Abstract
The plant toxin ricin is highly toxic for mammalian cells and is of concern for bioterrorism. Ricin belongs to a family of functionally related toxins, collectively referred to as ribosome inactivating proteins (RIPs), which disable ribosomes and halt protein synthesis. Currently there are no specific antidotes against ricin or related RIPs. The enzymatic subunit of ricin is an N-glycosidase that depurinates a universally conserved adenine residue within the sarcin/ricin loop (SRL) of the 28S rRNA. This depurination activity inhibits translation and its biochemistry has been intensively studied. Yet, recent developments paint a more complex picture of toxicity, with ribosomal proteins and cellular signaling pathways contributing to the potency of ricin. In particular, several studies have now established the importance of the ribosomal stalk structure in facilitating the depurination activity and ribosome specificity of ricin and other RIPs. This review highlights recent developments defining toxin-ribosome interactions and examines the significance of these interactions for toxicity and therapeutic intervention.
Keywords: Ricin, ribosome inactivating protein, ribosome, ribosomal stalk
1. Introduction
Ricin is a naturally occurring protein found in castor beans (Ricinus communis) that has a long and storied notoriety due to its potent toxicity (Olsnes, 2004). Castor beans are cultivated for oil production (Olsnes, 2004; Mutlu and Meier, 2010). Castor oil has been used in pharmaceutical and industrial applications and in the synthesis of biodiesel fuels (Mutlu and Meier, 2010). However, ricin remains at high levels in the castor cake after oil extraction, limits use of this by-product and causes health problems (Mutlu and Meier, 2010). Ricin belongs to a family of functionally and structurally related toxins of plant and bacterial origin that are collectively referred to as ribosome inactivating proteins (RIPs) due to their ability to depurinate the α-sarcin/ricin loop (SRL) of the large rRNA and inhibit protein synthesis (Endo et al., 1987; Endo and Tsurugi, 1987; Lord et al., 1994). Family members include the plant toxins pokeweed antiviral protein (PAP), trichosanthin (TCS) (from Trichosanthes kirilowii), abrin (from Abrus precatorius), saporin (from Saponaria officinalis) gelonin (from Gelonium multiflorum), and Shiga toxins (Stx) found in the bacterial species Shigella dysenteriae and Shiga-toxigenic Escherichia coli (de Virgilio et al., 2010). These Shiga toxin-producing pathogens are associated with severe gastrointestinal disease in humans and are responsible for significant morbidity and mortality world-wide (Paton and Paton, 1998; Bergan et al., 2012). The potent toxicity of ricin and other RIPs is being exploited for the development of targeted cancer therapies; yet this same property is feared as a formidable weapon of bioterrorism (Olsnes, 2004; Musshoff and Madea, 2009; de Virgilio et al., 2010). Ricin is a category B bioterrorism agent and to date, there are no US Food and Drug Administration-approved vaccines or therapeutics for either treatment or protection against ricin or other RIPs. Various therapeutic strategies against RIPs have been investigated, the majority of which have involved either inhibition of enzymatic activity (Yan et al., 1997; Miller et al., 2002; Mandal et al., 2008; Bai et al., 2009; 2010; Pang et al., 2011; Pruet et al., 2011; Wahome et al., 2011) or disruption of toxin uptake or trafficking (Paton et al., 2000; 2001; Saenz et al., 2007; Stechmann et al., 2010; Yermakova and Mantis, 2011; Mukhopadhyay and Linstedt, 2012; Park et al., 2012; Rasooly et al., 2012; Yermakova et al., 2012). Although some of these approaches have shown modest efficacy, a more complete understanding of the activity of ricin and other RIPs will be required to develop improved inhibitors of these toxins.
2. Path of ricin to the ribosome
Ricin is comprised of two subunits: a 32 kDa enzymatic subunit (RTA) and a 34 kDa galactose/N-acetylgalactosamine-binding subunit (RTB) that are coupled by a single disulfide bond (Olsnes and Pihl, 1972; 1973). RTB is a lectin that facilitates uptake of the toxin into mammalian cells through interactions with glycoproteins or glycolipids at the cell surface (Lord et al., 1994). Following endocytic uptake, a limited amount of ricin proceeds to the trans-Golgi network and undergoes retrograde trafficking to the endoplasmic reticulum (ER) (Yoshida et al., 1991; Rapak et al., 1997; Lord and Roberts, 1998; Sandvig et al., 2010). Within the ER lumen, a resident protein disulfide isomerase facilitates reductive separation of RTA and RTB (Spooner et al., 2004). Subsequently RTA is thought to exploit cellular pathways that function to transport misfolded proteins across the ER membrane into the cytosol for degradation by proteasomes (Simpson et al., 1999; Stolz and Wolf, 2010; Spooner and Lord, 2012). RTA utilizes specific components of the endoplasmic reticulum (ER) associated degradation (ERAD) pathway for dislocation, and has unique characteristics compared with typical ERAD substrates (Spooner and Lord, 2012).
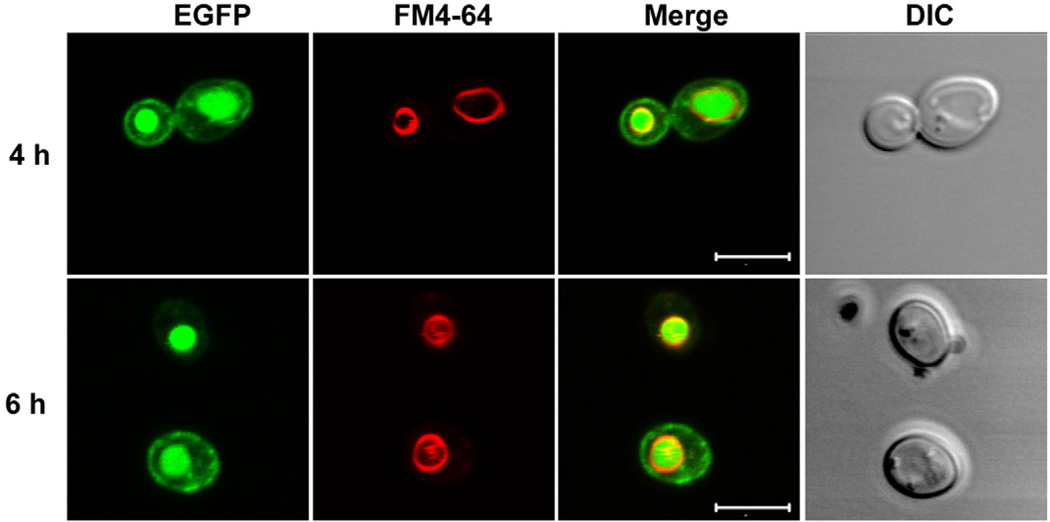
Recent work from our group examined the structural features within RTA itself that affect transport out of the ER into the cytoplasm, a process that indirectly affects subsequent ribosome depurination and cytotoxicity (Yan et al., 2012). Ricin is synthesized as a glycoprotein in Ricinus communis (Lord, 1985). N-glycosylation occurs on asparagines 10 and 236 in RTA (Rutenber et al., 1991). The role of glycosylation in transport of RTA out of the ER was not known. Recombinant nonglycosylated RTA inhibits protein synthesis almost as effectively as plant-derived glycosylated RTA in vitro (Schlossman et al., 1989), suggesting that glycosylation does not affect catalytic activity. Since very few RTA molecules reach the ER in mammalian cells and are difficult to visualize, we used yeast as a model to study the intracellular trafficking of RTA by expressing enhanced green fluorescent protein (EGFP) tagged RTA with its native signal sequence (Yan et al., 2012). The precursor form of RTA (preRTA) was initially targeted to the ER by the native N-terminal signal peptide and was subsequently transported to the vacuole. The preRTA accumulated in the vacuole at 4 and 6 hours post induction when expressed from the GAL1 promoter in yeast (Fig. 1). The mature form of RTA without the N-terminal extension was not localized in the ER or in the vacuole and remained in the cytosol. When the two glycosylation sites in RTA were mutated, the cytosolic mature form was fully active and toxic, indicating that the mutations did not affect its catalytic activity. However, the nonglycosylated precursor form had reduced level of depurination and toxicity, indicating that N-glycosylation affected transport of RTA to the cytosol (Yan et al., 2012). Mutations of N-glycosylation sites also delayed transport of RTA to the vacuole, indicating that N-glycosylation promotes transport of RTA out of the ER. The timing of depurination coincided with the timing of vacuole transport, suggesting that RTA may enter the cytosol during anterograde transport to the vacuole (Yan et al., 2012). A C-terminal hydrophobic sequence within RTA was critical for transport across the ER membrane (Yan et al., 2012). A C-terminal mutation (P250A) was also found to reduce transport of RTA from the ER to the cytosol in mammalian cells (Sokołowska et al., 2011). Collectively, these results demonstrated that N-glycosylation and the C-terminal hydrophobic sequence contribute to the toxicity of RTA by promoting its transport out of the ER (Yan et al., 2012).
