Drosophila gene encoding myosin alkali light chain result in different carboxyl-terminal amino acid sequences. Proc Natl Acad Sci U S A. 1985 Jan;82(2):449–453. doi: 10.1073/pnas.82.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Hodgetts R. B. An analysis of dopa decarboxylase expression during embryogenesis in Drosophila melanogaster. Dev Biol. 1985 Jan;107(1):142–155. doi: 10.1016/0012-1606(85)90383-5. [DOI] [PubMed] [Google Scholar]
- Graves B. J., Schubiger G. Cell cycle changes during growth and differentiation of imaginal leg discs in Drosophila melanogaster. Dev Biol. 1982 Sep;93(1):104–110. doi: 10.1016/0012-1606(82)90243-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Sloan J. S., Kelly J. D. A Drosophila metabolic gene transcript is alternatively processed. Cell. 1983 Sep;34(2):405–414. doi: 10.1016/0092-8674(83)90374-4. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Davidson N. Isolation and characterization of the dopa decarboxylase gene of Drosophila melanogaster. Mol Cell Biol. 1981 Jun;1(6):475–485. doi: 10.1128/mcb.1.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K., Riddiford L. M. Hormonal regulation of dopa decarboxylase during a larval molt. Dev Biol. 1985 Aug;110(2):509–513. doi: 10.1016/0012-1606(85)90109-5. [DOI] [PubMed] [Google Scholar]
- Kraminsky G. P., Clark W. C., Estelle M. A., Gietz R. D., Sage B. A., O'Connor J. D., Hodgetts R. B. Induction of translatable mRNA for dopa decarboxylase in Drosophila: an early response to ecdysterone. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4175–4179. doi: 10.1073/pnas.77.7.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Tempel B. L. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983 May 5;303(5912):67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- Lunan K. D., Mitchell H. K. The metabolism of tyrosine-O-phosphate in Drosophila. Arch Biochem Biophys. 1969 Jul;132(2):450–456. doi: 10.1016/0003-9861(69)90388-9. [DOI] [PubMed] [Google Scholar]
- McCaman M. W., McCaman R. E., Lees G. J. Liquid cation exchange--a basis for sensitive radiometric assays for aromatic amino acid decarboxylases. Anal Biochem. 1972 Jan;45(1):242–252. doi: 10.1016/0003-2697(72)90024-3. [DOI] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Schimke R. T. Interaction of estrogen and progesterone in chick oviduct development. II. Effects of estrogen and progesterone on tubular gland cell function. J Cell Biol. 1969 Oct;43(1):123–137. doi: 10.1083/jcb.43.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. I. Independent regulation of ovalbumin, conalbumin, ovomucoid, and lysozyme induction. J Biol Chem. 1972 Oct 25;247(20):6450–6461. [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. II. Modulation of polypeptide elongation and initiation rates by estrogen and progesterone. J Biol Chem. 1972 Nov 10;247(21):6770–6780. [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Differential processing of RNA transcribed from the single-copy Drosophila myosin heavy chain gene produces four mRNAs that encode two polypeptides. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2128–2132. doi: 10.1073/pnas.83.7.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Savakis C., Koehler M. M., Cherbas P. cDNA clones for the ecdysone-inducible polypeptide (EIP) mRNAs of Drosophila Kc cells. EMBO J. 1984 Jan;3(1):235–243. doi: 10.1002/j.1460-2075.1984.tb01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholnick S. B., Morgan B. A., Hirsh J. The cloned dopa decarboxylase gene is developmentally regulated when reintegrated into the Drosophila genome. Cell. 1983 Aug;34(1):37–45. doi: 10.1016/0092-8674(83)90134-4. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Gietz R. D., Hodgetts R. B. Analysis of the transcription unit adjacent to the 3'-end of the dopa decarboxylase gene in Drosophila melanogaster. Dev Biol. 1986 Mar;114(1):260–264. doi: 10.1016/0012-1606(86)90402-1. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Gietz R. D., Hodgetts R. B. Overlapping transcription units in the dopa decarboxylase region of Drosophila. Nature. 1986 Jul 17;322(6076):279–281. doi: 10.1038/322279a0. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Stevens B., O'Connor J. D., Hodgetts R. B. A novel form of DOPA decarboxylase produced in drosophila cells in response to 20-hydroxyecdysone. Can J Biochem Cell Biol. 1983 Jul;61(7):818–825. doi: 10.1139/o83-104. [DOI] [PubMed] [Google Scholar]
- Stevens B., Alvarez C. M., Bohman R., O'Connor J. D. An ecdysteroid-induced alteration in the cell cycle of cultured Drosophila cells. Cell. 1980 Dec;22(3):675–682. doi: 10.1016/0092-8674(80)90543-7. [DOI] [PubMed] [Google Scholar]
- Tata J. R. The expression of the vitellogenin gene. Cell. 1976 Sep;9(1):1–14. doi: 10.1016/0092-8674(76)90047-7. [DOI] [PubMed] [Google Scholar]
- Thireos G., Griffin-Shea R., Kafatos F. C. Untranslated mRNA for a chorion protein of Drosophila melanogaster accumulates transiently at the onset of specific gene amplification. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5789–5793. doi: 10.1073/pnas.77.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]