Abstract
Objective
To evaluate novel hormonal therapies in patients with unresectable Benign Metastasizing Leiomyoma (BML) disease.
Design
Case series.
Setting
National Institutes of Health (NIH).
Patients
5 subjects with the diagnosis of BML based on imaging and/or histopathologic diagnosis.
Interventions
Four patients were treated with single or combination therapy of Leuprolide acetate and/or an aromatase inhibitor. One patient was treated with an antiprogestin (CDB-2914).
Main Outcome Measures
Response to therapy was measured by tumor burden on cross-sectional imaging employing RECIST (Response Evaluation Criteria in Solid Tumours) 1.1 guidelines.
Results
Four patients treated with single or combination therapy of Leuprolide acetate and/or an aromatase inhibitor demonstrated stable disease with reduction in tumor burden. The fifth patient treated with antiprogestin (CDB-2914) had degeneration of her tumor, progression of its size and an improvement in symptoms.
Conclusions
Hormonal treatment with GnRH agonism and/or aromatase inhibition may be a therapeutic option to reduce tumor burden in unresectable BML disease or for those patients who wish to avoid surgical intervention. RECIST 1.1 guidelines, while traditionally used to evaluate tumor response to cancer therapeutics, may be useful in evaluating BML tumor burden response to hormonal therapy.
Keywords: benign metastasizing leiomyoma, uterine leiomyoma, hormone therapy, intravenous leiomyomatosis, leiomyomatosis peritonealis disseminata
Introduction
Since Steiner first described benign metastasizing leiomyoma (BML) in 1939, there have been over 150 cases documented in the literature (1). This rare disease is characterized by well-differentiated smooth muscle tumors occurring outside the uterine corpus in women who have a history of histologically benign leiomyomas. Most often these smooth muscle tumors are seen in the lungs, but are also found in the lymph nodes, abdomen, deep soft tissues, heart, bone and central nervous system (2). A majority of those afflicted are pre-menopausal women, more than 50% presenting after the 3rd decade of life (3). Almost all have had a history of uterine surgery, on average 10 years prior to presentation. Not infrequently, diagnosis of BML has been found on routine imaging of an asymptomatic patient, while some patients present with dyspnea and respiratory distress (4).
The pathogenesis of BML remains controversial and may overlap with other benign smooth muscle neoplasms such as intravenous leiomyomatosis (IVL) and leiomyomatosis peritonealis disseminata (LPD), which are characterized by macroscopic smooth muscle tumorlets found intravenously and diffuse peritoneal and omental implants, respectively (5,6). Three theories have been proposed regarding metastatic spread: metastatic uterine leiomyoma with invasive implants, implantation of smooth muscle tumors by IVL or mechanical means, and multifocal but independent proliferation of smooth muscle tissue (2). Despite being associated with distant metastases, BML lesions are distinct from more aggressive leiomyosarcomas, demonstrating low mitotic counts and lack of nuclear pleomorphism, which are characteristic of benign leiomyomas. The cytogenetic profile of BML lesions has been found in 3% of normal uterine leiomyomas, indicating that BML arises from a minority of uterine leiomyomas that have metastatic potential (7). There is convincing evidence that BML lesions are of uterine origin given that clonality analysis of pulmonary lesions and uterine tissue has demonstrated an identical monoclonal X-chromosome inactivation pattern (8). Furthermore, most BML lesions and uterine leiomyomas express estrogen and progesterone receptors similar to uterine smooth muscle (9).
While traditionally surgical resection of metastatic lesions was the mainstay of treatment with variable long-term results, more recent management has revolved around surgical or medical castration (10,11,12). Clinical evidence of hormonal influence is suggested by reports of BML lesion regression during menopause (13) and following pregnancy (14,15). Conversely disease progression has been observed in patients on hormone replacement therapy (HRT) and oral contraceptives (16). Surprisingly, a growing number of reports indicate that despite surgical castration, some lesions continue to progress, indicating that hormonal therapy might be a vital adjunct or primary treatment of BML (3,17,18).
Materials and Methods
We present a case series of five patients diagnosed with BML who were treated at the National Institutes of Health (NIH) between 2006 and 2012 with medical regimens, including an aromatase inhibitor, GnRH agonist, CDB-2914, or a combination of these medications. We report the response to therapy as measured by tumor burden on cross-sectional imaging employing RECIST 1.1 guidelines. All leiomyomatous lesions (up to a maximum of 5 lesions total) were recorded in at least one dimension. Complete response was defined as disappearance of target lesions, partial response was defined as at least a 30% decrease in the sum of diameters of target lesions, progressive disease was defined as at least a 20% increase in the sum of diameters of target lesions, and stable disease was defined as insufficient regression or increase in disease to qualify as “response” or “progression.” (18)
Results
Case 1
In 2006, a 44 year-old nulligravid African-American female presented with lower extremity edema, leg pain and renal failure eventually requiring bilateral nephrostomy tube placement. At that time, she was found to have a large abdominal mass and new lung nodules on imaging. Past surgical history was notable for a hysterectomy at age 32, secondary to a symptomatic fibroid uterus. Histopathology of the CT-guided biopsy of the abdomino-pelvic mass was estrogen and progesterone receptor positive and consistent with benign leiomyoma. The patient was initially started on Raloxifene and Leuprolide (3.75 mg/4 weeks) with no decrease in tumor burden or symptoms over a 10-month period. Her therapy was subsequently changed to Letrozole (2.5 mg/daily). Approximately 8 months later, the patient underwent loop sigmoid colostomy for small bowel obstruction. At the time her abdominal mass was unable to be resected due to proximity of external iliac vessels. Subsequently, she had a series of uterine artery emoblizations performed but with continued abdominal and leg pain. Given her persistent symptoms, she was started on a new regimen of Leuprolide (3.75 mg/3 weeks) and Letrozole (1mg/daily) approximately 2 years after her last hormonal treatment. While on this treatment her abdominal mass and lung nodules have been stable by RECIST 1.1 criteria (9.2% decrease in total tumor burden size), and she has had no new symptoms for the last two years.