Fig. 1.
Transport of preRTA to the vacuole in Saccharomyces cerevisiae. Wild type yeast cells carrying preRTA-EGFP were grown in SD medium with glucose and induced with galactose for 4 and 6 hours. Yeast cells were treated with FM4-64 to stain the vacuole. Intracellular localization of preRTA-EGFP in yeast was analyzed with a Zeiss LSM 710 Confocal Microscope. Merged images show localization of preRTA-EGFP relative to the vacuole. Scale bars represent 5 µm.
It is thought that partial unfolding of RTA facilitates its dislocation from the ER to the cytosol. Indeed RTA exhibits conformational lability and partial unfolding in vitro (Argent et al., 2000). Partially unfolded RTA was compact, retained significant secondary structure but lacked stable tertiary structure. Furthermore, compared to native RTA, RTA folding intermediates exhibited increased protease sensitivity and increased exposure of hydrophobic residues, as evidenced by an increased ability to bind to the hydrophobic fluorescent probe, anilino naphthalene sulfonate (Argent et al., 2000). All these properties are consistent with a molten globular state (Ptitsyn, 1995), observed for other proteins that traverse membranes (van der Goot et al., 1992). It is thought that specific conditions within cellular compartments or extra-cellular milieu, including changes in pH or temperature, or reduction of disulfide bonds, trigger partial unfolding into molten globular states permissive of membrane translocation (van der Goot et al., 1992). Remarkably, re-folding of RTA in vitro was stimulated by the addition of ribosomes leading the authors to propose that ribosomes support refolding of RTA following its dislocation from the ER to the cytosol (Argent et al., 2000). Once ricin has gained access to the cytosol it is thought that RTA evades proteasomal degradation due to a paucity of lysine residues, which can be polyubiquitinated, a process that mediates targeting to the proteasome (Hazes and Read, 1997).
3. The role of ribosomal proteins in ricin activity and specificity
It has long been established that RTA inhibits translation through its N-glycosidase activity and its ability to cleave a specific adenine residue of the universally conserved SRL of 28S ribosomal RNA, a process termed depurination (Endo and Tsurugi, 1987). The SRL is part of the GTPase center of the ribosome and is critical for ribosome function as it facilitates the binding and activation of translational GTPases that regulate protein synthesis (Agrawal et al., 1998; Clementi and Polacek, 2010; Voorhees et al., 2010; Shi et al., 2012). Although the SRL has been established as a common substrate for all RIPs, early work suggested that ricin might also interact with other ribosomal elements. In particular, it was observed that although the Km of RTA for rat ribosomes and naked 28S rRNA was similar, RTA exhibited 105-fold higher catalytic activity (kcat) on rat ribosomes than on naked 28S rRNA (Endo and Tsurugi, 1988), hinting at the importance of ribosomal proteins in facilitating the depurination activity. Consequently, several studies have examined the role of ribosomal proteins proximal to the SRL and their role in promoting depurination. Initially chemical cross-linking in mammalian cells suggested that internalized ricin is proximal to the stalk protein P0 and the ribosomal protein L9 (Vater et al., 1995), although this study did not demonstrate either a direct interaction of these ribosomal proteins with ricin or a direct requirement for these proteins in ricin activity. A subsequent study revealed that Shiga toxin 1 (Stx1) and RTA can interact with a short peptide corresponding to a conserved C-terminal region of the ribosomal stalk proteins, P0, P1 and P2, which form the ribosomal stalk (McCluskey et al., 2008). A type I RIP, trichosanthin (TCS) also interacts with the ribosomal proteins P0, P1 and P2 in vitro (Chan et al., 2001; 2007). By expressing RTA in yeast mutants lacking stalk proteins, we provided the first in vivo evidence that the ribosomal stalk is the docking site for RTA on the ribosome and is critical for RTA to depurinate the SRL (Chiou et al., 2008).
The ribosomal stalk is a dynamic, lateral protuberance of the large ribosomal subunit (60S), in proximity to the SRL, which functions to recruit translation factors to the ribosome and facilitates GTPase activation by EF-Tu and EF-G (Mohr et al., 2002; Tchórzewski, 2002; Gonzalo and Reboud, 2003; Diaconu et al., 2005; Bernadó et al., 2010). The stalk is relatively well conserved among eukaryotes (Ballesta and Remacha, 1996; Gonzalo and Reboud, 2003; Grela et al., 2008a). Despite homology between the stalk structures from yeast and humans, there are notable differences. The human ribosomal stalk structure is comprised of ribosomal phosphoproteins (P0, P1, and P2). The P proteins are assembled into a pentameric protein complex of a single P0 protein bound by two heterodimers of acidic ribosomal proteins P1 and P2 (Grela et al., 2008b; Lee et al., 2012). The yeast ribosomal stalk is also comprised of a pentameric structure but yeast possesses two different isoforms of P1 and P2 (P1α, P1β, P2α, P2β) (Ballesta and Remacha, 1996; Grela et al., 2010). Human P1 shares approximately 40–47% sequence identity with P1α and P1β while human P2 shares 53–56% sequence identity with P2α and P2β. Although the prokaryotic stalk exhibits similar organization and function to the eukaryotic stalk (Wahl and Möller, 2002; Gonzalo and Reboud, 2003), the prokaryotic stalk proteins share very little sequence identity with their eukaryotic counterparts and are not considered to be homologous, but rather functionally analogous (Grela et al., 2008a). Recently, we examined whether the human stalk proteins facilitate the interaction of RTA with human ribosomes using small interfering RNA-mediated knockdown of P1/P2 expression in human cells (May et al., 2012). We showed that depurination activity of RTA is lower when P1 and P2 levels are reduced in human cells. Ribosomes from P1/P2 depleted cells had reduced ability to bind RTA (Fig. 2A). Furthermore P1/P2 depleted ribosomes showed over 3-fold lower depurination levels than wild type (WT) ribosomes (Fig. 2B), demonstrating the importance of human P1 and P2 proteins in enabling RTA to bind to human ribosomes and depurinate the SRL (May et al., 2012).
