Abstract
We investigated the presence and alteration of lymphatic vessels in joints of arthritic mice using a whole-slide imaging system. Joints and long bone sections were cut from paraffin blocks of two mouse models of arthritis: meniscal-ligamentous injury (MLI)-induced osteoarthritis (OA) and TNF transgene (TNF-Tg)-induced rheumatoid arthritis (RA). MLI-OA mice were fed a high fat diet to accelerate OA development. TNF-Tg mice were treated with lymphatic growth factor VEGF-C virus to stimulate lymphangiogenesis. Sections were double immunofluorescence stained with anti-podoplanin and alpha-smooth muscle action. The area and number of lymphatic capillaries and mature lymphatic vessels were determined using a whole-slide imaging system and its associated software. Lymphatic vessels in joints were distributed in soft tissues mainly around the joint capsule, ligaments, fat pads and muscles. In long bones, enriched lymphatic vessels were present in the periosteal areas adjacent to the blood vessels. Occasionally, lymphatic vessels were observed in the cortical bone. Increased lymphatic capillaries, but decreased mature lymphatic vessels, were detected in both OA and RA joints. VEGF-C treatment increased lymphatic capillary and mature vessel formation in RA joints. We demonstrated decreased mature lymphatic vessels in the joints of mouse models of severe OA and RA. VEGF-C treatment increased the lymphatic vessel number and area in RA joints. Our findings suggest that the lymphatic system may play an important role in arthritis pathogenesis and treatment.
Keywords: lymphatic vessels, mouse models, osteoarthritis, rheumatoid arthritis, VEGF-C, whole-slide imaging
The lymphatic system is essential for both maintaining tissue fluid balance and immune response, and it is involved in tumor metastasis, wound healing and inflammation (Tammela and Alitalo 2010). Several investigators have reported increased lymphatic vasculature in joints of patients or mice with chronic inflammatory arthritis (Polzer et al. 2008, Zhang et al. 2007). In these studies, immunohistochemical (IHC) staining with antibodies that recognize lymphatic endothelium was used to stain EDTA-decalcified paraffin embedded sections. Commonly used lymphatic endothelial markers include lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) and podoplanin. LYVE-1 is a transmembrane receptor that binds to glycosaminoglycan hyaluronan (Adams and Alitalo 2007, Jackson 2004). LYVE-1 is expressed on all lymphatic endothelial cells during embryonic development, but it is restricted to lymphatic capillaries postnatally (Jackson 2004). Podoplanin is a small, mucin type transmembrane glycoprotein that normally is expressed on lymphatic, but not blood vessel, endothelial cells (Fujiwara et al. 1995, Weninger et al. 1999). Functionally, podoplanin appears to play a more critical role than LYVE-1, because podoplanin knockout mice show severe impairment of lymphatic development in lung and deep tissues (Schacht et al. 2003), while LYVE-1 knockout mice develop normal lymphatics (Gale et al. 2007).
Although IHC data provide valuable information about the involvement of lymphatic vessels in arthritis, it cannot distinguish mature lymphatic vessels from lymphatic capillaries. It is important to distinguish these structures, because lymphatic drainage is carried out only by mature lymphatic vessels so that drainage is affected by factors that regulate lymphatic vessel maturation.[Q1] Mature lymphatic vessels are covered by smooth muscle cells that express alpha smooth muscle actin (αSMA). Thus, mature lymphatic vessels can be identified by double immunofluorescence staining with antibodies against lymphatic endothelium and αSMA (Peyrol et al. 1992). Vessels that have characteristics of lymphatic vessels (thin walls, open lumen, no red blood cells) and are stained by lymphatic endothelial markers and αSMA are defined as mature lymphatic vessels. To date, most studies of lymphatic vessel maturation have been carried out on soft tissue frozen sections or using whole-mount immunostaining of a thin layer of tissue, such as skin (Dellinger et al. 2008, Muthuchamy et al. 2003).
Adjacent bones in joints are hard tissues that must undergo long-term decalcification before sectioning. This procedure makes immunofluorescence difficult, because it weakens or destroys antigens. It is even more difficult to perform histomorphometric analysis on immunofluorescence stained sections, because identification of lymphatic vessels requires the use of high magnification microscopy so that only a small area can be observed before the fluorescence is quenched. Owing to these technical challenges, the distribution and changes in mature lymphatic vessels have not been studied in animal models of arthritis.
We used a whole-slide digital imaging system and double immunofluorescence stained joint and long bone sections. We could identify both capillary and mature lymphatic vessels. Using this system, we examined the distribution of lymphatic vessels in bones of wild type (WT) mice and changes in lymphatic vessels in joint sections from two mouse models of chronic arthritis: osteoarthritis (OA) and rheumatoid arthritis (RA). We found enriched lymphatic vessels in the periosteal regions of long bones and in the joint capsules of WT mice. We demonstrated significantly decreased mature lymphatic vessels in the joints of OA and RA mice. These findings indicate that whole-slide digital imaging is an excellent tool for studying lymphatics in hard tissues. Moreover, they suggest that the lymphatic system may play an important role in arthritis pathogenesis and therapy.