Case 2
In 2008, a 49 year-old Caucasian female presented with pelvic pain secondary to a palpable abdominal mass and was found to have multiple pelvic lesions and subpleural pulmonary nodules on imaging consistent with a diagnosis of BML. Her surgical history was notable for an abdominal myomectomy at age 30, followed by two Caesarean sections, her last at age 41, where incidental “abdominal studding” was noted on the operative report. One month after diagnosis, she was started on Leuprolide (3.75 mg/3 weeks) and Anastrazole (1mg/daily). Three months later, repeat imaging showed decreased tumor burden. Leuprolide acetate dose interval was increased to 3.75 mg/4 weeks and Anastrazole (1 mg/daily) was continued. Approximately two months later, she developed disabling arthritis, and following a consult with rheumatology, she discontinued Anastrazole and her Leuprolide acetate regimen was changed to 11.25 mg/3 months. Repeat imaging two months later showed a slight increase in mass size, therefore she was restarted on Anastrazole (1mg/daily) and Leuprolide (3.75mg/3 weeks). Her large pelvic tumor has since demonstrated interval decrease in mass size with a stable response by RECIST 1.1 (22.0% reduction in size of overall disease), and she reports improved symptoms of pelvic pain. She has continued on this hormonal regimen to date and although surgery has been recommended, she strongly desires to continue with medical management.
Case 3
In 2009, a 43 year-old Hispanic female with a long history of symptomatic uterine fibroids was diagnosed with BML after new pulmonary nodules were found on CT scan. The patient’s surgical history is notable for an abdominal myomectomy at age 37, uterine artery embolization at age 39, and total abdominal hysterectomy at age 42 due to recurrent fibroid growth. Her surgical course was also complicated by two incidences of bowel obstruction requiring surgical intervention. Upon her diagnosis of BML in 2009, she was started on Leuprolide 3.75 mg/3 weeks. A follow-up visit three months later revealed improvement in pelvic pain. Due to vasomotor symptoms from GnRH agonism, her regimen was subsequently adjusted to Leuprolide (3.75 mg/monthly) and Norethindrone (0.35 mg/daily). Over 13 months of therapy, disease burden has demonstrated radiographic evidence of a stable response to therapy by RECIST 1.1, with overall reduction in tumor size by 20.4%.
Case 4
In 2007, a 55 year-old Caucasian female presented to the ER with increasing shortness of breath and near syncope and was found to have bilateral pulmonary emboli and discrete lung nodules. Imaging revealed an inferior vena cava (IVC) and right atrial mass thought to be a thrombus. Further work-up revealed a large abdomino-pelvic mass. Her surgical history involved a myomectomy at age 42 for a symptomatic fibroid uterus. She subsequently underwent resection of the intracardiac thrombus three months after presentation. Histopathology of the mass revealed spindle cells with degeneration consistent with a benign leiomyoma. Post-operatively she was started on Letrozole (5mg/daily) for two months. The patient noted improvement of pelvic pressure symptoms and follow-up radiographic studies revealed stable size of her abdominal mass by RECIST 1.1 (overall tumor burden decrease of 3.6 %). Unfortunately, the patient was lost to follow-up after treatment and subsequently stopped hormonal therapy. She returned to the NIH in 2012 and she reported ongoing symptoms related to her IVC mass.
Case 5
In 2005, a 36 year-old Caucasian female with a known history of leiomyomatosis peritonealis disseminata presented with shortness of breath and was found to have a pleural effusion and pulmonary metastases consistent with a diagnosis of BML. Her prior surgical history was notable for two hysteroscopic myomectomies in 1998 and 1999. In 2002 she had an abdominal myomectomy where multiple omental and peritoneal metastases were found with pathology consistent with benign leiomyoma. Following diagnosis of BML she was treated with Leuprolide (3.75 mg/3 weeks) with resolution of her dyspnea and pleural effusion. However, she had little improvement in pelvic tumor burden. In 2007 she developed acute abdominal pain secondary to a bowel obstruction and she underwent exploratory laparotomy and partial bowel resection. Postoperatively she was started on the GnRH antagonist Ganirelix (250 μg SQ/daily) with minimal response. Abdominal hysterectomy and bilaterally salpingo-oophorectomy was offered to patient, but she declined further surgical intervention. She subsequently received an investigational drug, selective progesterone modulator (SPRM), CDB-2914, under compassionate exemption. Over three months, the patient’s symptoms significantly improved. Radiographic imaging revealed enlargement of her pelvic dominant mass but with marked degenerative changes. She continued on CDB-2914 for approximately four more years with no new symptoms, but repeat imaging demonstrated increasing size of the large pelvic mass (2.4 cm/annually) without growth of other target lesions. Specifically analyzing her large pelvic mass by RECIST 1.1 guidelines, her disease was progressive (tumor burden increase by 44.3%). Uterine artery embolization was attempted, but without significant decrease in the size of her pelvic mass. She underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy and a debulking procedure in 2012, following 5 years of CDB-2914 treatment. She is currently asymptomatic and has recovered fully.
Discussion
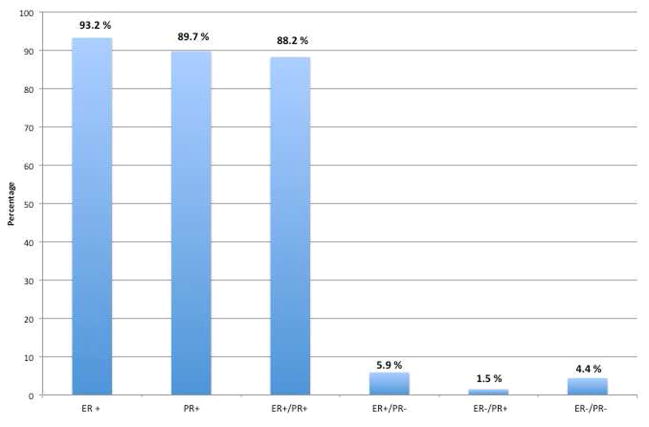
The first hormonal treatments for BML were primarily offered to patients who refused further surgical intervention or had unresectable masses. The use of hormonal therapy was based on the hypothesis that BML lesions had hormonal receptors. The finding that many lesions regressed following surgical castration, removal of exogenous estrogen, and following pregnancy, supported this premise (20,21). In 1980, Cramer was the first to demonstrate that high levels of estrogen receptors were present on metastatic leiomyoma lesions (22). Since then, several case reports have analyzed BML lesions for hormone receptor status. Of the 74 cases reviewed testing for estrogen receptors (ER), 93.2% were positive, and of the 68 cases testing for progesterone receptors (PR), 89.7% were positive (Figure 1). The vast majority of BML lesions are ER/PR receptor positive, explaining why BML patients have responded to hormonal therapy in the past.