Fig. 2.
The effect of P1/P2-depletion on RTA activity in vitro. Human embryonic kidney (HEK293T) cells stably transfected with doxycycline-inducible P2 RNAi were treated with doxycycline (0.1µg/ml for 96 h) to knock-down of P1 and P2 protein levels. Ribosomes were isolated for biochemical analyses. (A) The interaction of RTA with WT and P1/P2-depleted human ribosomes measured using surface plasmon resonance. N-terminal His-tagged RTA was coupled to an NTA chip on the Biacore 3000 and used as a ligand for WT (5 nM) or P1/P2-depleted (5 nM) ribosomes. (B) The relative in vitro activity of RTA (0.1nM) on WT and P1/P2-depleted ribosomes (2 pmol) compared over a time course of 0–10 minutes. rRNA was extracted post-treatment and depurination measured by qRT-PCR. Data indicate the fold increases in depurination relative to untreated (no toxin) WT ribosomes (n=3).
The interaction of ricin with the ribosomal stalk is significant for several reasons. Firstly, species-dependent differences in the stalk structure may account for the differences in the activity of RTA on ribosomes from different organisms. Although RTA depurinates naked 23S rRNA from E. coli, it does not depurinate E. coli ribosomes (Endo and Tsurugi, 1988), suggesting that even if a suitable substrate for ricin is present, the ribosomal context is important for RTA to access it. Given the established importance of the stalk proteins in facilitating the interaction of RTA with ribosomes in eukaryotes, and their significant divergence between prokaryotes and eukaryotes, the stalk proteins may be responsible for the species specificity of ricin and ultimately determine the susceptibility of the SRL to depurination. Paradoxically, Stx1, which also interacts with the eukaryotic stalk to gain access the SRL, can depurinate E. coli ribosomes (Skinner and Jackson, 1998; Suh et al., 1998). It is not yet known if the interaction of Shiga toxins with the prokaryotic ribosome is stalk-dependent.
Secondly, several RIPs, including ricin, Shiga toxins and trichosanthin have been reported to interact with the ribosomal stalk (Chan et al., 2001; 2007; Chiou et al., 2008; McCluskey et al., 2008; Li et al., 2009; Too et al., 2009; Chiou et al., 2011; May et al., 2012; McCluskey et al., 2012). Phylogenetic analyses of these RIPs suggest that the ability to interact with the ribosomal stalk arose independently by convergent evolution (Lapadula et al., 2012). It has been suggested that RIP interaction with the stalk may lack strong sequence constraints, thereby enabling different proteins to readily evolve to acquire this ability. Intriguingly, it was proposed that convergent evolution might signify that the ability to interact with the stalk provides an adaptive advantage.
Thirdly, another significant feature of the stalk proteins is that their amount on the ribosome does not remain constant but is dependent on the metabolic state of the cell. There is a cytoplasmic pool of free P1/P2 proteins, which can exchange with the ribosome-bound P1/P2 proteins (Zinker and Warner, 1976; Tsurugi and Ogata, 1985). Ribosomes from exponentially growing yeast cultures contain 40% higher levels of P1/P2 proteins than ribosomes from stationary phase cultures (Saenz-Robles et al., 1990). Moreover, ribosomes within polysomes are enriched in P proteins compared to free 80S ribosomes (Saenz-Robles et al., 1990). Additionally, while most ribosomes contain the typical pentameric composition of (P1α/P2β)-P0-(P1β/P2α), variations in stalk composition occur in vivo (García-Marcos et al., 2008; Cardenas et al., 2012). Yeast mutants possessing only one of the four possible P1/P2 combinations (i.e. either P1α/P2β, P1β/P2β, P1α/P2α or P1β/P2α) each exhibited phenotypic variation relating to translational efficiency and cell growth (Cardenas et al., 2012). In other yeast mutants the absence of each type of P1/P2 heterodimer reduced the rate of cell growth by varying degrees (Cardenas et al., 2012). Collectively, these data along with earlier studies suggest that not all ribosomal P proteins play the same role and that variations in the stalk structure may play a regulatory role within the cell (Ballesta and Remacha, 1996; Qiu et al., 2006; Krokowski et al., 2007; Grela et al., 2010; Cardenas et al., 2012). Ribosome heterogeneity with respect to the stalk structure may affect RIP activity such that the relative translational activity and metabolic state of cells may correlate with sensitivity to RIPs. Recent results suggest that RIPs differ in their requirements for the ribosomal stalk. Cytoplasmic stalk proteins stimulated ribosome depurination by Stx1 by facilitating access of the toxin to the ribosome (Chiou et al., 2011). In contrast, Stx2 was less dependent on the stalk proteins for activity, indicating that Stx1 and Stx2 differ in their requirements for the ribosomal stalk (Chiou et al., 2011). Further studies are required to understand the relationship between stalk composition and the relative activities of various RIPs.
Finally the interaction of ricin with the ribosomal stalk proteins may represent a promising target for protection against ricin. Indeed work from our lab comparing the toxicity of RTA variants in yeast has demonstrated that mutations, which disrupt the interaction of RTA with ribosomal proteins, dramatically reduced RTA activity and toxicity (Li and Tumer, submitted). The structure of a peptide (SDDDMGFGLFD) corresponding to the last 11 amino acids of the stalk proteins in complex with TCS has been reported (Too et al., 2009). According to this structure, the acidic amino acids at the amino end of the peptide interact with the positively charged amino acids in TCS, while the hydrophobic part of the carboxy end of the peptide is inserted into a hydrophobic pocket of TCS (Too et al., 2009; Lee et al., 2012). A recent study revealed that A1 chain of Stx1 interacted transiently with this peptide. The anionic tripeptide and hydrophobic tetrapeptide motifs within this peptide (SDDDMGFGLFD) were recognized by the A1 chain of Stx1 (McCluskey et al., 2012). These results have provided new molecular leads for the development of RIP antidotes.
