Abstract
Study Design
Laboratory based controlled in vivo study
Objective
To determine the in vivo effects of oral glucosamine sulfate on intervertebral disc degeneration
Summary of Background Data
Although glucosamine has demonstrated beneficial effect in articular cartilage, clinical benefit is uncertain. A CDC report from 2009 reported that many patients are using glucosamine supplementation for low back pain (LBP), without significant evidence to support its use. Because disc degeneration is a major contributor of LBP, we explored the effects of glucosamine on disc matrix homeostasis in an animal model of disc degeneration.
Methods
Eighteen skeletally mature New Zealand White rabbits were divided into four groups: control, annular puncture, glucosamine, and annular puncture+glucosamine. Glucosamine treated rabbits received daily oral supplementation with 107mg/day (weight based equivalent to human 1500mg/day). Annular puncture surgery involved puncturing the annulus fibrosus (AF) of 3 lumbar discs with a 16G needle to induce degeneration. Serial MRIs were obtained at 0, 4, 8, 12, and 20 weeks. Discs were harvested at 20 weeks for determination of glycosaminoglycan(GAG) content, relative gene expression measured by RT-PCR, and histological analyses.
Results
The MRI index and NP area of injured discs of glucosamine treated animals with annular puncture was found to be lower than that of degenerated discs from rabbits not supplemented with glucosamine. Consistent with this, decreased glycosaminoglycan was demonstrated in glucosamine fed animals, as determined by both histological and GAG content. Gene expression was consistent with a detrimental effect on matrix.
Conclusions
These data demonstrate that the net effect on matrix in an animal model in vivo, as measured by gene expression, MRI, histology, and total proteoglycan is anti-anabolic. This raises concern over this commonly used supplement, and future research is needed to establish the clinical relevance of these findings.
Keywords: intervertebral disc, glucosamine, degeneration, matrix, annular puncture, glycosaminoglycan
Introduction
In a CDC report from 2004 on complementary and alternative medicine use among adults, low back pain was identified as the most common condition for which patients sought alternative treatments. Among adults using a non-vitamin, non-mineral supplement, 15% were using glucosamine, with over 5 million respondents using glucosamine within a previous 12 month period1. Clinical evidence for the efficacy of oral supplements exists for articular cartilage2, 3, as well as intervertebral disc degeneration4. In fact, similar efficacy has been shown when compared to NSAID use in a double blind trial, with a much more favorable side effect profile5. However, inconsistency exists in the literature due to variability in study design, patient inclusion, and glucosamine formulation. The GAIT trial failed to demonstrate improvement in pain and function related to knee osteoarthritis in two year follow up6. In a more recent randomized controlled trial of glucosamine for low back pain, no difference was noted in pain or pain related disability after 6 months of glucosamine supplementation7. With the lack of consensus in the literature, one can conclude that it is possible that subgroups of patients may derive benefit from glucosamine supplementation. However, to identify any potential biologic plausibility for a clinical effect, if one exists, identification of the biological mechanism of action of glucosamine is needed. Because all patients with low back pain do not have the same pathophysiology leading to their clinical presentation, understanding the metabolic processes affected by glucosamine would allow for prediction of which patients may respond to the treatment, and may lead to more directed clinical trials.
The hallmark of intervertebral disc degeneration is progressive loss of disc height related to loss of water and proteoglycan content. The integrity of the disc matrix depends on the catabolic and anabolic balance, which is affected by inflammatory cytokines and degradative enzymes in disc degeneration. The potentially therapeutic action of glucosamine, as studied in articular cartilage, is thought to occur through promoting glycosaminoglycan synthesis8, and through the inhibition of IL-1beta induced proteoglycan catabolic activity9, thereby shifting the matrix homeostatic balance. However, these effects have not been established in intervertebral disc. The goal of the current study was to explore the effects on glucosamine on disc matrix homeostasis, using an animal model, with the purpose of establishing the ground work for future clinical studies direceted against the specific pathophysiology affected by glucosamine.