Figure 1.
ER/PR receptor status of BML case reports 1986–2012.
The first attempts at medical treatment with hormonal agents utilized progesterone, which resulted in increased lesions as well as worsening symptoms (12,22). Since then, progesterone therapy has resulted in varied responses, some with regression of disease and symptomatic relief (4,23) (Table 1). Progesterone therapy might have been successful in some patients due to its ability to suppress the hypothalamic-pituitary-gonadal axis and reduce ovarian estrogen production (24). In addition, progesterone has been found to increase rates of inactivation of estradiol by increasing conversion to estrone in endometrial tissue (25) and decrease intracellular aromatase activity by up to 30% (26). The variance in response to progesterone therapy might be explained by the finding that progesterone can increase both Bcl-2, an apoptosis-inhibiting gene product, and tumor necrosis factor-α, a cytokine that induces apoptosis (27). In one of our cases we utilized an experimental selective progesterone receptor modulator (SPRM), CDB-2914 and found that despite symptomatic relief, a dominant pelvic mass continued to grow, ultimately needing resection (Case 5). Although it has been shown that CDB-2914 has demonstrated significant decrease in leiomyoma size (28), perhaps there is something unique about BML lesions requiring blockade of estrogen in addition to progesterone at the tumor site.
Table 1.
BML Case reports 1986–2012: Hormonal Treatment and Disease Response
| Case Reports | Treatment: GnRH agonism | Response |
|---|---|---|
| Hague, 1986 (35) | Buserelin × 3 months | + |
| Maheux, 1987 (48) | Buserelin × 6 months | 0, C |
| Jacobson, 1995 (30) | Buserelin × 42 months | +, C |
| Takemura, 1996 (43) | BSO + Buserelin × 20 months | 0, C |
| Tietze, 2000 (8) | GnRH agonist × 24 months | + |
| Alessi, 2003 (49) | Goserelin × 12 months | 0 |
| Nardo, 2003 (50) | GnRH agonist × 6 months | 0, C |
| Chan, 2005 (51) | Triptorelin × 3 months | + |
| Egberts, 2006 (52) | GnRH agonist × 12 months | 0, C |
| Arif, 2006 (42) | Gosrelin × 6 months | + |
| Tori, 2008 (53) | GnRH agonist × 10 months | + |
| Bodner-Adler, 2009 (54) | GnRH agonist × 6 months | 0 |
| Mizuno, 2011 (40) | GnRH agonist × 3 years | 0 |
| Silva, 2012 (55) | Goserelin × 2 months → BSO | − |
| Taveira-DaSilva, 2012 (56) | Lupron × 12 months | + |
|
| ||
| Treatment: Aromatase inhibition | ||
| Rivera, 2004 (24) | Raloxifene + Anastrazole × 24 months | +, C |
| Lupron + Anastrazole × 60 months | +, C | |
| Nasu, 2009 (57) | TAH/BSO→Anastrazole × 15 months | 0 |
| Yoon, 2011 (58) | TAH/BSO →Anastrazole × 4 months | + |
| Xiao, 2012 (59) | TAH/BSO→Anastrazole × 12 months | + |
|
| ||
| Treatment: Selective Estrogen Receptor Modulation | ||
| Evans, 1986 (12) | Tamoxifen × 4 months | − |
| Jacobson, 1995 (30) | Tamoxifen + Medroxyprogesterone × 9 months | −, S |
| Shin, 1996 (60) | Tamoxifen × unknown duration | −, C |
| Rivera, 2004 (24) | Tamoxifen × unknown duration | −, S |
| Kishore, 2004 (61) | Tamoxifen × 60 months | +, C |
| Benetti-Pinto, 2006 (33) | Raloxifene × 14 months | + |
| Jo, 2006 (62) | TAH/BSO + Tamoxifen × unknown duration | + |
| Awonuga, 2008 (16) | Tamoxifen × 1 month | − |
| Vaquero, 2009 (3) | Tamoxifen × 1 month | −, S |
| Ahmad, 2011 (63) | Tamoxifen × 6 months | − |
| Wongsripuemtet, 2012 (64) | Tamoxifen × 2 months | − |
|
| ||
| Treatment: Progesterone | ||
| Cramer, 1980 (22) | Progesterone × unknown duration | − |
| Stephenson, 1984 (65) | BSO→Megestrol × 48 months | + |
| Evans, 1986 (12) | Medroxyprogesterone × 3 months | −, S |
| Cho, 1989 (66) | BSO→Progesterone × unknown duration | 0, C |
| Cohen, 1993 (67) | Depo-Provera × 18 months→Megestrol × 8 months | − |
| Motegi, 1993 (68) | Medroxyprogesterone × 48 months | + |
| Hekster, 1994 (69) | Progesterone × unknown duration | −, S |
| Jautzke, 1996 (4) | Medroxyprogesterone × unknown duration | + |
| Goyle, 2003 (70) | Medroxyprogesterone × 3 months | −, S |
| Nardo, 2003 (71) | Provera × unknown duration | 0, C |
| Wentling, 2005 (72) | Megestrol × 3 months | +, C |
| Chan, 2005 (51) | Megestrol × 26 months | 0 |
| Londero, 2008 (73) | Megestrol × 6 months | + |
| Beck, 2012 (23) | Megestrol × 6 months | + |
(+) decrease in disease
(0) stable disease
(−) increase in disease
(C) clinical improvement
(S) no clinical improvement
Given the finding that BML lesions grew in response to oral contraceptives and HRT, many have attempted treatment with estrogen blockade. A growing body of evidence closely relates estrogen with tumorigenesis and growth of leiomyomas (29). The first attempt at estrogen blockade was made with Tamoxifen, a Selective Estrogen Receptor Modulator (SERM), which resulted in disease progression (12). Some case reports indicate that Tamoxifen provided symptomatic relief for BML patients, but very rarely did treatment cause regression of tumor nodules (23,30). (Table 1) The reports of unsuccessful results with Tamoxifen therapy can be attributed to its estrogen agonism on the myometrium (31), with one case report demonstrating the ability of Tamoxifen to promote extrauterine leiomyoma growth (32). Raloxifene, another SERM, which has a known estrogenic antagonist effect on the breast and uterus, has shown greater success in treatment of BML (33). Furthermore studies have shown that Raloxifene can induce apoptosis and reduce cell proliferation in postmenopausal women with leiomyomas, whereas Raloxifene in premenopausal women has had mixed effects (34). In one of the patients described in our series, GnRH agonism and Raloxifene treatment resulted in no improvement of BML lesions (Case 1). Given reports of success with Raloxifene therapy, it might have a role in the treatment of BML in postmenopausal women or those who have had a therapeutic oophorectomy.