Materials and methods
Mouse models of arthritis
All experiments involving mice were conducted following procedures approved by the University Committee on Animal Resources at the University of Rochester Medical Center. We used two mouse models of arthritis that have been used previously in our center (Li et al. 2004a,b, Sampson et al. 2011a,b, Zhou et al. 2011). One model was the meniscal ligamentous injury (MLI)-induced OA model; these mice were fed a high fat diet. Joints in MLI-OA mice develop OA-like changes when they are 2 months old. We reported earlier that high fat diet increases the severity of joint tissue damage in this model (Mooney et al. 2011). Male mice 10 weeks old on C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). These animals were administered an MLI on the right knee and sham surgery on the left knee. Mice were fed high fat diet (60% kcal; D12492) or low fat diet (10% kcal; D12450B, Open Source Diets, Research Diets Inc., New Brunswick, NJ) for 3 months immediately post-MLI, then sacrificed for the experiments.
The other model was the TNF transgene (TNF-Tg)-induced RA model. These mice were treated with an intra-articular injection of lymphatic growth factor, VEGF-C virus. TNF-Tg mice develop RA-like symptoms when they are 1.5 to 2 months old. We reported that VEGF-C decreases the disease severity in TNF-Tg mice (Zhou et al. 2011). TNF-Tg mice (line 3647) were obtained originally from Dr. G. Kollias and were on C57BL/6 background. One-half-month-old TNF-Tg mice received VEGF-C adeno-associated virus or luciferase virus for 4 months, then were sacrificed for the experiments.
Tissue harvest and immunofluorescence staining of lymphatic vessels
Tissues were fixed by cardiac perfusion using 10% neutral buffered formalin. Isolated knee or ankle joints were fixed for 3 days in 10% neutral buffered formalin, decalcified for 3 weeks in 10% w/v EDTA and embedded in paraffin. Sections 4 μm thick were used for hematoxylin and eosin (H & E) or double immunofluorescence staining as described previously (Zhou et al. 2011). Antigen retrieval was carried out using a digestion solution containing 20 μg/ml proteinase K for 7 min at 37° C. Sections were blocked in 5% BSA-PBST for 30 min at room temperature, then incubated with hamster monoclonal anti-mouse podoplanin (Abcam Inc., Cambridge, MA; cat. #ab11936; clone: 8.1.1) diluted 1:1,000 in 1% BSA-PBST [Q2] at 4° C overnight. After extensive washing, sections were incubated with Alexa Fluor 546-goat-anti-hamster (Invitrogen-Molecular Probes, Eugene, OR; cat. #A21111) diluted 1:400 in 1% BSA-PBST [Q2] and FITC-monoclonal anti-mouse αSMA (Sigma-Aldrich Corp., Saint Louis, MO; cat. #F3777; clone: 1A4) diluted 1:400 in 1% BSA-PBST [Q2] at room temperature for 2 h. The sections were mounted using the aqueous mounting medium, Vector H-1000 (Vector Laboratories, Inc., Burlingame, CA).[Q2]
Histomorphometric analysis
To evaluate the histopathologic changes of OA, semiquantitative grading of OA pathology was carried out on H & E stained sections using a murine scoring system established by the OARSI histopathology initiative (Glasson et al. 2010). Other histopathologic parameters of OA including joint space, tibial articular cartilage thickness and area were measured using OsteoMeasure software (OsteoMetrics, Decatur, GA) as we described previously (Wu et al. 2008). Each group contained three to seven mice and three sections from each joint were analyzed.
To assess the presence and alteration of lymphatic capillaries and mature lymphatic vessels, double immunofluorescence stained slides were scanned using a VS-ASW FL system (Virtual Slide-Acquisition Wizard for Fluorescence and Lumination system, Olympus, Japan [Q3]) with a x 20 lens and saved as multiple electronic images. Images were analyzed using the VS-110 software (Version 2.3). The region of interest was outlined by hand and included articular capsules and adjacent soft tissues. The total area of the region of interest was determined automatically by the VS-110 software. The area of lymphatic vessels within the entire region of interest was measured at the final magnification of 100 X by a grid counting method as we described previously (Boyce 1990, Zhang et al. 2007, Zhou et al. 2011). Two sections per animal were stained and data were combined. The podoplanin+/αSMA-vessels were defined as lymphatic capillaries and podoplanin+/αSMA+ vessels were defined as mature lymphatic vessels consistent with current criteria (Dellinger et al. 2008, Noda et al. 2010, Tammela et al. 2007, von der Weid and Zawieja 2004).
Imaging cytometry
Cells were isolated from ankle joints of two TNF-Tg mice, stained with FITC-CD11b (BD Bioscience, San Jose, CA) and PE-podoplanin antibodies (BD Bioscience) for 15 min at room temperature in the dark. Isotype controls (FITC-conjugated mouse IgG2aκ, clone G155 178, and PE-CyTM5 mouse IgG1κ, clone MOPC-21; BD Biosciences) were included. Imaging cytometry was performed in a flow cytometer (BD FACSAriaTM, Becton Dickinson) using BD FACSDiva software systems (Becton Dickinson). Monocytes first were gated in a forward scatter/sideward scatter dot plot, then two-color fluorescence was measured within the monocyte gate. The percentages of podoplanin+/CD11b- and podoplanin+/CD11b+ cells were determined.