4. Models for the interaction of RTA with ribosomes and the ribosomal stalk
Several studies have modeled the kinetics of the interaction between ricin and the ribosome. An early analytical ultracentrifugation study of RTA:ribosome complexes determined that RTA interacts with the 60S ribosomal subunit with a molar stoichiometry of 1:1 (Hedblom et al., 1978). Notably, although RTA was not able to interact with isolated 40S subunits, the binding of RTA to 80S ribosomes was approximately 3.5-fold stronger than binding to the isolated 60S subunits, suggesting that elements of the 40S subunit may facilitate optimal binding of ricin to the ribosome. Subsequently, Hongo et al. (2002) were the first to report real-time kinetic analyses of the interaction of RTA and ribosomes using surface plasmon resonance (SPR). Global analyses of association and dissociation data indicated that the interaction did not fit a simple 1:1 binding model (Honjo et al., 2002). Instead, the data fit a conformational change model whereby upon binding of RTA to the ribosome a conformational change occurred, resulting in the formation of a high affinity complex. Similar binding kinetics was observed for RTA interacting with ribosomes that had already been depurinated. A potential caveat to the study by Honjo et al. (2002) is that complex interaction models (including the conformational model) have higher degrees of freedom, with the result that a good statistical fit is not sufficient to prove the model. Further experimental approaches are needed.
Having identified a role for ribosomal stalk proteins in the interaction of RTA with ribosomes (Chiou et al., 2008), our group sought to compare the binding kinetics of RTA with wild type (WT) yeast ribosomes and those obtained from a yeast mutant devoid of P1 (ΔP1α, ΔP1β) and P2 (ΔP2α, ΔP2β) proteins (Li et al., 2009). These comparisons revealed two types of interactions between RTA and WT ribosomes consisting of a saturable, P1/P2 protein-dependent interaction with a rapid association and dissociation rate, and a nonsaturable P1/P2-independent interaction with a much slower association and dissociation rate. The salt sensitivity of both interactions suggested that they were dominated by electrostatic interactions. The faster stalk-specific interaction was stronger than the slower interaction, which was not specific to the stalk. RTA interacted with the yeast mutant ribosomes lacking an intact stalk by a single type of interaction, which was similar to the slow interaction with the WT ribosomes. Since the conformational change model could not account for the observed differences in the interactions of RTA with WT and mutant ribosomes lacking an intact stalk, we proposed a two-step binding model that could explain the interaction with both types of ribosomes (Fig. 3). According to this model, initially the slow and nonspecific electrostatic interactions concentrate RTA molecules on the surface of the ribosome and guide RTA to the stalk (Fig. 3, Step 1). Proximity to the stalk enables the faster, more specific interaction of RTA with the ribosomal stalk that ultimately delivers RTA to the SRL (Fig. 3, Step 2). Both interactions work together allowing RTA to depurinate the SRL at a much higher rate on intact ribosomes than on the naked 28S rRNA (Li et al., 2009). Examination of the interaction of RTA with mammalian (human and rat) ribosomes also produced binding curves consistent with this two-step binding model (May et al., 2012).
Fig. 3.
Two-step binding model for ribosome depurination by RTA. Step one: slow, stalk-independent, electrostatic AB1 interactions concentrate RTA molecules on the ribosomal surface, increasing their local concentration and facilitating diffusion of RTA toward the stalk. Step two: once RTA molecules are proximal to the stalk faster, stalk-specific AB2 interactions occur, with the stalk ultimately facilitating delivery of RTA to the sarcin/ricin loop (SRL) of the large rRNA. AB1 and AB2 interactions work together on ribosomes to deliver RTA to the SRL enabling RTA to depurinate the SRL at a very high rate.
To further test this model, we examined the interaction between RTA and the native pentameric stalk complex isolated from yeast (Li et al., 2010). In contrast to the interaction of RTA with WT ribosomes, the interaction of RTA with the native pentameric stalk fit well with a single step interaction model. The dissociation rate of RTA from the stalk pentamer was much slower than its dissociation rate from the intact ribosome, indicating that RTA binding may stabilize the stalk complex. The observation that there was only one type of interaction between RTA and the stalk pentamer further supported the two-step binding model for the interaction of RTA with ribosomes (Li et al., 2010). To determine which part of the stalk pentamer was responsible for RTA binding, a trimeric form of the stalk, which contained either the ΔP1α/ΔP2β or the ΔP1β/ΔP2α heterodimer was isolated from yeast and the interaction of each trimer with RTA was compared using SPR (Li et al., 2010). RTA interacted with each stalk trimer via a single step binding model. The association rate of the interaction of RTA with the stalk pentamer, which contains two P1/P2 heterodimers, was 2-fold greater than its association rate with the stalk trimers, which contain only one P1/P2 heterodimer, indicating that ribosomes that contain two heterodimers recruit RTA twice as fast as ribosomes that contain a single heterodimer. These results demonstrated for the first time that multiple copies of the stalk proteins affect the rates of association and dissociation of RTA with ribosomes and accelerate the recruitment of RTA to the ribosome for depurination (Li et al., 2010).
5. Modes of action of ricin on the ribosome that trigger cellular signaling and apoptosis
While RIP-ribosome interaction studies have proven extremely fruitful, a longstanding question remains largely unanswered: what are the consequences of depurination? It was initially thought that RIPs simply cause cell death by arresting protein synthesis. Indeed it has been shown that ricin can alter inflammatory signaling as a direct result of protein synthesis inhibition due to the disappearance of labile protein(s) that normally suppress inflammation (Lindauer et al., 2010). However, seminal work by Iordanov et al. (1997) demonstrated that cellular stress responses can be triggered specifically by depurination of the SRL by ricin, but not necessarily resulting from translation inhibition (Iordanov et al., 1997). These experiments showed that anisomycin and aminohexose pyrimidine nucleoside antibiotics, which interact with the 28S rRNA in the peptidyl transferase center, as well as ricin and α-sarcin, which target the SRL, were all potent activators of cJun NH2-terminal kinases (JNKs). JNKs are known to regulate signaling pathways in mammalian cells that facilitate either cellular recovery or apoptosis in response to conditions of stress (Lin, 2002; Dhanasekaran and Reddy, 2008). Notably, Iordanov et al. found that other antibiotics and toxins that inhibit protein synthesis by alternate mechanisms did not activate JNKs. The authors proposed that the 28S rRNA specifically acts as a sensor for stress pathways, and named this “the ribotoxic stress response” (Iordanov et al., 1997; Jandhyala et al., 2012). The double-stranded RNA–activated protein kinase (PKR) has been proposed as a sensor for ribosomal damage caused by ricin and Shiga toxin (Gray et al., 2008). Using PKR inhibitors C16 and 2-aminopurine (2-AP), it was demonstrated that ricin and Shiga toxin induced PKR-dependent expression of the inflammatory cytokine IL-8. Although further work is required to characterize the PKR-dependent signaling caused by ricin, PKR is known to induce proinflammatory and apoptotic responses (Williams, 2001; García et al., 2006; Guerra et al., 2006) and to phosphorylate the eukaryotic initiation factor 2α (eIF-2α), thereby reducing protein synthesis (Galabru and Hovanessian, 1987; Zhu et al., 1997; Kumar et al., 1999). PKR associates with the ribosome near the peptidyl transferase center (Zhu et al., 1997; Kumar et al., 1999), placing it in a prime position to act as a sensor for 28S rRNA damage. It was proposed that RIP-mediated damage of the SRL alters the secondary structure of the rRNA that is recognized by PKR (Gray et al., 2008; Tesh, 2012). Interestingly, a recent study comparing the activity of RTA variants in mammalian cells showed that while apoptosis induction correlated linearly with protein synthesis inhibition, depurination did not (Jetzt et al., 2012). Jetz et al. (2012) concluded that absolute levels of depurination might not be important, but that a threshold level of depurination is required to trigger stress and apoptotic signaling. The lack of correlation between depurination levels and the levels of protein synthesis inhibition imply that depurination of the ribosome by ricin may not be solely responsible for the observed translation inhibition. Similar findings have also been observed in yeast for ricin and PAP (Hudak et al., 2004; Li et al., 2007). Cellular stress responses have been demonstrated to shutdown protein synthesis transiently while the cell attempts to recover (Brewer and Diehl, 2000; Harding et al., 2000; Uesono, 2002). It would be of interest to determine if ricin modulates stress responses, which inhibit protein synthesis, such as PKR-dependent phosphorylation of eIF-2α. Ricin inhibits activation of the unfolded protein response (UPR) in yeast (Parikh et al., 2008) and in mammalian cells (Wang et al., 2011). Inhibition of the UPR enhanced the cytotoxicity of ricin in both systems. Defining these cellular signaling events may open up new avenues for therapeutic intervention for ricin and other RIPs. To this end, Wahome et al. (2012) recently performed a high-throughput screen of small molecule libraries and identified three compounds that delayed ricin-induced cell death. All three compounds appeared to function not by interfering with the translation inhibitory activity of ricin, but by blocking downstream stress and/or inflammatory signaling pathways associated with the toxin-mediated apoptosis (Wahome et al., 2012).