GnRH agonism, resulting in medical castration, has been very effective in the treatment of BML. The first reported case, by Hague in 1986, found that disease was significantly decreased after three months of treatment (35). In our survey of case reports, GnRH agonism has predominantly provided both symptomatic relief and reduction in metastastic lesions (Table 1). In addition to suppressing ovarian steroidogeneis, GnRH agonism can inhibit the expression of aromatase P450, an estrogen synthetase, which catalyzes androgens to estrogen, thereby decreasing in-situ estrogen production (36). GnRH agonism was sufficient enough to decrease pelvic lesions in one patient in our series (Case 3), while in another case GnRH agonism alone, without aromatase inhibition, resulted in disease progression (Case 2). Furthermore, another patient on GnRH agonism alone had improvement of pulmonary disease, but no significant reduction in pelvic tumor burden, and thus therapy was changed (Case 5).
Recent case reports have begun to utilize aromatase inhibitors to decrease BML lesions with resounding beneficial effects (Table 1). Given the knowledge that leiomyomas have been found to over-express aromatase p450, aromatase inhibitors can further create a local hypoestrogenic milieu which may inhibit cell growth (37). Studies demonstrate that aromatase therapy has been successful in decreasing uterine leiomyoma size (38, 39). Typically, aromatase inhibition has been employed in combination with medical or surgical castration. In one case we saw tumor burden reduction and stable disease in response to GnRH agonism and aromatase inhibition (Case 2), and in another patient we found stable disease of pelvic masses with aromatase therapy alone (Case 4).
Our case series mirrors that of current case reports, with the finding that patients diagnosed with BML treated with GnRH agonism and/or aromatase inhibition had stable disease and a reduction in tumor burden. With the help of RECIST guidelines we were able to evaluate in a fastidious manner BML response to hormonal therapy (Figure 2). To date, few case reports have utilized this rigorous standard for measuring disease response or progression in BML (40). Some randomized control trials measuring leiomyoma response to hormonal therapy utilize ultrasound measurement, but these are often operator dependent, and the technique used can obscure measurement (38,29). While volumetric assessment of leiomyomas with MRI imaging might be a good measure of tumor response (28), it is difficult to implement in BML given the widely metastatic nature of the disease. Although the RECIST guidelines were formulated to assess tumor burden in response to cancer therapeutics, they might serve a role in measuring the response of benign yet metastasizing tumors like BML.
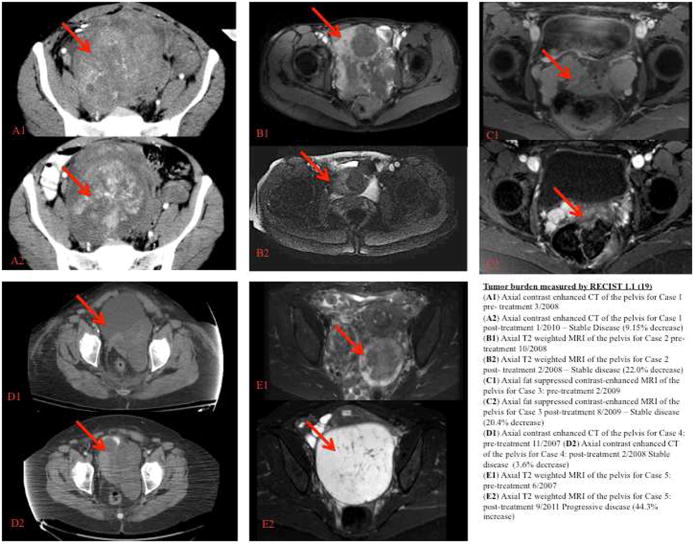
Figure 2. Tumor burden measured by RECIST 1.1 (19).