Statistical analysis
Data are presented as means ± SE. Statistical analyses were performed using SPSS 16.0 software. Differences between two groups were compared using Student’s t-test, and p < 0.05 was considered significant.
Results
Identification and distribution of capillary and mature lymphatic vessels in normal joints of WT mice
To study the changes in capillary and mature lymphatic vessel formation in the joints of arthritic mouse models, we performed double immunofluorescence staining on paraffin sections. These sections were cut from paraffin blocks that had undergone EDTA decalcification for 3 weeks and had been stored at room temperature for 2-5 years. Sections were stained with anti-podoplanin antibody followed by Fluor546-labeled secondary antibody and FITC-anti-αSMA antibody. Under our staining conditions, podoplanin+ vessels have red, αSMA+ vessels have green, and podoplanin+/αSMA+ vessels have yellow fluorescence. Consistent with current criteria (Dellinger et al. 2008, Noda et al. 2010, Tammela et al. 2007, von der Weid and Zawieja 2004), we defined podoplanin+/αSMA-vessels as lymphatic capillaries, because they are not covered by smooth muscle cells, which express αSMA, while podoplanin+/αSMA+ vessels were defined as mature lymphatic vessels, because they are surrounded by a thin layer of αSMA+ smooth muscle cells.
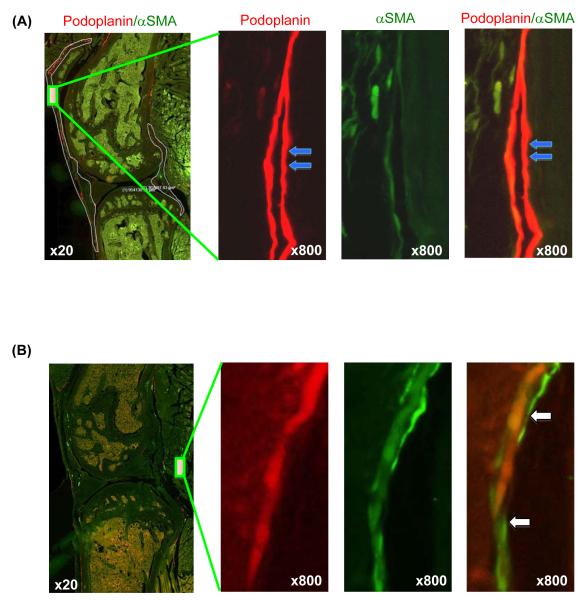
We scanned the entire section using a VS-ASW FL (Olympus) analysis system with a x 20 objective lens, then enlarged the region of interest to x 100-1000 magnification to observe the fine structures of the lymphatic vessels. We observed that anti-podoplanin antibody stained only vessels. The podoplanin+ vessels have thin walls and most of them are long and flat with closed lumens characteristics of lymphatic vessels (Fig. 1A). Other types of tissues and cells were podoplanin negative. Positive staining with anti-αSMA antibody also occurred only with vessels. By contrast to podoplanin+ vessels, most of the αSMA+ vessels have thick walls and are round with open lumens, which indicates that they are small blood vessels. A small percentage of podoplanin+ vessels were stained positive for αSMA; these structures are defined as mature lymphatic vessels (Fig. 1B).
Fig. 1.
Identification of lymphatic capillaries and mature lymphatic vessels. Paraffin embedded knee sections of a 6-month-old WT mouse stained with anti-podoplanin and anti-αSMA antibodies. Sections were scanned using a x 20 lens and the VS-ASW FL system. Images were observed under x 100–1000 magnification to distinguish lymphatic capillaries and mature lymphatic vessels. A) Left panel: low magnification (x 20) showing the region of interest. Areas that contain lymphatic vessels are outlined. Right panels: enlargements (x 800) of the region indicated in the left image showing a podoplanin+/αSMA-lymphatic capillary (blue arrows). B) Left panel: low magnification (x 20) showing the region of interest. Right panels: enlargements of the region indicated in the left panel showing a podoplanin+/αSMA+ mature lymphatic vessel (white arrows).
We found that numerous podoplanin+ lymphatic vessels were present in the soft tissues around the knee joint including the synovial membrane, fat pad, fibrous layer of the articular capsule, articular ligaments, and patellar ligament region. Lymphatic vessels were localized mainly in the fat pad near the meniscus, in fibrous tissues adjacent to the synovial membrane, and between ligament/tendon and bone (Fig. 1). No podoplanin staining was detected within hard tissues such as menisci, articular cartilage and subchondral bone. We evaluated the alteration of lymphatic vessels in a region that included all lymphatic vessels as outlined in Fig. 1A.