Materials and Methods
Glucosamine supplement
Glucosamine sulfate (#488) was obtained as a crystalline powder from Wonder Labs, Inc., White House TN. All glucosamine utilized for the current study was obtained from a single lot (06902), with certificate of analysis provided by the company demonstrating excellent specifications, with < 10cfu/gm yeast, negative for salmonella and E. coli and <10 ppm of heavy metals. This glucosamine sulfate preparation (lot 06902) was separately analyzed by an independent, private laboratory (BioQuant Inc. San Diego, Ca) and found to have over 93% purity with no measureable heavy metal contamination.
Bioavailability
A total of six skeletally mature female New Zealand White rabbits were used to determine the intra-discal bioavailiability of oral glucosamine sulfate. All rabbits were approximately 4 Kg upon purchase. Three rabbits were orally fed 85mg per day (21.4 mg/kg) of glucosamine sulfate via syringe for 30 days, while the other three controls received no glucosamine supplementation. After thirty days the 5 lumbar intervertebral discs were harvested and the lumbar nucleus pulposus (NP) tissues isolated and consolidated for each animal. The tissue was shipped snap frozen in liquid nitrogen to BioQuant for blinded analysis of glucosamine content within the NP tissues, determined via the High Performance Liquid Chromatography (HPLC) method.
Animal model
Eighteen skeletally mature New Zealand White rabbits, approximately 5 Kg upon purchase, were divided into four groups: control (n=3), glucosamine-fed (n=4), annular puncture (n=6), and annular puncture+glucosamine-fed (n=5). Glucosamine treated rabbits received daily oral supplementation with 107mg/day (21.4 mg/Kg, weight based equivalent to human 1500 mg/day) for 30 days prior to the onset of the study, and throughout the 20 weeks of imaging analysis until sacrifice (see Figure 1). The annular puncture model for disc degeneration in the rabbit was used as previously described and validated10. Before initiation of the study, the protocol was approved by the University of Pittsburgh IACUC (protocol #0901832). Rabbits in the annular puncture group were tranquilized by intramuscular injection of xylazine (5mg/kg) and ketamine (35 mg/kg). They were placed under general anesthesia and the L2-3, L3-4, and L4-5 discs were punctured with a 16 gauge hypodermic needle to a depth of 5mm in the anterolateral annulus fibrosus on the left side.
Figure 1.
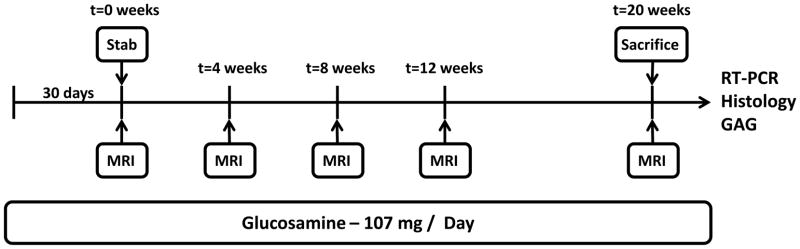
Rabbits were pre-fed glucosamine for 30 days. All animals underwent baseline MRIs immediately prior to annular puncture. Rabbits were followed with serial MRIs until sacrifice at 20wks, at which time spines were harvested and additional outcome measures were performed.
Measurement of MRI index
MRI scans were obtained using a 3.0 Tesla magnet after rabbits were tranquilized using xylazine and ketamine as listed above. All animals, including controls, had an MRI of the lumbar spine one day prior to the puncture procedure. Animals were placed supine with the lumbar region centered over a 5 inch diameter circular surface coil, and coronal T2 weighted localizer image obtained to establish position. Mid-sagittal sections were selected using T2 weighted imaging (TR/TE = 3,500/109 ms, slice thickness of 3mm) to highlight the signal from the nucleus pulposus,. The nucleus pulposus area of each disc was outlined using a semi-automated contour segmentation methods, previously validated to detect rabbit disc degeneration and shown to have excellent intraclass correlation11, from which the average signal intensity was computed using MATLAB software (Natick, MA). The MRI index was computed as the product of nucleus pulposus area and average signal intensity as previously described10. This analysis provides information on both the architecture of the disc (area) and the relative preservation of water and proteoglycan content in the disc (index). Values are reported as % change from each animals t=0 scan to account for differences between animals. The rabbits were sacrificed after their final MRI scans at 20 weeks (Figure 1).