(A1) Axial contrast enhanced CT of the pelvis for Case 1 pre - treatment 3/2008
(A2) Axial contrast enhanced CT of the pelvis for Case 1 post - treatment 1/2010 – Stable Disease (9.15% decrease)
(B1) Axial T2 weighted MRI of the pelvis for Case 2 pre - treatment 10/2008
(B2) Axial T2 weighted MRI of the pelvis for Case 2 post - treatment 2/2008 – Stable disease (22.0% decrease)
(C1) Axial fat suppressed contrast-enhanced MRI of the pelvis for Case 3: pre - treatment 2/2009
(C2) Axial fat suppressed contrast-enhanced MRI of the pelvis for Case 3 post - treatment 8/2009 – Stable disease (20.4% decrease)
(D1) Axial contrast enhanced CT of the pelvis for Case 4: pre - treatment 11/2007
(D2) Axial contrast enhanced CT of the pelvis for Case 4: post - treatment 2/2008 - Stable disease (3.6% decrease)
(E1) Axial T2 weighted MRI of the pelvis for Case 5: pre-treatment 6/2007
(E2) Axial T2 weighted MRI of the pelvis for Case 5: post-treatment 9/2011 - Progressive disease (44.3% progression)
The one caveat to using the RECIST guidelines or any other type of volumetric system for measuring BML disease is that they do no take into account cystic or vascular changes within a lesion. The RECIST guidelines might not fully capture a response to therapy if the lesions stay the same size but undergo cystic degeneration. For instance in Case 5, the patient had a progression of BML disease after CDB-2914 treatment, but her dominant pelvic mass also underwent cystic degeneration (Figure 2). Although the RECIST guidelines are extremely helpful in evaluating overall tumor burden in BML, attention must also be given to the characteristics of lesions after therapy as to not miss a potential therapeutic treatment.
Our series is limited by the fact that two of our cases lack histopathologic confirmation of distant metastases (Case 2,3). While these patients lack histologic diagnosis, the available radiologic evidence supports the diagnosis of BML. The combination of new findings of lung nodules in relatively healthy women with a prior history of uterine surgery for leiomyomas strongly supports the diagnosis of BML, and does not require the patient to undergo the risks associated with pulmonary biopsies (41). In the cases reviewed here, pulmonary nodules also stabilized or regressed with therapy. In one patient, histologic diagnosis may have been associated with significant morbidity, given close proximity of lung nodules to the heart (Case 1). Another case demonstrates the reported close pathogenesis between IVL and BML, as the patient presented with an IVC/cardiac tumor consistent with intravenous leiomyomatosis as well as pulmonary lesions consistent with BML (5, 42, 43) (Case 4).
This case series suggests that hormonal therapy may be used in the treatment of BML, specifically by employing synergistic combinations with individual dosing. One patient who received high dose Anastrazole at 5 mg/daily (Case 4) instead of traditional dosing at 1 mg/daily, for example, illustrates that higher doses may be critical in obtaining disease regression in BML patients. Initial studies on Anastrazole found doses of 1 mg and 10 mg equipotent in suppressing estradiol levels (44), but a recent study found large variations in drug metabolism and drug effect, indicating that Anastrazole therapy might need to be individualized (45). In three of our patients (Case 1, 2, 3) we found that more frequent dosing of Lupron (3.75 mg/3 weeks) than the standard regimen of Lupron (3.75 mg/4 weeks) was necessary for effective disease stabilization. These findings are consistent with the results of other authors who demonstrated variability in response to standard doses of Lupron, and ultimately adjusted frequency of dosing based on end organ response by measuring sex steroid levels (46, 47). In our case series, specific doses and combinations of medications were effective for some patients and not for others. Future hormonal treatments of BML could involve monitoring of therapy response with serum sex-steroids levels, and adjusting doses/frequency as needed.
The historical treatment of BML by therapeutic oophorectomy has been challenged by case reports demonstrating hormonal treatment of BML can be an effective alternative (Table 1). Furthermore, despite oophorectomy or menopause, hormonal treatment might be necessary due to continued disease progression. For young women who desire to retain fertility or for those where oophorectomy is not surgically possible, hormonal treatment can be a therapeutic option. We have found that individually tailored combination therapy might have an added benefit given the synergistic effects of different medications. Although the rarity of this disease precludes the identification of standard treatment through the use of clinical trials, GnRH agonism with or without aromatase inhibition has demonstrated success in selected patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steiner PE. Metastasizing fibroleiomyoma of the uterus: Report of a case and review of literature. Am J Path. 1939;15:89–110. [PMC free article] [PubMed] [Google Scholar]
- 2.Awonuga AO, Shavell VI, Imudia AN, Rotas M, Diamond MP, Puscheck EE. Pathogenesis of Benign Metastasizing Leiomyoma. Obstet Gynecol Surv. 2010;65:189–195. doi: 10.1097/OGX.0b013e3181d60f93. [DOI] [PubMed] [Google Scholar]