6. Conclusions and future directions
Whereas inhibiting the enzymatic activity or the cellular uptake and trafficking of ricin and other RIPs was once the goal of therapeutic intervention, more nuanced strategies are being formulated in step with new discoveries defining toxin-ribosome interactions and the cellular signals that are triggered at the ribosomal surface. The case for involvement of the ribosomal stalk proteins in the activity of ricin and other RIPs is compelling. The aim now is to understand how stalk proteins contribute mechanistically to these interactions and to define the functional regions within toxin and stalk structure. As more is learned in this area, perhaps the molecular basis for the species specificity of RIPs and the relationship between the stalk composition and RIP activity will be clarified. Finally, ample evidence points to cellular signaling as an important contributor to ricin toxicity. Understanding how ribosomal damage is sensed and which stress pathways respond will yield a more holistic appreciation of ricin toxicity and reveal additional targets for the development of countermeasures against ricin and related RIPs.
Highlights.
This article covers the talk that was presented at the 17th World Congress of the International Society of Toxinology and Venom Week 2012 meeting in Honolulu, Hawaii on July 8–13, 2012.
This is a review article, which summarizes recent developments in the interaction of ricin with ribosomal proteins that contribute to its potency.
Several recent studies have now established the importance of the ribosomal stalk structure in facilitating the depurination activity and ribosome specificity of ricin and other ribosome inactivating proteins.
This review highlights recent developments, which define toxin-ribosome interactions and examines the significance of these interactions for toxicity and therapeutic intervention.
Acknowledgements
The authors gratefully acknowledge Jennifer Nielsen-Kahn, Xiao-Ping Li, Marcin Grabowicz and Przemysław Grela and for critically reading the manuscript. This work was supported by the National Institutes of Health grants AI072425, TW008418 and S10RR025424 to NET.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
There are no conflicts of interest.
References
- Agrawal RK, Penczek P, Grassucci RA, Frank J. Visualization of elongation factor G on the Escherichia coli 70S ribosome: the mechanism of translocation. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6134–6138. doi: 10.1073/pnas.95.11.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argent RH, Parrott AM, Day PJ, Roberts LM, Stockley PG, Lord JM, Radford SE. Ribosome-mediated folding of partially unfolded ricin A-chain. J. Biol. Chem. 2000;275:9263–9269. doi: 10.1074/jbc.275.13.9263. [DOI] [PubMed] [Google Scholar]
- Bai Y, Monzingo AF, Robertus JD. The X-ray structure of ricin A chain with a novel inhibitor. Arch. Biochem. Biophys. 2009;483:23–28. doi: 10.1016/j.abb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Bai Y, Watt B, Wahome PG, Mantis NJ, Robertus JD. Identification of new classes of ricin toxin inhibitors by virtual screening. Toxicon. 2010;56:526–534. doi: 10.1016/j.toxicon.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta JP, Remacha M. The large ribosomal subunit stalk as a regulatory element of the eukaryotic translational machinery. Prog. Nucleic Acid Res. Mol. Biol. 1996;55:157–193. doi: 10.1016/s0079-6603(08)60193-2. [DOI] [PubMed] [Google Scholar]
- Bergan J, Dyve Lingelem AB, Simm R, Skotland T, Sandvig K. Shiga toxins. Toxicon. 2012;60:1085–1107. doi: 10.1016/j.toxicon.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Bernadó P, Modig K, Grela P, Svergun DI, Tchórzewski M, Pons M, Akke M. Structure and dynamics of ribosomal protein L12: an ensemble model based on SAXS and NMR relaxation. Biophys. J. 2010;98:2374–2382. doi: 10.1016/j.bpj.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas D, Revuelta-Cervantes J, Jimenez-Diaz A, Camargo H, Remacha M, Ballesta JPG. P1 and P2 protein heterodimer binding to the P0 protein of Saccharomyces cerevisiae is relatively non-specific and a source of ribosomal heterogeneity. Nucleic Acids Res. 2012;40:4520–4529. doi: 10.1093/nar/gks036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DSB, Chu L-O, Lee K-M, Too PHM, Ma K-W, Sze KH, Zhu G, Shaw P-C, Wong K-B. Interaction between trichosanthin, a ribosome-inactivating protein, and the ribosomal stalk protein P2 by chemical shift perturbation and mutagenesis analyses. Nucleic Acids Res. 2007;35:1660–1672. doi: 10.1093/nar/gkm065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Hung FS, Chan DS, Shaw PC. Trichosanthin interacts with acidic ribosomal proteins P0 and P1 and mitotic checkpoint protein MAD2B. Eur. J. Biochem. 2001;268:2107–2112. doi: 10.1046/j.1432-1327.2001.02091.x. [DOI] [PubMed] [Google Scholar]
- Chiou J-C, Li X-P, Remacha M, Ballesta JPG, Tumer NE. The ribosomal stalk is required for ribosome binding, depurination of the rRNA and cytotoxicity of ricin A chain in Saccharomyces cerevisiae. Mol. Microbiol. 2008;70:1441–1452. doi: 10.1111/j.1365-2958.2008.06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou J-C, Li X-P, Remacha M, Ballesta JPG, Tumer NE. Shiga toxin 1 is more dependent on the P proteins of the ribosomal stalk for depurination activity than Shiga toxin 2. Int. J. Biochem. Cell Biol. 2011;43:1792–1801. doi: 10.1016/j.biocel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi N, Polacek N. Ribosome-associated GTPases: the role of RNA for GTPase activation. RNA Biol. 2010;7:521–527. doi: 10.4161/rna.7.5.12467. [DOI] [PubMed] [Google Scholar]
- de Virgilio M, Lombardi A, Caliandro R, Fabbrini MS. Ribosome-inactivating proteins: from plant defense to tumor attack. Toxins (Basel) 2010;2:2699–2737. doi: 10.3390/toxins2112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconu M, Kothe U, Schlünzen F, Fischer N, Harms JM, Tonevitsky AG, Stark H, Rodnina MV, Wahl MC. Structural basis for the function of the ribosomal L7/12 stalk in factor binding and GTPase activation. Cell. 2005;121:991–1004. doi: 10.1016/j.cell.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 1987;262:5908–5912. [PubMed] [Google Scholar]