Histological analysis
At the time of sacrifice, the L4-5 disc from each animal was removed, fixed and decalcified using Decalcifier I (Surgipath, #00442) for two weeks, and embedded in paraffin before sectioning. The discs were sectioned at a thickness of 5 um in the sagittal plane and mounted on super frost plus glass slides. Following de-paraffinization in xylene substitute and re-hydration through graded ethanol wash to water, the sections were stained with Hematoxylin and Eosin (for morphological changes), Safranin O (for regional proteoglycan changes) and Alcian Blue (Alcian Blue stain kit, Leica, Catalog No: 38016ss3, for proteoglycan changes) according to standard histological protocols and analyzed qualitatively under Nikon E800 microscope for evidence of changes in the nucleus pulposus and annulus fibrosus. Comparisons were made between the L4-5 disc of control, glucosamine fed, annular puncture, and annular puncture + glucosamine-fed animals.
Gene Expression Analysis
The nucleus pulposus and annulus fibrosus regions were removed from the L2-3 and L3-4 discs of each lumbar spine at the time of sacrifice. To isolate RNA, nucleus pulposus or annulus fibrosus disc tissue was minced separately, dispersed in QIAzol (Qiagen) and homogenized (using an Ika-Werker Ultra-Turax T8). Proteins and particulates was removed by phenol/chloroform extraction, and nucleic acids recovered by ethanol precipitation. RNA isolation was accomplished using Qiagen miniprep kit including a DNAse step to remove genomic material per the manufacturer’s instructions. RNA concentration and quality was measured using a Nanodrop spectrophotometer. RT-PCR was performed using Sybr-Green and custom designed and validated primers for collagen I, II, and aggrecan, using Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the housekeeping gene (see Table 1). Relative gene expression changes were reported using the comparative Ct method as previously described12.
Table 1.
Primer sequences used for RT-PCR
| Primer | Forward Sequence (5′ - 3′ | Reverse Sequence (5′ - 3′ |
|---|---|---|
| GAPDH | GATGCTGGTGCCGAGTAC | GCTGAGATGATGACCCTTTTGG |
| Collagen I | ATGGATGAGGAAACTGGCAACT | GCCATCGACAAGAACAGTGTAAGT |
| Collagen II | AAGAGGTATAATGATAAGGATGTGTGGAA | CAGTCCCCGTGTCACAGACA |
| Aggrecan | GCTACGGAGACAAGGATGAGTTC | CGTAAAAGACCTCACCCTCCAT |
Total GAG content
At the time of sacrifice, the nucleus pulposus and annulus fibrosus was dissected out of the T11-12 and T12-L1 discs (non-annular puncture discs) to determine the effect of glucosamine on total glycosaminoglycan content. Digestion of the tissue to remove interfering proteins were accomplished in papain (0.25 mg/mL) in 20 mM phosphate buffer, 1 mM EDTA, 2 mM dithiothreitol at 60 C for 1 hour, followed by addition of iodoacetic acid to a 10mM final concentration as reported previously13. Samples were prepared in 1,9 dimethylmethylene blue buffer, pH 3.5 and the optical density read at 540 nm and compared to known chondroitin sulfate standards. Results were normalized to tissue weight in milligrams.