- 3.Vaquero ME, Magrina JF, Leslie KO. Uterine smooth-muscle tumors with unusual growth patterns. J Minim Invasive Gynecol. 2009;16(3):263–8. doi: 10.1016/j.jmig.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Jautzke G, Müller-Ruchholtz E, Thalmann U. Immunohistological detection of estrogen and progesterone receptors in multiple and well differentiated leiomyomatous lung tumors in women with uterine leiomyomas (so-called benign metastasizing leiomyomas). A report on 5 cases. Pathol Res Pract. 1996;192:215–23. doi: 10.1016/S0344-0338(96)80224-X. [DOI] [PubMed] [Google Scholar]
- 5.Pitts S, Oberstein EM, Glassberg MK. Benign metastasizing leiomyoma and lymphangioleiomyomatosis: sex-specific disease? Clin Chest Med. 2004;25:343–60. doi: 10.1016/j.ccm.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Canzonieri V, Piazza M, Blandamura S, Carbone A. Leiomyomatosis with vascular invasion. A unified pathogenesis regarding leiomyoma with vascular invasion, benign metastasizing leiomyoma and intravenous leiomyomatosis. Virchow Archiv. 1994;425:541–5. doi: 10.1007/BF00197559. [DOI] [PubMed] [Google Scholar]
- 7.Nucci MR, Drapkin R, Dal Cin P, Fletcher CDM, Fletcher JA. Distinctive cytogenetic profile in benign metastasizing leiomyoma: Pathogenetic implications. Am J Surg Pathol. 2007;31:737–743. doi: 10.1097/01.pas.0000213414.15633.4e. [DOI] [PubMed] [Google Scholar]
- 8.Tietze L, Günther K, Hörbe A, Pawlik C, Klosterhalfen B, Handt S, et al. Benign metastasizing leiomyoma: A cytogenetically balanced but clonal disease. Human Pathology. 2000;31:126–128. doi: 10.1016/s0046-8177(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 9.Kayser K, Zink S, Schneider T, Dienemann H, Andre S, Kaltner H, et al. Benign metastasizing leiomyoma of the uterus: documentation of clinical, immunohistochemical and lectin-histochemical data of ten cases. Virchow Arch. 2000;437:284–92. doi: 10.1007/s004280000207. [DOI] [PubMed] [Google Scholar]
- 10.Banner AS, Carrington CB, Emory WB, Kittle F, Leonard G, Ringus J, et al. Efficacy of oophorectomy in lymphangioleiomyomatosis and benign metastasizing leiomyoma. NEJM. 1981;305:204–9. doi: 10.1056/NEJM198107233050406. [DOI] [PubMed] [Google Scholar]
- 11.Uchida T, Tokumaru T, Kojima H, Nakagawaji K, Imaizumi M, Abe T. A case of multiple leiomyomatous lesions of the lung: an analysis of flow cytometry and hormone receptors. Surg Today. 1992;22:265–8. doi: 10.1007/BF00308833. [DOI] [PubMed] [Google Scholar]
- 12.Evans AJ, Wiltshaw E, Kochanowski SJ, MacFarlane A, Sears RT. Metastasizing leiomyoma of the uterus and hormonal manipulations. Case report. Br J Obstet Gynecol. 1986;93:646–8. doi: 10.1111/j.1471-0528.1986.tb08043.x. [DOI] [PubMed] [Google Scholar]
- 13.Arai T, Yasuda Y, Takaya T, Shibayama M. Natural decrease of benign metastasizing leiomyoma. Chest. 2000;117:921–2. doi: 10.1378/chest.117.3.921. [DOI] [PubMed] [Google Scholar]
- 14.Thomas EO, Gordon J, Smith-Thomas S, Cramer S. Diffuse uterine leiomyomatosis with uterine rupture and benign metastatic lesions of the bone. Obtet Gynecol. 2007;109:528–30. doi: 10.1097/01.AOG.0000237314.07944.32. [DOI] [PubMed] [Google Scholar]
- 15.Horstmann JP, Giuseppe GP, Harman JA, Cole NG, Grinspan S. Spontaneous regression of pulmonary leiomyomas during pregnancy. Cancer. 1977;39:314–21. doi: 10.1002/1097-0142(197701)39:1<314::aid-cncr2820390148>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Awonuga AO, Rotas M, Imudia AN, Choi C, Khulpateea N. Recurrent benign metastasizing leiomyoma after hysterectomy and bilateral salpingo-oophorectomy. Arch Gynecol Obstet. 2008;278:373–6. doi: 10.1007/s00404-008-0581-z. [DOI] [PubMed] [Google Scholar]
- 17.Ariel IM, Trinidad S. Pulmonary metastases from a uterine “leiomyoma.” Report of case: evaluation of differential diagnosis and treatment policies. Am J Obstet Gynecol. 1966;94:110–6. [PubMed] [Google Scholar]
- 18.Abu-Rustum NF, Curtin JP, Burt M, Jones WB. Regression of uterine low-grade smooth muscle tumors metastatic to the lung after oophorectomy. Obstet Gynecol. 1997;89:850–2. doi: 10.1016/s0029-7844(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 11) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Clark DH, Weed JC. Metastasizing leiomyoma: A case report. Am J Obstet Gynecol. 1977;127:672–3. doi: 10.1016/0002-9378(77)90373-8. [DOI] [PubMed] [Google Scholar]
- 21.Abell MR, Littler ER. Benign metastasizing uterine leiomyoma: Multiple Lymph Nodal Metastases. Cancer. 1975;36:2206–13. doi: 10.1002/cncr.2820360938. [DOI] [PubMed] [Google Scholar]
- 22.Cramer SF, Meyer JS, Kraner JF, Camel M, Mazur MT, Tenenbaum MS. Metastasizing leiomyoma of the uterus: S-phase fraction, estrogen receptor and ultrastructure. Cancer. 1980;45:932–7. doi: 10.1002/1097-0142(19800301)45:5<932::aid-cncr2820450516>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Beck MM, Biswas B, D’Souza A, Kumar R. Benign Metastasising leiomyoma after hysterectomy and bilateral salpingo-oophorectomy. Hong Kong Med J. 2012;18:153–5. [PubMed] [Google Scholar]
- 24.Rivera JA, Christopoulos S, Small D, Trifiro M. Hormonal manipulation of benign metastasizing leiomyomas: Report of two cases and review of the literature. Clin Endocrinol Metab. 2004;89:3183–8. doi: 10.1210/jc.2003-032021. [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Fang A, Gurates B, Tamura M, Miller J, Ferrer K, et al. Stromal PRs mediate induction of 17β-hydroxysteroid dehydrogenase Type 2 expression in human endometrial epithelium: A paracrine mechanism for inactivation of E2. Mol Endocrin. 2001;15:2093–2105. doi: 10.1210/mend.15.12.0742. [DOI] [PubMed] [Google Scholar]