Distribution of lymphatic vessels in long bones of WT mice
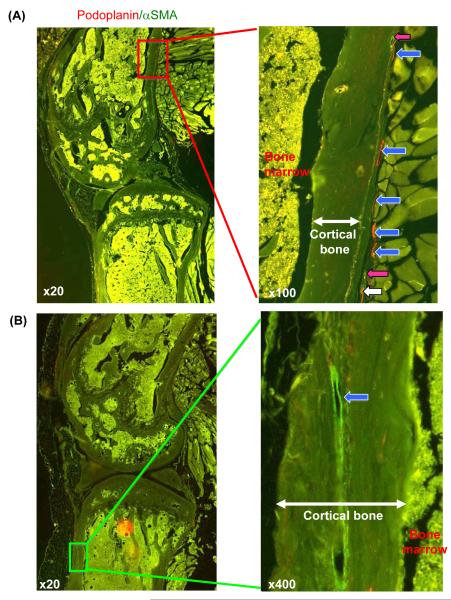
Previous reports using routine light microscopy found no lymphatic vessels in bone sections (Edwards et al. 2008, Zhang et al. 2008a). We re-examined lymphatic vasculature in bone sections using the VS-ASW FL whole-slide imaging system. We observed many capillaries and mature lymphatic vessels in the periosteal area, both of which usually are present adjacent to blood vessels [Q4] (Fig. 2A, right panel). Occasionally, we observed lymphatic vessels in the cortical bone (Fig. 2B, right panel). We found that nearly all the vessels could be observed clearly at low magnification as shown in Fig. 2, and higher magnification was required only occasionally to identify small vessels. Therefore, we used x 100 magnification to assess the percentage of the tissue area occupied by lymphatic vessels.
Fig. 2.
Lymphatic vessels in bone. A) Left panel: low magnification micrograph (x 20) showing the region of interest. Right panel: high magnification (x 100) showing numerous lymphatic vessels in the periosteum area including lymphatic capillaries (blue arrows), mature lymphatic vessels (white arrow) and blood vessels (pink arrows). B) Left panel: low magnification (x 20) showing the region of interest. Right panel: high magnification (x 400) showing a lymphatic capillary (blue arrow) within the cortical bone of the tibia.
Decreased area of mature lymphatic vessels in OA mice fed a high fat diet
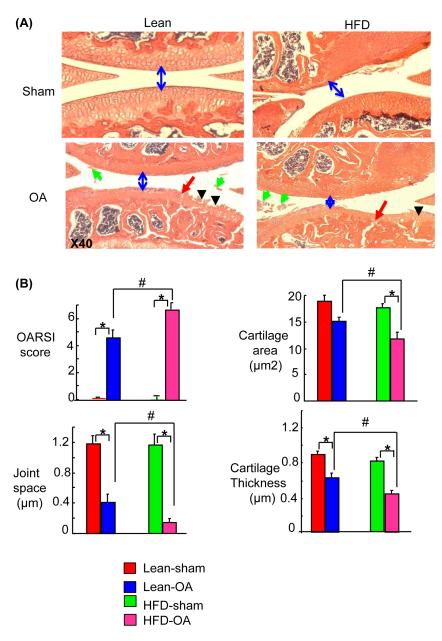
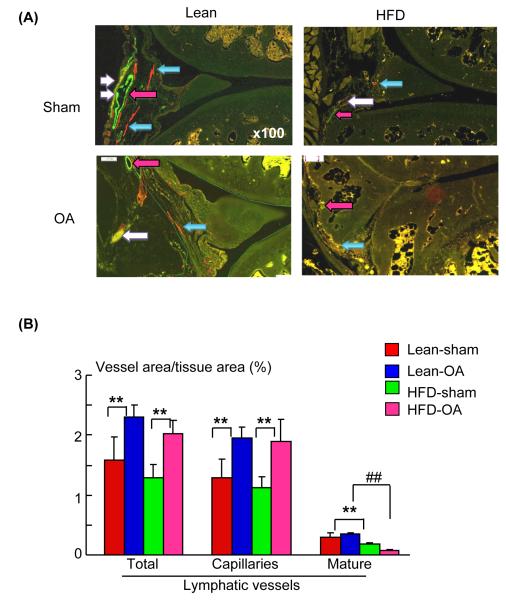
The lymphatic system plays a critical role in general body metabolism and decreased lymphatic function has been reported recently in rats with metabolic syndrome (Zawieja et al. 2012). We reported earlier that high fat diet increases the severity of joint damage in MLI-OA mice (Mooney et al. 2011). We examined lymphatic vessel changes in sections from high fat diet or lean diet fed mice that received either MLI or a sham operation. Histological analysis confirmed the loss of articular cartilage in MLI-OA knees, which was accelerated by high fat diet (Fig. 3A, B). OA knees had increased area and number of lymphatic capillaries in both lean and high fat diet groups. The area and number of mature lymphatic vessels, however, were decreased significantly in sections from high fat diet-fed mice (Fig. 4A, B).
Fig. 3.
Severe tissue damage in OA mice fed with a high fat diet. Knee joints from mice that received either MLI or sham operation, then were fed with a high fat diet or lean diet for 3 months. A) H & E stained sections show more severe OA histological changes in high fat diet fed mice, including narrowed joint space (blue arrows), clefts (red arrows), floating cartilage (green arrows), articular cartilage loss and cartilage erosion (black arrow). B) Histomorphometric analyses of H & E sections. Values are mean ± SD of 3-7 mice. * p < 0.05 vs. corresponding control mice; # p < 0.05 vs. OA mice fed a lean diet. [Q8]
Fig. 4.