Statistical Analysis
To evaluate the MRI outcome measures (MRI index and area), a mixed-measures general linear model (or ANOVA) was used to examine the effect of time (a repeated, within-group comparison) and the effect of treatment (a between-group comparison). Simple planned comparisons (chosen based on original hypotheses) and Gabriel’s post-hoc test (chosen due to the unequal sample sizes in some groups) were performed to examine differences between groups. In addition, one way repeated measures ANOVA was used to identify the effect of time within each group. For gene expression and total GAG analysis, 95% confidence intervals were calculated to determine statistical significance at p<0.05 level. The confidence intervals were calculated based on the t-distribution because of the small sample size. Computations were performed using Microsoft Excel and SPSS v 17.0 statistical software.
Results
Bioavailability
The mean concentration of glucosamine within the NP was 2.26μg/g NP tissue in the treatment group, compared to 0.052 μg/g in the control group. This difference was statistically significant (p=0.043), demonstrating intradiscal bioavailability of orally dosed glucosamine sulfate.
General
No adverse events were noted in any of the animals fed glucosamine which could be attributed to the supplementation. However, in the annular puncture + glucosamine group, one animal’s hindlimb became trapped within the cage, resulting in a fracture and necessitating sacrifice of the animal at 8 weeks, after the 8 week MRI timepoint.
MRI index
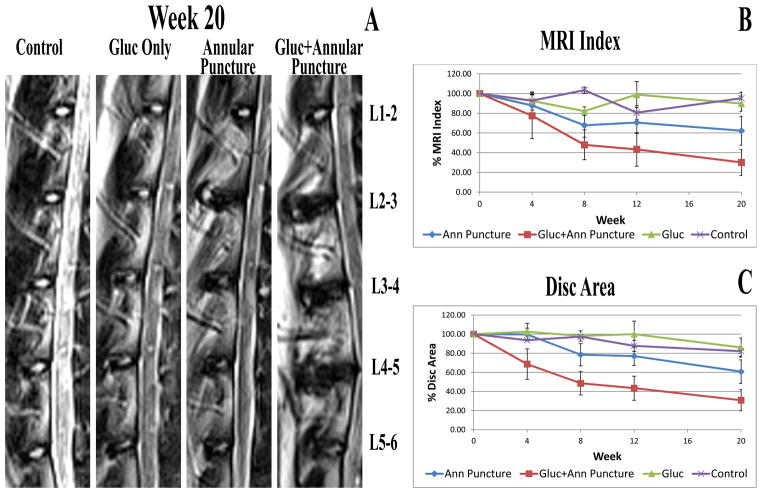
The MRI index and NP area of injured discs of glucosamine treated animals with annular puncture was found to be lower than that of degenerated discs from rabbits not supplemented with glucosamine. (See Figure 2). Upon statistical analysis, MRI index demonstrated a statistically significant effect of time, but not group. Change over time was significant for annular puncture and annular puncture + glucosamine fed groups. There was no significant change over time in the control or glucosamine fed group. However, NP area demonstrated statistical significance for both time (p<0.001) and group (0.029) effects. Similar to MRI index, change over time was significant for annular puncture (p<0.0001) and annular puncture + glucosamine fed (p=0.02) groups, but not control and glucosamine fed groups. Importantly, the NP area demonstrated a statistically significant effect of time at 8, 12, and 20 week timepoints for annular puncture + glucosamine fed, whereas the annular puncture alone did not show a significant time effect until the 20 week timepoint, demonstrating more rapid decline in NP area in the glucosamine fed group. Regarding group effects, the difference between annular puncture and annular puncture + glucosamine fed was statistically significant (p=0.036). However, statistical significance was not maintained after Gabriel pos-hoc correction for multiple comparisons. There was no significant difference seen in the MRI index or NP area of other lumbar discs (non-intervention discs) in animals with and without glucosamine supplementation (data not shown).
Figure 2.
A. Representative mid sagittal T2 weighted MRI images of animals from each group at the 20 week timepoint. B. MRI index calculated at each timepoint. C. NP area calculated at each timepoint.