- 26.Perel E, Daniilescu D, Kharlip L, Blackstein M, Killinger DW. Steroid modulation of aromatase activity in human culture breast carcinoma cells. J Steroid Biochem. 1988;29:393–9. doi: 10.1016/0022-4731(88)90248-8. [DOI] [PubMed] [Google Scholar]
- 27.Maruo T, Matsuo H, Shimomura Y, Kurachi O, Gao Z. Effects of progesterone on growth factor expression in human uterine leiomyoma. Steroids. 2003;68:817–24. doi: 10.1016/j.steroids.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111:1129–36. doi: 10.1097/AOG.0b013e3181705d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruo T, Ohara N, Wang J, Matsuo H. Sex Steroidal regulation of uterine leiomyoma growth and apoptosis. Human Repro. 2004;10:207–220. doi: 10.1093/humupd/dmh019. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson TZ, Rainey EJ, Turton CW. Pulmonary benign metastasizing leiomyoma: response to treatment with gosrelin. Thorax. 1995;50:1225–6. doi: 10.1136/thx.50.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasu K, Takai N, Nishida M, Narahara H. Tumorigenic effects of tamoxifen on the female genital tract. Clin Med Pathol. 2008;1:17–34. doi: 10.4137/cpath.s487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayya J, Minkoff H. Tamoxifen and growth of extrauterine leiomyoma. Eur J Obstet Gynecol. 2008;141:90–1. doi: 10.1016/j.ejogrb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Benetti-Pinto CL, Soares PM, Petta CA, De Angelo-Andrade LA. Pulmonary benign metastasizing leiomyoma: a report of 2 cases with different outcomes. J Reprod Med. 2006;51:715–8. [PubMed] [Google Scholar]
- 34.Palomba S, Orio F, Russo T, Falbo A, Tolino A, Lombardi G. Antiproliferative and proapoptotic effects of raloxifene on uterine leiomyomas in postmenopausal women. Fertil Steril. 2005;84:154–61. doi: 10.1016/j.fertnstert.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 35.Hague WM, Abdulwahid NA, Jacobs HS, Craft I. Use of LHRH analogue to obtain reversible castration in a patient with benign metastasizing leiomyoma. Br J Obstet Gynecol. 1986;93:455–60. [PubMed] [Google Scholar]
- 36.Shozu M, Sumitani H, Segawa T, Yang H-J, Murakami K, Inoue M. Inhibition of in situ expression of aromatase P450 in leiomyoma of the uterus by leuprorelin acetate. J Clin Endocrinol Metab. 2001;86:5405–11. doi: 10.1210/jcem.86.11.8026. [DOI] [PubMed] [Google Scholar]
- 37.Sumitani H, Shozu M, Segawa T, Murakami K, Yang H-J, Shimada K. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrin. 2000;141:3852–61. doi: 10.1210/endo.141.10.7719. [DOI] [PubMed] [Google Scholar]
- 38.Shozu M, Murakami K, Segawa T, Kasai T, Inoue M. Successful treatment of symptomatic uterine leiomyoma in perimenopausal with nonsteroidal aromatase inhibitor. Fertil Steril. 2003;79:628–31. doi: 10.1016/s0015-0282(02)04761-1. [DOI] [PubMed] [Google Scholar]
- 39.Parsanezhad ME, Azmoon M, Alborzi S, Rajaeefard A, Zarei A, Kazerooni R, et al. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril. 2010;93:192–8. doi: 10.1016/j.fertnstert.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno M, Nawa A, Nakanishi T, Yatabe Y. Clinical benefit of endocrine therapy for benign metastasizing leiomyoma. Int J Clin Oncol. 2001;16:587–91. doi: 10.1007/s10147-010-0156-4. [DOI] [PubMed] [Google Scholar]
- 41.Fasih N, Prasad Shanbhogue AK, Macdonald DB, Fraser-Hill MA, Papdatos D, Kielar AZ, et al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics. 2008;28:1931–48. doi: 10.1148/rg.287085095. [DOI] [PubMed] [Google Scholar]
- 42.Arif S, Ganesan R, Spooner D. Intravascular leiomyomatosis and benign metastasizing leiomyoma: an unusual case. Int J Gynecol Cancer. 2006;16:1448–50. doi: 10.1111/j.1525-1438.2006.00607.x. [DOI] [PubMed] [Google Scholar]
- 43.Takemura G, Takatsu Y, Kaitani K, Ono M, Ando F, Tanada S, et al. Metastasizing uterine leiomyoma. A case with cardiac and pulmonary metastasis. Pathol Res Pract. 1996;192:622–9. doi: 10.1016/S0344-0338(96)80116-6. [DOI] [PubMed] [Google Scholar]
- 44.Kelly CM, Buzdar AU. Anastrazole. Expert Opin Drug Saf. 2010;9:996–1003. doi: 10.1517/14740338.2010.515977. [DOI] [PubMed] [Google Scholar]
- 45.Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010;70:3278–86. doi: 10.1158/0008-5472.CAN-09-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka T. Long-term personalized GnRH agonist therapy without estrogen supplementation for recurrent endometriotic catamenial pneumothorax—case report. Clin Exp Obstet Gynecol. 2007;34:179–81. [PubMed] [Google Scholar]
- 47.Pathak AS, Pacificar JS, Shaprio CE, Williams SG. Determining dosing intervals for luteinizing hormone releasing hormone agonist based on serum testosterone levels: A prospective study. J Urol. 2007;177:2132–35. doi: 10.1016/j.juro.2007.01.157. [DOI] [PubMed] [Google Scholar]
- 48.Maheux R, Samson Y, Farid NR, Parent JG, Jean C. Utilization of luteinizing hormone-releasing hormone agonist in pulmonary leiomyomatosis. Fertil Steril. 1987;48:315–7. doi: 10.1016/s0015-0282(16)59362-5. [DOI] [PubMed] [Google Scholar]