High fat diet reduced the area of mature lymphatic vessels in joint sections of OA mice. Knee sections from high fat diet fed mice that were described in Fig. 3. A) Sections double stained with anti-podoplanin and αSMA antibodies and scanned as described for Fig. 1. High magnification (x 100) showing decreased mature lymphatic vessels in high fat diet-OA knees (white arrows). Lymphatic capillaries and blood vessels are shown by blue and pink color arrows, respectively. B) Histomorphometric analyses were performed at the final magnification of x 100. Values are the mean ± SD of 3-7 mice. * p < 0.05 or ** p < 0.01 vs. corresponding control mice; # p < 0.05 or ## p < 0.01 vs. OA mice fed a lean diet. [Q9]
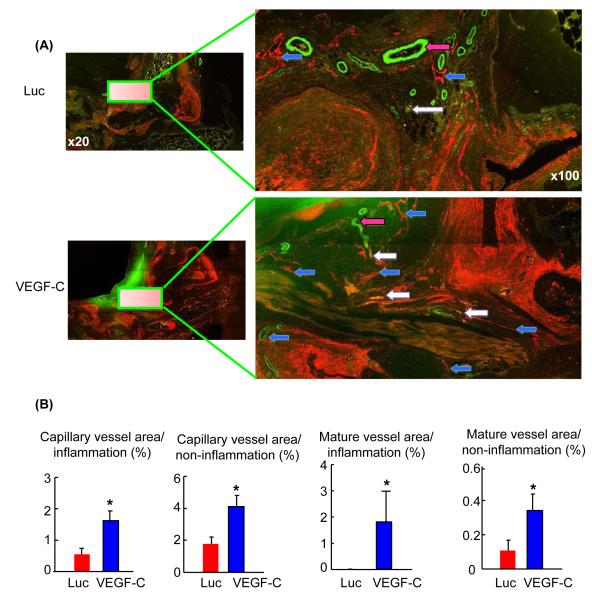
Decreased area of mature lymphatic vessels in RA joints, which are prevented [Q5] by VEGF-C treatment
Our earlier studies revealed that TNF-Tg mice begin to develop ankle synovitis when they are 1.5 months old; joint lesions progress thereafter, with significantly increased numbers of LYVE-1+ lymphatic vessels (Zhang et al. 2007). To determine whether there are changes in mature lymphatic vessels, we stained joint sections from 2- and 5-month-old TNF-Tg mice. We found a similar change in podoplanin+/αSMA-lymphatic capillaries in 2- and 5-month-old TNF-Tg mice (area capillary vessels/tissue area: 2.5 ± 0.25% vs. 2.9 ± 0.31%). Mature lymphatic vessels, however, were detected rarely in 5-month-old TNF-Tg mice compared to 2-month-old (area mature vessels/tissue area: 0.005 ± 0.001% vs. 0.5 ± 0.15%, p < 0.05), which indicates impaired lymphatic vessel maturation in TNF-Tg mice with severe arthritis. We reported that VEGF-C reduces inflammation and improves the lymph draining function of TNF-Tg mice (Zhou et al. 2011). It is not known, however, whether VEGF-C affects lymphatic maturation. To examine this, we double immunofluorescence stained sections from VEGF-C- or control virus treated TNF-Tg mice with anti-podoplanin and αSMA antibodies. Increased area of capillary and mature lymphatic vessels was observed in VEGF-C-treated joints (Fig. 5A). We have reported that lymphatic vessels are localized mainly in soft tissues surrounding the inflammatory pannus in RA joints (Zhang et al. 2007, Zhou et al. 2011). VEGF-C treatment increased the area of capillary and mature lymphatic vessels in both the inflammatory and non-inflammatory areas (Fig. 5B).
Fig. 5.
VEGF-C treatment increased the area of mature lymphatic vessels in TNF-Tg RA mice. Ankle sections from 5.5-month-old TNF-Tg mice that received VEGF-C virus or Luc control virus for 4 months. Sections were double stained with anti-podoplanin and αSMA antibodies and scanned as described for Fig. 1. A) High magnification (x 100) showing increased capillary (blue arrows) and mature lymphatic vessels (white arrows) in VEGF-C-treated joints. Blood vessels are indicated by pink arrows. B) Histomorphometric analysis was performed at a final magnification of x 100. The percentage of lymphatic vessel area over inflammatory and non-inflammatory area was assessed. Values are mean + SD of 4-5 mice. * p < 0.05 vs. Luc-treated joints.