- Endo Y, Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 1987;262:8128–8130. [PubMed] [Google Scholar]
- Endo Y, Tsurugi K. The RNA N-glycosidase activity of ricin A-chain. The characteristics of the enzymatic activity of ricin A-chain with ribosomes and with rRNA. J. Biol. Chem. 1988;263:8735–8739. [PubMed] [Google Scholar]
- Galabru J, Hovanessian A. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J. Biol. Chem. 1987;262:15538–15544. [PubMed] [Google Scholar]
- García MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Marcos A, Sánchez SA, Parada P, Eid J, Jameson DM, Remacha M, Gratton E, Ballesta JPG. Yeast ribosomal stalk heterogeneity in vivo shown by two-photon FCS and molecular brightness analysis. Biophys. J. 2008;94:2884–2890. doi: 10.1529/biophysj.107.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo P, Reboud J-P. The puzzling lateral flexible stalk of the ribosome. Biol. Cell. 2003;95:179–193. doi: 10.1016/s0248-4900(03)00034-0. [DOI] [PubMed] [Google Scholar]
- Gray JS, Bae HK, Li JCB, Lau AS, Pestka JJ. Double-stranded RNA-activated protein kinase mediates induction of interleukin-8 expression by deoxynivalenol, Shiga toxin 1, and ricin in monocytes. Toxicol. Sci. 2008;105:322–330. doi: 10.1093/toxsci/kfn128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grela P, Bernadó P, Svergun D, Kwiatowski J, Abramczyk D, Grankowski N, Tchórzewski M. Structural relationships among the ribosomal stalk proteins from the three domains of life. J. Mol. Evol. 2008a;67:154–167. doi: 10.1007/s00239-008-9132-2. [DOI] [PubMed] [Google Scholar]
- Grela P, Krokowski D, Gordiyenko Y, Krowarsch D, Robinson CV, Otlewski J, Grankowski N, Tchórzewski M. Biophysical properties of the eukaryotic ribosomal stalk. Biochemistry. 2010;49:924–933. doi: 10.1021/bi901811s. [DOI] [PubMed] [Google Scholar]
- Grela P, Sawa-Makarska J, Gordiyenko Y, Robinson CV, Grankowski N, Tchórzewski M. Structural properties of the human acidic ribosomal P proteins forming the P1–P2 heterocomplex. J. Biol. Chem. 2008b;143:169–177. doi: 10.1093/jb/mvm207. [DOI] [PubMed] [Google Scholar]
- Guerra S, López-Fernández LA, García MA, Zaballos A, Esteban M. Human gene profiling in response to the active protein kinase, interferon-induced serine/threonine protein kinase (PKR), in infected cells. Involvement of the transcription factor ATF-3 IN PKR-induced apoptosis. J. Biol. Chem. 2006;281:18734–18745. doi: 10.1074/jbc.M511983200. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated Translation Initiation Controls Stress-Induced Gene Expression in Mammalian Cells. Molecular Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Hazes B, Read RJ. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry. 1997;36:11051–11054. doi: 10.1021/bi971383p. [DOI] [PubMed] [Google Scholar]
- Hedblom ML, Cawley DB, Boguslawski S, Houston LL. Binding of ricin A chain to rat liver ribosomes: Relationship to ribosome inactivation. J. Supramol. Struct. 1978;9:253–268. doi: 10.1002/jss.400090210. [DOI] [PubMed] [Google Scholar]
- Honjo E, Watanabe K, Tsukamoto T. Real-time kinetic analyses of the interaction of ricin toxin A-chain with ribosomes prove a conformational change involved in complex formation. J. Biochem. 2002;131:267–275. doi: 10.1093/oxfordjournals.jbchem.a003098. [DOI] [PubMed] [Google Scholar]
- Hudak KA, Parikh BA, Di R, Baricevic M, Santana M, Seskar M, Tumer NE. Generation of pokeweed antiviral protein mutations in Saccharomyces cerevisiae: evidence that ribosome depurination is not sufficient for cytotoxicity. Nucleic Acids Res. 2004;32:4244–4256. doi: 10.1093/nar/gkh757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol. Cell. Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandhyala DM, Thorpe CM, Magun B. Ricin and Shiga toxins: effects on host cell signal transduction. Curr. Top. Microbiol. Immunol. 2012;357:41–65. doi: 10.1007/82_2011_181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetzt AE, Cheng J-S, Li X-P, Tumer NE, Cohick WS. A relatively low level of ribosome depurination by mutant forms of ricin toxin A chain can trigger protein synthesis inhibition, cell signaling and apoptosis in mammalian cells. Int. J. Biochem. Cell Biol. 2012;44:2204–2211. doi: 10.1016/j.biocel.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokowski D, Tchórzewski M, Boguszewska A, McKay AR, Maslen SL, Robinson CV, Grankowski N. Elevated copy number of L-A virus in yeast mutant strains defective in ribosomal stalk. Biochem. Biophys. Res. Commun. 2007;355:575–580. doi: 10.1016/j.bbrc.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Kumar KU, Srivastava SP, Kaufman RJ. Double-stranded RNA-activated protein kinase (PKR) is negatively regulated by 60S ribosomal subunit protein L18. Mol. Cell. Biol. 1999;19:1116–1125. doi: 10.1128/mcb.19.2.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapadula WJ, Sanchez-Puerta MV, Juri Ayub M. Convergent evolution led ribosome inactivating proteins to interact with ribosomal stalk. Toxicon. 2012;59:427–432. doi: 10.1016/j.toxicon.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Lee K-M, Yu CW-H, Chiu TY-H, Sze KH, Shaw P-C, Wong K-B. Solution structure of the dimerization domain of the eukaryotic stalk P1/P2 complex reveals the structural organization of eukaryotic stalk complex. Nucleic Acids Res. 2012;40:3172–3182. doi: 10.1093/nar/gkr1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-P, Baricevic M, Saidasan H, Tumer NE. Ribosome depurination is not sufficient for ricin-mediated cell death in Saccharomyces cerevisiae. Infect. Immun. 2007;75:417–428. doi: 10.1128/IAI.01295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-P, Chiou J-C, Remacha M, Ballesta JPG, Tumer NE. A two-step binding model proposed for the electrostatic interactions of ricin a chain with ribosomes. Biochemistry. 2009;48:3853–3863. doi: 10.1021/bi802371h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-P, Grela P, Krokowski D, Tchórzewski M, Tumer NE. Pentameric organization of the ribosomal stalk accelerates recruitment of ricin a chain to the ribosome for depurination. J. Biol. Chem. 2010;285:41463–41471. doi: 10.1074/jbc.M110.171793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. Activation of the JNK signaling pathway: Breaking the brake on apoptosis. Bioessays. 2002;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- Lindauer M, Wong J, Magun B. Ricin Toxin Activates the NALP3 Inflammasome. Toxins (Basel) 2010;2:1500–1514. doi: 10.3390/toxins2061500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JM. Precursors of ricin and Ricinus communis agglutinin. Glycosylation and processing during synthesis and intracellular transport. Eur. J. Biochem. 1985;146:411–416. doi: 10.1111/j.1432-1033.1985.tb08667.x. [DOI] [PubMed] [Google Scholar]