- 49.Alessi G, Lemmerling M, Vereecken L, De Waele L. Benign metastasizing leiomyoma to skull base and spine: a report of two cases. Clin Neurol Neurosurg. 2003;105:170–4. doi: 10.1016/s0303-8467(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 50.Nardo LG, Iyer L, Reginald PW. Benign pulmonary metastasizing leiomyomatosis in pregnancy: a rare complication after cesarean section. Acta Obstet Gynecol Scand. 2003;82:770–2. doi: 10.1034/j.1600-0412.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 51.Chan JW, Law WL, Cheung SO, Lee MP, Ng CK, Lee S. Benign metastasising leiomyoma: a rare but possible cause of bilateral pulmonary nodules in Chinese patients. Hong Kong Med J. 2005;11:303–6. [PubMed] [Google Scholar]
- 52.Egberts JH, Schafmayer C, Bauerschlag DO, Janig U, Tepel J. Benign abdominal and pulmonary metastasizing leiomyoma of the uterus. Arch Gynecol Obstet. 2006;274:319–22. doi: 10.1007/s00404-006-0165-8. [DOI] [PubMed] [Google Scholar]
- 53.Tori M, Akamatsu H, Mizutani S, Yoshidome K, Oyama T, Ueshima S, et al. Multiple benign metastasizing leiomyomas in the pelvic lymph nodes and biceps muscle: report of a case. Surg Today. 2008;38:432–5. doi: 10.1007/s00595-007-3609-2. [DOI] [PubMed] [Google Scholar]
- 54.Bodner-Adler B, Bartl, Wagner G. Intravenous leiomyomatosis of the uterus with pulmonary metastases or a case with benign metastasizing leiomyoma? Anticancer Res. 2009;29:495–6. [PubMed] [Google Scholar]
- 55.Silva I, Tome V, Oliveira J. Benign metastasising leiomyoma: a progressive disease despite chemical and surgical castration. BMJ Case Rep. 2012 doi: 10.1136/bcr.01.2012.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taveira-DaSilva AM, Alford CE, Levens ED, Kotz HL, Moss J. Favorable response to antigonadal therapy for a benign metastasizing leiomyoma. Obstet Gynecol. 2012;119:438–42. doi: 10.1097/AOG.0b013e318240090e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nasu K, Tsuno A, Takai N, Narahara H. A case of benign metastasizing leiomyoma treated by surgical castration followed by an aromatase inhibitor, anastrozole. Arch Gynecol Obstet. 2009;279:255–7. doi: 10.1007/s00404-008-0698-0. [DOI] [PubMed] [Google Scholar]
- 58.Yoon G, Kim TJ, Sung CO, Choi CH, Lee JW, Lee JH, et al. Benign metastasizing leiomyoma with multiple lymph node metastasis: a case report. Cancer Res Treat. 2011;43:131–3. doi: 10.4143/crt.2011.43.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao H, Li B, Li W, Feng X, Wu L. A rare case of benign abdominal wall and pelvic metastasizing leiomyomas following hysterectomy. J Obstet Gynaecol. 2012;32:198–9. doi: 10.3109/01443615.2011.622059. [DOI] [PubMed] [Google Scholar]
- 60.Shin MS, Fulmer JD, Ho KJ. Unusual computed tomographic manifestations of benign metastasizing leiomyomas as cavitary nodular lesions or interstitial lung disease. Clin Imaging. 1996;20:45–9. doi: 10.1016/0899-7071(94)00076-x. [DOI] [PubMed] [Google Scholar]
- 61.Kishore R, Richards AP, Evans N. Benign metastasizing leiomyoma. Clin Radiology Extra. 2004;54:29–31. [Google Scholar]
- 62.Jo JH, Lee JH, Kim DC, Kim SH, Kwon HC, Kim JS, et al. A case of benign metastasizing leiomyoma with multiple metastasis to the soft tissue, skeletal muscle, lung and breast. Korean K Intern Med. 2006;21:199–201. doi: 10.3904/kjim.2006.21.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmad SZ, Anupama R, Vijaykumar DK. Benign metastasizing leiomyoma – case report and review of literature. Eur J Obstet Gynecol Reprod Biol. 2011;159:240–1. doi: 10.1016/j.ejogrb.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 64.Wongsripuemtet J, Ruangchira-urai R, Stern EJ, Kanne JP, Muangman N. Benign metastasizing leiomyoma. J Thorac Imaging. 2012;27:W41–3. doi: 10.1097/RTI.0b013e318215cc26. [DOI] [PubMed] [Google Scholar]
- 65.Stephenson CA, Henley FT, Goldstein AR. Benign metastasizing leiomyoma. Ala J Med Sci. 1984;21:78–81. [PubMed] [Google Scholar]
- 66.Cho KR, Woodruff JD, Epstein JI. Leiomyoma of the uterus with multiple extrauterine smooth muscle tumors: a case report suggesting multifocal origin. Hum Pathol. 1989;20:80–3. doi: 10.1016/0046-8177(89)90207-4. [DOI] [PubMed] [Google Scholar]
- 67.Cohen JD, Robbins HI. Response of benign metastasizing leiomyoma to progestin withdrawal. Case report. Eur J Gynaecol Oncol. 1993;14:44–5. [PubMed] [Google Scholar]
- 68.Motegi M, Takayanagi N, Sando Y, Ubukata M, Imai S, Suzuki T, et al. A case of so-called benign metastasizing leiomyoma responsive to progesterone. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31:890–5. [PubMed] [Google Scholar]
- 69.Hekster RE, Lambooy N, van Hall EV, Kazzaz BA, van Rijssel EJ. Hormone-dependent spinal leiomyoma. Surg Neurol. 1994;41:330–3. doi: 10.1016/0090-3019(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 70.Goyle KK, Moore DF, Jr, Garrett C, Goyle V. Benign metastasizing leiomyomatosis: case report and review. 2003;26:473–6. doi: 10.1097/01.coc.0000037737.78080.E3. [DOI] [PubMed] [Google Scholar]
- 71.Nardo LG, Iyer L, Reginald PW. Benign pulmonary metastasizing leiomyomatosis in pregnancy: a rare complication after cesarean section. Acta Obstet Gynecol Scand. 2003;82:770–2. doi: 10.1034/j.1600-0412.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 72.Wentling GK, Sevin BU, Geiger XJ, Bridges MD. Benign metastasizing leiomyoma responsive to megestrol: case report and review of literature. Int J Gynecol Cancer. 2005;15:1213–7. doi: 10.1111/j.1525-1438.2005.00190.x. [DOI] [PubMed] [Google Scholar]
- 73.Londero AP, Perego P, Mangioni C, Lelle RJ, Londero F, Marchesoni D. Locally relapsed and metastatic uterine leiomyoma: a case report. J Med Case Rep. 2008;2:308. doi: 10.1186/1752-1947-2-308. [DOI] [PMC free article] [PubMed] [Google Scholar]