Histological Analysis
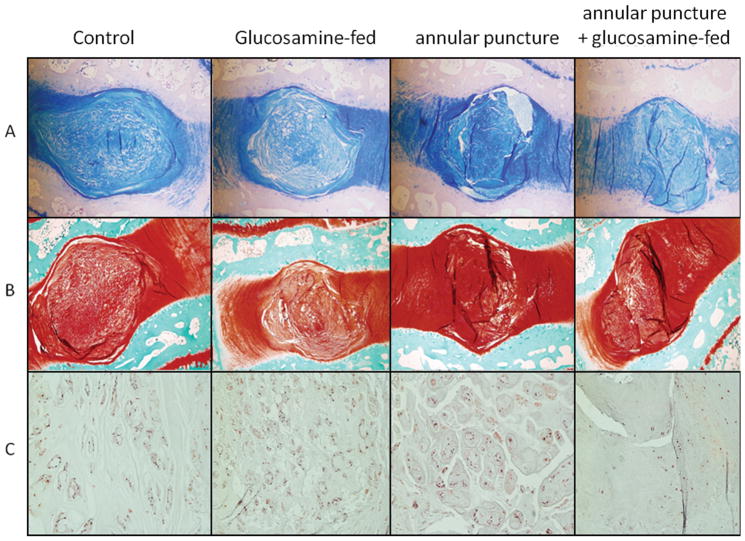
Glucosamine fed animals demonstrated decreased safranin-O and alcian blue staining in the intervertebral disc, indicating lower glycosaminoglycan content in both uninjured and annular puncture groups (see Figure 3A and B). In addition, in discs that underwent annular puncture, development of hypertrophic chondrocytes was noted, whereas in discs from glucosamine fed animals with annular puncture, decreased cellularity was noted (Figure 3C).
Figure 3.
A. Alcian blue staining of representative discs from each group imaged at 20X. B. Safranin-O staining of representative discs from each group imaged at 20X. C. H+E staining of representative NP from each group imaged at 100X.
Gene Expression Analysis
In the annulus fibrosus, glucosamine administration resulted in slightly increased collagen I expression in uninjured (11%) and injured (15%) discs compared to uninjured and injured controls respectively. However, collagen II was decreased in uninjured (88%) and injured (51%) discs in response to glucosamine administration. Similarly, aggrecan gene expression was decreased in uninjured (41%) and injured discs (52%) in glucosamine fed animals. In the NP, aggrecan gene expression was increased modestly (20%) in uninjured discs in response to glucosamine administration, but decreased (79%) in injured discs. However, these changes did not reach statistical significance, and low mRNA yield from degenerated discs precluded reliable analysis of additional genes in the NP.
Total GAG content
Non-injured (T11-12 and T12-L1) discs of glucosamine treated rabbits had statistically significantly lower GAG content in the NP and AF compared to discs of untreated rabbits (See Figure 4), consistent with findings from other outcome measures above demonstrating negative effect on disc homeostasis.
Figure 4.
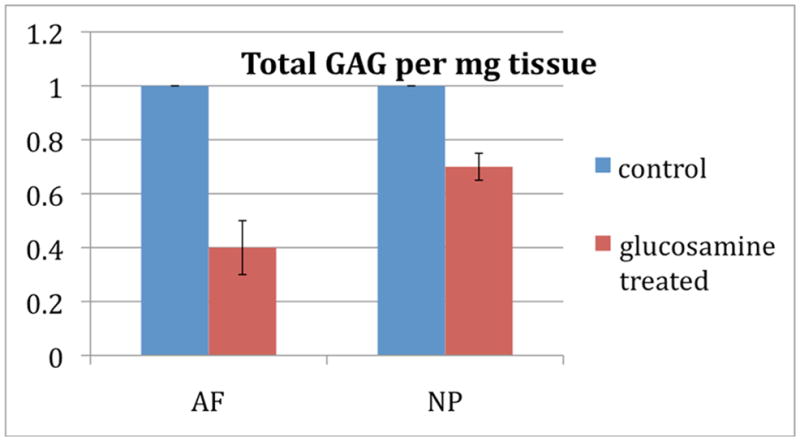
Total glycosaminoglycan per mg of tissue of glucosamine fed animals normalized to control. Non-injured discs of glucosamine treated rabbits had lower GAG content in the NP and AF compared to discs of untreated control rabbits, and these changes were statistically significant with p<0.05.