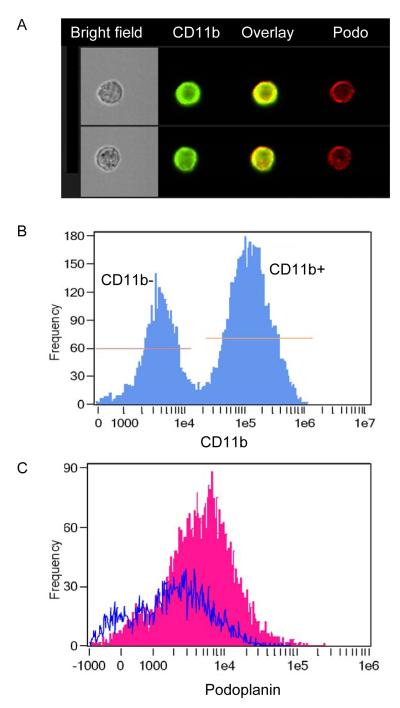
We observed that, unlike OA sections where only lymphatic vessels were stained positively by anti-podoplanin antibody (Figs. 1-4), there were many podoplanin+ cells in RA joint sections (Fig. 5). Because CD11b-expressing monocytes may also express podoplanin (Maruyama et al. 2005) and function as bone marrow-derived lymphatic endothelial progenitor cells (Park et al. 2011), we wanted to know whether podoplanin+ cells in our RA sections were CD11b+ cells, because CD11b+ cells are composed of a major cell type in the inflammatory synovium of TNF-Tg mice (Zhang et al. 2008b). Consequently, we isolated cells from joints of TNF-Tg mice, stained them with anti-podoplanin and CD11b antibodies, and performed imaging stream analysis. Podoplanin was co-localized with CD11b protein on the cell surface (Fig. 6A). More than 65% of podoplanin+ cells are CD11b+, while only 34% of podoplanin+ cells are CD11b− (Fig. 6B, C), which suggests that most podoplanin+ cells within the pannus are myeloid inflammatory cells. Despite these podoplanin+ monocytes, we were able to identify and analyze podoplanin+ lymphatic vessels in the RA sections.
Fig. 6.
Analysis of surface expression of podoplanin on joint cells from TNF-Tg mice. Cells were isolated from ankle joints of two TNF-Tg mice, stained with FITC-CD11b and PE-podoplanin antibodies and subjected to imaging cytometry analysis. A) Representative imagery from CD11b+ cells shows the surface expression of podoplanin. Yellow signal in “overlay” column is the combination of green (CD11b signal) and red (podoplanin) signals. B) Histogram shows that 66% of podoplanin+ cells express CD11b, while only 34% of podoplanin+ cells are CD11b-negative. C) Histogram overlay of podoplanin staining on CD11b+ (pink) and CD11b− (blue) cells. The mean fluorescence intensity of podoplanin CD11b+ cells is 7613, while for CD11b-cells it is 3332.
Discussion
We used a whole-slide imaging system to examine the distribution and alteration of lymphatic capillaries and mature lymphatic vessels in joint and long bone sections from normal mice and mice with chronic arthritis including OA and RA. We found that both lymphatic capillaries and mature vessels were present in the various soft tissues of normal joints and in the periosteum of long bones. Lymphatic vessels were located mainly in the loose connective and adipose tissues adjacent to the synovial membrane within the joint capsule. The areas of the lymphatic capillaries were increased significantly in joints with mild arthritis. Areas of the lymphatic vessels, mainly mature lymphatic vessels, were decreased markedly in joints with severe arthritis in both OA and RA. VEGF-C increased lymphatic vessel maturation in RA joints. Our findings suggest that the lymphatic system may play a critical role in arthritis pathogenesis and therapy, and these factors require further investigation. Our findings also indicate that the whole-slide imaging system is an excellent tool for investigating lymphatic vasculature in hard tissues.
The presence of numerous lymphatic vessels in the periosteum is particularly interesting. Periosteum plays a critical role in bone repair by providing blood supply and mesenchymal stem cells (Schonmeyr et al. 2009, Zhang et al. 2005). It is not known, however, whether lymphatic-mediated events also contribute to bone repair. Because sufficient lymphatic drainage helps resolve inflammation, and because the inflammatory reaction is one of the key components of bone repair, it is conceivable that the lymphatic system plays a role in this process. This possibility must be examined in the future. The IHC staining method did not detect lymphatic vessels in bone marrow (Edwards et al. 2008, Zhang et al. 2008a), which indicates that fluid clearance in the marrow cavities is by venous sinusoids rather than lymphatics. With the help of the VS-ASW FL system, we occasionally (~ 10% of samples) observed lymphatic vessels in bone cortex and marrow cavities (data not shown). The exact role of these vessels in bone metabolism, however, is not clear at this time.
OA is a degenerative disease of the joints that is associated with high levels of catalytic enzymes and their products in the articular space. IHC staining of joint sections with anti-LYVE-1 or podoplanin antibodies indicated that the number of lymphatic vessels was increased (Xu et al. 2003) or decreased (Walsh et al. 2012) in OA patients. The discrepancy may be due to the sampling site and/or different patient populations. Capillary and mature lymphatic vessels were not distinguished in these descriptive studies. We found markedly decreased cartilage and mature lymphatic vessels in OA mice fed a high fat diet (Fig. 4). There are two possible explanations for this. One is that the pathological process associated with OA progression inhibits lymphatic vessel maturation, as seen in RA joints. Another possibility is that high fat diet influences maturation of lymphatic vessels. It was reported recently that hypercholesterolemic mice exhibit decreased smooth muscle cell coverage [Q6] and decreased lymphatic transport of fluid (Lim et al. 2009). In humans, a strong correlation has been shown between type 2 diabetes and OA (Holliday et al. 2011). Our earlier study also indicated that a high fat diet leads to weight gain, diabetes and progression of OA (Mooney et al. 2011). Thus, the possibility that a high fat diet accelerates OA progression by hindering the maturation of lymphatic capillaries in OA joints must be investigated in the future. In particular, investigation of whether a high fat diet affects lymphatic drainage function is required.