- Lord JM, Roberts LM. Toxin entry: retrograde transport through the secretory pathway. J. Cell Biol. 1998;140:733–736. doi: 10.1083/jcb.140.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB. J. 1994;8:201–208. [PubMed] [Google Scholar]
- Mandal S, Li WT, Bai Y, Robertus JD, Kerwin SM. Synthesis of 2-substituted 9-oxa-guanines {5-aminooxazolo[5,4-d]pyrimidin-7(6H)-ones} and 9-oxa-2-thioxanthines {5-mercaptooxazolo[5,4-d]pyrimidin-7(6H)-ones} Beilstein. J. Org. Chem. 2008;4:26. doi: 10.3762/bjoc.4.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May KL, Li XP, Azorín FM, Ballesta J, Grela P, Tchórzewski M, Tumer NE. The P1/P2 proteins of the human ribosomal stalk are required for ribosome binding and depurination by ricin in human cells. FEBS. J. 2012;279:3925–3936. doi: 10.1111/j.1742-4658.2012.08752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey AJ, Bolewska-Pedyczak E, Jarvik N, Chen G, Sidhu SS, Gariépy J. Charged and hydrophobic surfaces on the A chain of shiga-like toxin 1 recognize the C-terminal domain of ribosomal stalk proteins. PLoS ONE. 2012;7:e31191. doi: 10.1371/journal.pone.0031191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey AJ, Poon GMK, Bolewska-Pedyczak E, Srikumar T, Jeram SM, Raught B, Gariépy J. The catalytic subunit of shiga-like toxin 1 interacts with ribosomal stalk proteins and is inhibited by their conserved C-terminal domain. J. Mol. Biol. 2008;378:375–386. doi: 10.1016/j.jmb.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Ravikumar K, Shen H, Suh J-K, Kerwin SM, Robertus JD. Structure-based design and characterization of novel platforms for ricin and shiga toxin inhibition. J. Med. Chem. 2002;45:90–98. doi: 10.1021/jm010186s. [DOI] [PubMed] [Google Scholar]
- Mohr D, Wintermeyer W, Rodnina MV. GTPase activation of elongation factors Tu and G on the ribosome. Biochemistry. 2002;41:12520–12528. doi: 10.1021/bi026301y. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Linstedt AD. Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science. 2012;335:332–335. doi: 10.1126/science.1215930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musshoff F, Madea B. Ricin poisoning and forensic toxicology. Drug Test Anal. 2009;1:184–191. doi: 10.1002/dta.27. [DOI] [PubMed] [Google Scholar]
- Mutlu H, Meier MAR. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 2010;112:10–30. [Google Scholar]
- Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004;44:361–370. doi: 10.1016/j.toxicon.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Olsnes S, Pihl A. Treatment of abrin and ricin with -mercaptoethanol opposite effects on their toxicity in mice and their ability to inhibit protein synthesis in a cell-free system. FEBS Lett. 1972;28:48–50. doi: 10.1016/0014-5793(72)80674-4. [DOI] [PubMed] [Google Scholar]
- Olsnes S, Pihl A. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry. 1973;12:3121–3126. doi: 10.1021/bi00740a028. [DOI] [PubMed] [Google Scholar]
- Pang Y-P, Park JG, Wang S, Vummenthala A, Mishra RK, McLaughlin JE, Di R, Kahn JN, Tumer NE, Janosi L, Davis J, Millard CB. Small-molecule inhibitor leads of ribosome-inactivating proteins developed using the doorstop approach. PLoS ONE. 2011;6:e17883. doi: 10.1371/journal.pone.0017883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh BA, Tortora A, Li X-P, Tumer NE. Ricin inhibits activation of the unfolded protein response by preventing splicing of the HAC1 mRNA. J. Biol. Chem. 2008;283:6145–6153. doi: 10.1074/jbc.M707981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Kahn JN, Tumer NE, Pang Y-P. Chemical structure of Retro-2, a compound that protects cells against ribosome-inactivating proteins. Sci Rep. 2012;2:631. doi: 10.1038/srep00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton AW, Morona R, Paton JC. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat. Med. 2000;6:265–270. doi: 10.1038/73111. [DOI] [PubMed] [Google Scholar]
- Paton AW, Morona R, Paton JC. Neutralization of Shiga toxins Stx1, Stx2c, and Stx2e by recombinant bacteria expressing mimics of globotriose and globotetraose. Infect. Immun. 2001;69:1967–1970. doi: 10.1128/IAI.69.3.1967-1970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruet JM, Jasheway KR, Manzano LA, Bai Y, Anslyn EV, Robertus JD. 7-Substituted pterins provide a new direction for ricin A chain inhibitors. Eur. J. Med. Chem. 2011;46:3608–3615. doi: 10.1016/j.ejmech.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptitsyn OB. Molten globule and protein folding. Advan. Protein Chem. 1995;47:83–229. doi: 10.1016/s0065-3233(08)60546-x. [DOI] [PubMed] [Google Scholar]
- Qiu D, Parada P, Marcos AG, Cárdenas D, Remacha M, Ballesta JPG. Different roles of P1 and P2 Saccharomyces cerevisiae ribosomal stalk proteins revealed by cross-linking. Mol. Microbiol. 2006;62:1191–1202. doi: 10.1111/j.1365-2958.2006.05445.x. [DOI] [PubMed] [Google Scholar]
- Rapak A, Falnes PO, Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3783–3788. doi: 10.1073/pnas.94.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasooly R, He X, Friedman M. Milk inhibits the biological activity of ricin. J. Biol. Chem. 2012;287:27924–27929. doi: 10.1074/jbc.M112.362988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenber E, Katzin BJ, Ernst S, Collins EJ, Mlsna D, Ready MP, Robertus JD. Crystallographic refinement of ricin to 2.5 A. Proteins. 1991;10:240–250. doi: 10.1002/prot.340100308. [DOI] [PubMed] [Google Scholar]