Discussion
In this study, glucosamine appears to negatively affect disc matrix as observed by decrease total disc GAG content, decrease in the MRI index and NP area, worsening proteoglycan and cellularity on histological analysis, and decreased matrix gene expression. To our knowledge, this is the first study to suggest a negative effect of glucosamine. All prior studies, including those in articular cartilage, have demonstrated either no significant effect or beneficial effects on matrix homeostasis, including anti-catabolic effects, such as inhibition of matrix metalloproteinases or aggrecanases14,15, or improved anabolic activity16, In addition, our previous in vitro research on the effects of glucosamine on inflammation in the intervertebral disc paralleled evidence from articular cartilage, demonstrating anti-inflammatory activity17,18.
However, we have now demonstrated a detrimental effect of glucosamine on matrix homeostasis in discs in vivo, raising concern over the widespread use of this unregulated supplement Interestingly, changes in response to glucosamine were noted in non-annular puncture groups in the GAG and histological outcome measure, but not in MRI index or NP area. This may relate to the inherent sensitivities of the two outcome measures to stage of degeneration, and represents an important area of future investigation. MRI findings are more sensitive to later stage degenerative changes19, and would therefore be more likely to pick up differences in the punctured animals. To the contrary, in a degenerated disc with low remaining proteoglycan content, it is possible that incremental loss of proteoglycan would not be detectable. However, there remains the potential for biologic differences to exist in the effects of glucosamine on normal and degenerative discs. This could not be reconciled in the current study because the other outcome measures utilized, namely PCR and histology, preclude GAG analysis from the same disc. Therefore, GAG analysis could not performed on injured discs. However, the histological results are consistent with the MRI findings, both indicating that glucosamine treatment resulted in further loss of disc proteoglycan matrix in punctured discs.
To determine if orally administered glucosamine reached disc tissue, we determined the bioavailability of glucosamine in the NP. The bioavailability data demonstrated a significant increase in intradiscal concentrations of glucosamine after oral supplementation, consistent with concentrations found previously in vitro to have biologic effects on disc cells20. Therefore, it is plausible that the effect on disc matrix observed in this study is a direct effect of glucosamine on disc cell metabolic activity, either anabolically, catabolically, or both. Our outcome measures did not allow us to distinguish between a decrease in matrix synthesis vs. an increase in matrix catabolism, and therefore the mechanism of the induced effect remains unknown. It is also possible that the oral supplementation induced a systemic effect, which may have affected disc matrix homeostasis. In addition, we chose glucosamine sulfate for use in our study based on previous research suggesting superior oral bioavailability21. However, it remains possible that other clinically used formulations of glucosamine, such as glucosamine HCl, could demonstrate different effects.