RA is an inflammatory erosive disease associated with pain, swelling and stiffness of affected joints (Bergman 2006). We reported earlier that lymphatic drainage is associated with the onset, progression and flare-up of RA (Li et al. 2010, 2011) and that lymphatic growth factor, VEGF-C, promotes resolution of inflammation in RA joints by promoting lymphatic vessel formation and lymph drainage (Zhou et al. 2011). In our earlier studies, however, we did not distinguish capillaries from mature lymphatic vessels. We do not know whether the severity of RA is related to impaired lymphatic vessel maturation or whether it is affected by VEGF-C. This is important, because many studies have demonstrated that inflammation induces lymphangiogenesis, but that inflammation-induced lymphatic vessels are not sufficiently functional. We found that mature lymphatic vessels were observed rarely in the inflammatory pannus of TNF-Tg mice and that VEGF-C treatment significantly increased the areas of capillary and mature lymphatic vessels, which suggests that VEGF-C not only stimulates lymphangiogenesis, but also lymphatic vessel maturation. This raises the possibility of using VEGF-C as a new lymphatic therapy for patients with arthritis.
The whole-slide imaging is an excellent tool for studying lymphatics in hard tissues. With this new tool, we have begun to explore the presence, distribution, and alteration of lymphatic capillaries and mature vessels in mouse models of arthritis, which may lead to the development of new mechanisms and treatments for patients with arthritis.
Acknowledgments
The authors thank Dr. Timothy Bushnell for imaging cytometry analysis, Ms Yanyun Li for technical assistance with the slide-scanning, and Ms Evelyn Henderson for the initial manuscript editing. This work was supported by research grants from National Institute of Health PHS awards (AR48697 to LX, AR43510 and 1S10RR027340-01 to BFB), Dr. Shi’s salary was supported by grants from National Key Constructive Disciplines of China (T0303 to JXS) and Putuo Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China. A portion of Dr. Liang’s salary was supported by grants from the National Natural Science Foundation of China (81173278 to YJW) and the National Basic Research Program of China (2010CB530400 to YJW).
Footnotes
Declaration of conflict of interest The authors declare that they have no competing interests.
References
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Bergman MJ. Social and economic impact of inflammatory arthritis. Postgrad. Med. 2006;Spec. No: 5-11 [Q7] [PubMed] [Google Scholar]
- Boyce BF. Bone biopsy and histomorphometry in metabolic bone disease. In: Stevenson JC, editor. NewTechniques in Metabolic Bone Disease. Butterworths; London: 1990. pp. 110–131. [Google Scholar]
- Dellinger M, Hunter R, Bernas M, Gale N, Yancopoulos G, Erickson R, Witte M. Defective remodeling and maturation of the lymphatic vasculature in angiopoietin-2 deficient mice. Dev. Biol. 2008;319:309–320. doi: 10.1016/j.ydbio.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JR, Williams K, Kindblom LG, Meis-Kindblom JM, Hogendoorn PC, Hughes D, Forsyth RG, Jackson D, Athanasou NA. Lymphatics and bone. Hum. Pathol. 2008;39:49–55. doi: 10.1016/j.humpath.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Kato S, Itonaga I, Torisu T, Masumi S. Fine structure and distribution of lymphatics in the synovial membrane of monkey and human knee joints. A study using an enzyme-histochemical method. Int. Ortho. 1995;19:396–402. doi: 10.1007/BF00178358. [DOI] [PubMed] [Google Scholar]
- Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, Thurston G, Jackson DG. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol. Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Holliday KL, McWilliams DF, Maciewicz RA, Zhang W, Doherty M. Lifetime body mass index, other anthropometric measures of obesity and risk of knee or hip osteoarthritis in the GOAL case-control study. Osteoarthr. Cartil. 2011;19:37–43. doi: 10.1016/j.joca.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526–538. doi: 10.1111/j.1600-0463.2004.apm11207-0811.x. [DOI] [PubMed] [Google Scholar]
- Li J, Kuzin I, Moshkani S, Proulx ST, Xing L, Skrombolas D, Dunn R, Sanz I, Schwarz EM, Bottaro A. Expanded CD23(+)/CD21(hi) B cells in inflamed lymph nodes are associated with the onset of inflammatory-erosive arthritis in TNF-transgenic mice and are targets of anti-CD20 therapy. J. Immunol. 2010;184:6142–6150. doi: 10.4049/jimmunol.0903489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhou Q, Wood RW, Kuzin I, Bottaro A, Ritchlin CT, Xing L, Schwarz EM. CD23(+)/CD21(hi) B-cell translocation and ipsilateral lymph node collapse is associated with asymmetric arthritic flare in TNF-Tg mice. Arthr. Res. Ther. 2011;13:R138. doi: 10.1186/ar3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Schwarz EM, O’Keefe RJ, Ma L, Boyce BF, Xing L. RANK signaling is not required for TNFalpha-mediated increase in CD11(hi) osteoclast precursors but is essential for mature osteoclast formation in TNFalpha-mediated inflammatory arthritis. J. Bone Miner. Res. 2004;19:207–213. doi: 10.1359/JBMR.0301233. [DOI] [PubMed] [Google Scholar]
- Li P, Schwarz EM, O’Keefe RJ, Ma L, Looney RJ, Ritchlin CT, Boyce BF, Xing L. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthr. Rheum. 2004;50:265–276. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ, Angeli V. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am. J. Pathol. 2009;175:1328–1337. doi: 10.2353/ajpath.2009.080963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Li M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Sampson ER, Lerea J, Rosier RN, Zuscik MJ. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthr. Res. Ther. 2011;13:R198. doi: 10.1186/ar3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. 2003;17:920–922. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- Noda Y, Amano I, Hata M, Kojima H, Sawa Y. Immunohistochemical examination on the distribution of cells expressed lymphatic endothelial marker podoplanin and LYVE-1 in the mouse tongue tissue. Acta Histochem. Cytochem. 2010;43:61–68. doi: 10.1267/ahc.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrol S, Gindre D, Cordier JF, Loire R, Grimaud JA. Characterization of the smooth muscle cell infiltrate and associated connective matrix of lymphangiomyomatosis. Immunohistochemical and ultrastructural study of two cases. J. Pathol. 1992;168:387–395. doi: 10.1002/path.1711680409. [DOI] [PubMed] [Google Scholar]
- Park C, Lee JY, Yoon YS. Role of bone marrow-derived lymphatic endothelial progenitor cells for lymphatic neovascularization. Trends Cardiovasc. Med. 2011;21:135–140. doi: 10.1016/j.tcm.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzer K, Baeten D, Soleiman A, Distler J, Gerlag DM, Tak PP, Schett G, Zwerina J. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Ann. Rheum. Dis. 2008;67:1610–1616. doi: 10.1136/ard.2007.083394. [DOI] [PubMed] [Google Scholar]
- Sampson ER, Beck CA, Ketz J, Canary KL, Hilton MJ, Awad H, Schwarz EM, Chen D, O’Keefe RJ, Rosier RN, Zuscik MJ. Establishment of an index with increased sensitivity for assessing murine arthritis. J. Ortho. Res. 2011;29:1145–1151. doi: 10.1002/jor.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson ER, Hilton MJ, Tian Y, Chen D, Schwarz EM, Mooney RA, Bukata SV, O’Keefe RJ, Avad H, Puzas JE, Rosier RN, Zuscik MJ. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci. Transl. Med. 2011;3:101ra93. doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonmeyr B, Clavin N, Avraham T, Longo V, Mehrara BJ. Synthesis of a tissue-engineered periosteum with acellular dermal matrix and cultured mesenchymal stem cells. Tissue Eng. Part A. 2009;15:1833–1841. doi: 10.1089/ten.tea.2008.0446. [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo-Ramadan U, Yla-Herttuala S, Petrova TV, Alitalo K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat. Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int. J. Biochem. Cell Biol. 2004;36:1147–1153. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Verghese P, Cook GJ, McWilliams DF, Mapp PI, Ashraf S, Wilson D. Lymphatic vessels in osteoarthritic human knees. Osteoarthr. Cartil. 2012;20:405–412. doi: 10.1016/j.joca.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Weninger W, Partanen TA, Briteneder-Geleff S, Mayer C, Kowalski H, Mildner M, Pammer J, Sturzl M, Kerjaschki D, Alitalo K, Tschachler E. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi’s sarcoma tumor cells. Lab. Invest. 1999;79:243–251. [PubMed] [Google Scholar]
- Wu Q, Kim KO, Sampson ER, Chen D, Awad H, O’Brien T, Puzas JE, Drissi H, Schwarz EM, O’Keefe RJ, Zuscik MJ, Rosier RN. Induction of an osteoarthritis-like phenotype and degradation of phosphorylated Smad3 by Smurf2 in transgenic mice. Arthr. Rheum. 2008;58:3132–3244. doi: 10.1002/art.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Edwards J, Banerji S, Prevo R, Jackson DG, Athanasou NA. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann. Rheum. Dis. 2003;62:1227–1229. doi: 10.1136/ard.2003.005876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H643–653. doi: 10.1152/ajpheart.00606.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Guo R, Lu Y, Zhao L, Zhou Q, Schwarz EM, Huang J, Chen D, Jin ZG, Boyce BF, Xing L. VEGF-C, a lymphatic growth factor, is a RANKL target gene in osteoclasts that enhances osteoclastic bone resorption through an autocrine mechanism. J. Biol. Chem. 2008;283:13491–13499. doi: 10.1074/jbc.M708055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Guo R, Schwarz EM, Boyce BF, Xing L. TNF inhibits production of stromal cell-derived factor 1 by bone stromal cells and increases osteoclast precursor mobilization from bone marrow to peripheral blood. Arthr. Res. Ther. 2008;10:R37. doi: 10.1186/ar2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lu Y, Proulx ST, Guo Z, Yao Z, Schwarz EM, Boyce BF, Xing L. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthr. Res. Ther. 2007;9:R118. doi: 10.1186/ar2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O’Keefe RJ, Guldberg RE. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J. Bone Miner. Res. 2005;20:2124–2137. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Guo R, Wood R, Boyce BF, Liang Q, Wang YJ, Schwarz EM, Xing L. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthr. Rheum. 2011;63:2318–2328. doi: 10.1002/art.30421. [DOI] [PMC free article] [PubMed] [Google Scholar]