The limitations of the study include limitations inherent in an animal model study, including the inability to directly translate this to an expected effect in humans. However, we utilized a well-validated rabbit model of disc degeneration, which mimics human degeneration biochemically22. In addition, we chose the supplement for use in the study based on its known purity. However, given the variability of commercially available glucosamine supplements, these results may not generalize to other formulations. We also chose weight based equivalent dosing due to the lack of information about rates of glucosamine metabolism in rabbits. However, because rabbits often demonstrate more rapid metabolism of oral agents compared to humans, the dosing used in this study may be lower than typical human doses. Although we demonstrated bioavailability in the disc with oral glucosamine, based on standard conversions23 for metabolic rates, this weight based dosing may actually underestimate the human equivalent, and therefore, the detrimental effects may be even greater for typical human doses. In addition, we chose to pre-feed the animals for 30 days prior to administration of the annular puncture. The purpose of this, from an experimental design perspective, was to maximize the ability to observe any potential therapeutic benefit of glucosamine at dosing equivalent to typical human use. Clinically, this protocol may most closely model an individual who was already taking glucosamine, perhaps for other purposes, and experienced an annular injury. The results, therefore, cannot be extrapolated to chronic degeneration. However, we would posit that any negative effects on matrix would potentially be more detrimental during chronic degeneration, during which matrix homeostasis is already tenuous. Similarly, as glucosamine has been shown to be anti-inflammatory, it would be expected that any anti-inflammatory effects would be most beneficial early after an acute injury, and may therefore counteract the negative anabolic effects. This would suggest that the negative anabolic effects may actually be more detrimental later in the disease course, where such high levels of inflammation are not present. This represents an important topic for future research since in clinical practice, supplementation may not coincide with the timing of an acute injury.
In conclusion, while in vitro gene expression suggested an anti-inflammatory effect of oral glucosamine20, the net effect on matrix in vivo, as measured by gene expression, MRI, histology, and total proteoglycan is anti-anabolic. This may in part help to explain the conflicting reports of efficacy, as there is biological plausibility for a therapeutic effect under conditions of predominate inflammation, but not of matrix loss. Because all patients with low back pain do not have the same pathophysiology, understanding the pathways affected by glucosamine may allow for prediction of which patients may respond to the treatment. This has the potential to facilitate more directed clinical trials with greater chance of demonstrating efficacy or harm, which represents an important avenue for future study of this widely used nutritional supplement.
Key Points.
Glucosamine is a nutritional supplement commonly used in patients with disc degeneration and back pain, despite lack of evidence for efficacy.
In an animal model, oral glucosamine resulted in decreased intervertebral disc matrix health, as assessed by MRI, histology, total glycosaminoglycan content, and gene expression.
Human studies are required to determine the clinical relevance of these findings.
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s). This work was supported by NIH/NCCAM grant K08 AT004718. Relevant financial activities outside the submitted work: Grants, Patents, Payment for Lecture, Royalties.
Footnotes
Level of Evidence: NA
References
- 1.Barnes P, Powell-Griner E, McFann K, Nahin R. Complementary and alternative medicine use among adults: United States 2002. Adv Data. 2004;343:1–19. [PubMed] [Google Scholar]
- 2.Towheed TSB, Wells G, et al. Analgesia and non-aspirin, non-steroidal anti-inflammatory drugs for osteoarthritis of the hip. The Cochrane library Oxford; England: 2001. Update Software. [DOI] [PubMed] [Google Scholar]
- 3.Richy FBO, Ethgen O, et al. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis: a comprehensive meta-analysis. Arch Intern Med. 2003:163. doi: 10.1001/archinte.163.13.1514. [DOI] [PubMed] [Google Scholar]
- 4.van Blitterswijk W, van de Nes J, Wuisman P. Glucosamine and chondroitin sulfate supplementation to treat symptomatic disc degeneration: biochemical rationale and case report. BMC Complement Altern Med. 2003;3:2–9. doi: 10.1186/1472-6882-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu GGS, GIacovelli G, et al. Efficacy and safety of glucosamine sulfate versus ibuprofen in patients with knee osteoarthritis. Arzneimittelforschung. 1998;4:469–74. [PubMed] [Google Scholar]
- 6.Sawitzke ADSH, Finco MF, Dunlop DD, Harris CL, Singer NG, Bradley JD, Silver D, Jackson CG, Lane NE, Oddis CV, Wolfe F, Lisse J, Furst DE, Bingham CO, Reda DJ, Moskowitz RW, Williams HJ, Clegg DO. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459–64. doi: 10.1136/ard.2009.120469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkens P, Scheel IB, Grundnes O, Hellum C, Storheim K. Effect of glucosamine on pain-related disability in patients with chronic low back pain and degenerative lumbar osteoarthritis: a randomized controlled trial. JAMA. 2010;304:45–52. doi: 10.1001/jama.2010.893. [DOI] [PubMed] [Google Scholar]
- 8.Oegema TDL, Sandy J, et al. Effect of oral glucosamine on cartilage and meniscus in normal and chymopapain-injected knees of yuong rabbits. Arthritis Rheum. 2002;46:2495–503. doi: 10.1002/art.10499. [DOI] [PubMed] [Google Scholar]
- 9.Sandy JGD, Thompson V, Verscharen C. Chondrocyte-mediated catabolism of aggrecan: aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem J. 1998;335:59–66. doi: 10.1042/bj3350059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobajima S, Kompel JF, Kim JS, et al. A Slowly Progressive and Reproducible Animal Model of Intervertebral Disc Degeneration Characterized by MRI, X-Ray, and Histology. Spine. 2004;30:15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 11.Bechara BPLS, Bowman BW, Davies CE, Woods BI, Kanal E, Sowa GA, Kang JD. Application of a Semiautomated Contour Segmentation Tool to Identify the Intervertebral Nucleus Pulposus in MR Images. Am J Neuroradiol. 2010;31:1640–4. doi: 10.3174/ajnr.A2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livak K, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Farndale RBD, Barrett A. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue biochem biophysica acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 14.Piperno M, Reboul P, Hellio Le Graverand MP, Peschard MJ, Annefeld M, Richard M, Vignon E. Glucosamine sulfate modulates dysregulated activities of human osteoarthritic chondrocytes in vitro. Osteoarthritis Cartilage. 2000 May;8(3):207–12. doi: 10.1053/joca.1999.0291. [DOI] [PubMed] [Google Scholar]
- 15.Sandy JD, Gamett D, Thompson V, Verscharen C. Chondrocyte-mediated catabolism of aggrecan: aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem J. 1998 Oct 1;335(Pt 1):59–66. doi: 10.1042/bj3350059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi M, Kaga I, Takamori Y, Sakamoto K, Miyazawa K, Nagaoka I. Effects of glucosamine derivatives and uronic acids on the production of glycosaminoglycans by human synovial cells and chondrocytes. Int J Mol Med. 2011 Jun;27(6):821–7. doi: 10.3892/ijmm.2011.662. Epub 2011 Mar 31. [DOI] [PubMed] [Google Scholar]
- 17.Chan PCJ, Rosa G, Orth M. Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E2 in articular cartilage explants. Osteoarthr Cartilage. 2005;13:387–94. doi: 10.1016/j.joca.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Chan PCJ, Orth M. Short-term gene expression changes in cartilage explants stimulated with interleukin 1beta plus glucosamine and chondroitin sulfate. J Rheumotol. 2006;33:1329–40. [PubMed] [Google Scholar]
- 19.Moon CHJL, Kim JH, Sowa G, Vo N, Kang J, Bae KT. Quantitative Proton T2 and Sodium MR Imaging to Assess Intervertebral Disc Degeneration in a Rabbit Model. Spine. 2012;37:E1113–9. doi: 10.1097/BRS.0b013e3182583447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sowa GCP, Komperda K, Vo N, Balk J, Preuss H, Studer R, Kang J. Glucosamine Decreases Inflammatory Gene Expression in Intervertebral Disc Cells Exposed to Inflammatory or Mechanical Stress. Proceedings of the North American Spine Society; 2010; Orlando, Fl. [Google Scholar]
- 21.Meulyzer MVP, Beaudry F, Vinardell T, Richard H, Beauchamp G, Laverty S. Comparison of pharmacokinetics of glucosamine and synovial fluid levels following administration of glucosamine sulphate or glucosamine hydrochloride. Osteoarthr Cartilage. 2008;16:973–9. doi: 10.1016/j.joca.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Sobajima SSA, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5:14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 23.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB Journal. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]