- Saenz JB, Doggett TA, Haslam DB. Identification and characterization of small molecules that inhibit intracellular toxin transport. Infect. Immun. 2007;75:4552–4561. doi: 10.1128/IAI.00442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz-Robles MT, Remacha M, Vilella MD, Zinker S, Ballesta JP. The acidic ribosomal proteins as regulators of the eukaryotic ribosomal activity. Biochim. Biophys. Acta. 1990;1050:51–55. doi: 10.1016/0167-4781(90)90140-w. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Bergan J, Dyve A-B, Skotland T, Torgersen ML. Endocytosis and retrograde transport of Shiga toxin. Toxicon. 2010;56:1181–1185. doi: 10.1016/j.toxicon.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Schlossman D, Withers D, Welsh P, Alexander A, Robertus J, Frankel A. Role of glutamic acid 177 of the ricin toxin A chain in enzymatic inactivation of ribosomes. Mol. Cell. Biol. 1989;9:5012–5021. doi: 10.1128/mcb.9.11.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Khade PK, Sanbonmatsu KY, Joseph S. Functional role of the sarcin-ricin loop of the 23S rRNA in the elongation cycle of protein synthesis. J. Mol. Biol. 2012;419:125–138. doi: 10.1016/j.jmb.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JC, Roberts LM, Römisch K, Davey J, Wolf DH, Lord JM. Ricin A chain utilizes the endoplasmic reticulum-associated protein degradation pathway to enter the cytosol of yeast. FEBS Lett. 1999;459:80–84. doi: 10.1016/s0014-5793(99)01222-3. [DOI] [PubMed] [Google Scholar]
- Skinner L, Jackson M. Inhibition of prokaryotic translation by the Shiga toxin enzymatic subunit. Microbial Pathogenesis. 1998;24:117–122. doi: 10.1006/mpat.1997.0180. [DOI] [PubMed] [Google Scholar]
- Sokołowska I, Wälchli S, Węgrzyn G, Sandvig K, Słomińska-Wojewódzka M. A single point mutation in ricin A-chain increases toxin degradation and inhibits EDEM1-dependent ER retrotranslocation. Biochem. J. 2011;436:371–385. doi: 10.1042/BJ20101493. [DOI] [PubMed] [Google Scholar]
- Spooner RA, Lord JM. How Ricin and Shiga Toxin Reach the Cytosol of Target Cells: Retrotranslocation from the Endoplasmic Reticulum. Curr. Top. Microbiol. Immunol. 2012;357:19–40. doi: 10.1007/82_2011_154. [DOI] [PubMed] [Google Scholar]
- Spooner RA, Watson PD, Marsden CJ, Smith DC, Moore KAH, Cook JP, Lord JM, Roberts LM. Protein disulphide-isomerase reduces ricin to its A and B chains in the endoplasmic reticulum. Biochem. J. 2004;383:285–293. doi: 10.1042/BJ20040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechmann B, Bai S-K, Gobbo E, Lopez R, Merer G, Pinchard S, Panigai L, Tenza D, Raposo G, Beaumelle B, Sauvaire D, Gillet D, Johannes L, Barbier J. Inhibition of Retrograde Transport Protects Mice from Lethal Ricin Challenge. Cell. 2010;141:231–242. doi: 10.1016/j.cell.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Stolz A, Wolf DH. Endoplasmic reticulum associated protein degradation: A chaperone assisted journey to hell. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2010;1803:694–705. doi: 10.1016/j.bbamcr.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Suh J-K, Hovde CJ, Robertus JD. Shiga Toxin Attacks Bacterial Ribosomes as Effectively as Eukaryotic Ribosomes. Biochemistry. 1998;37:9394–9398. doi: 10.1021/bi980424u. [DOI] [PubMed] [Google Scholar]
- Tchórzewski M. The acidic ribosomal P proteins. Int. J. Biochem. Cell Biol. 2002;34:911–915. doi: 10.1016/s1357-2725(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Tesh VL. Activation of cell stress response pathways by Shiga toxins. Cell. Microbiol. 2012;14:1–9. doi: 10.1111/j.1462-5822.2011.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Too PH-M, Ma MK-W, Mak AN-S, Wong Y-T, Tung CK-C, Zhu G, Au SW-N, Wong K-B, Shaw P-C. The C-terminal fragment of the ribosomal P protein complexed to trichosanthin reveals the interaction between the ribosome-inactivating protein and the ribosome. Nucleic Acids Res. 2009;37:602–610. doi: 10.1093/nar/gkn922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugi K, Ogata K. Evidence for the exchangeability of acidic ribosomal proteins on cytoplasmic ribosomes in regenerating rat liver. J. Biochem. 1985;98:1427–1431. doi: 10.1093/oxfordjournals.jbchem.a135410. [DOI] [PubMed] [Google Scholar]
- Uesono Y. Transient Inhibition of Translation Initiation by Osmotic Stress. J. Biol. Chem. 2002;277:13848–13855. doi: 10.1074/jbc.M108848200. [DOI] [PubMed] [Google Scholar]
- van der Goot FG, Lakey JH, Pattus F. The molten globule intermediate for protein insertion or translocation through membranes. Trends Cell Biol. 1992;2:343–348. [PubMed] [Google Scholar]
- Vater CA, Bartle LM, Leszyk JD, Lambert JM, Goldmacher VS. Ricin A chain can be chemically cross-linked to the mammalian ribosomal proteins L9 and L10e. J. Biol. Chem. 1995;270:12933–12940. doi: 10.1074/jbc.270.21.12933. [DOI] [PubMed] [Google Scholar]
- Voorhees RM, Schmeing TM, Kelley AC, Ramakrishnan V. The Mechanism for Activation of GTP Hydrolysis on the Ribosome. Science. 2010;330:835–838. doi: 10.1126/science.1194460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl MC, Möller W. Structure and function of the acidic ribosomal stalk proteins. Curr. Protein Pept. Sci. 2002;3:93–106. doi: 10.2174/1389203023380756. [DOI] [PubMed] [Google Scholar]
- Wahome PG, Ahlawat S, Mantis NJ. Identification of small molecules that suppress ricin-induced stress-activated signaling pathways. PLoS ONE. 2012;7:e49075. doi: 10.1371/journal.pone.0049075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahome PG, Robertus JD, Mantis NJ. Small-molecule Inhibitors of ricin and shiga toxins. Curr. Top. Microbiol. Immunol. 2011;357:179–207. doi: 10.1007/82_2011_177. [DOI] [PubMed] [Google Scholar]
- Wang C-T, Jetzt AE, Cheng J-S, Cohick WS. Inhibition of the unfolded protein response by ricin A-chain enhances its cytotoxicity in mammalian cells. Toxins (Basel) 2011;3:453–468. doi: 10.3390/toxins3050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR. Signal integration via PKR. Sci. STKE 2001. 2001:re2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- Yan Q, Li X-P, Tumer NE. N-glycosylation does not affect the catalytic activity of ricin A chain but stimulates cytotoxicity by promoting its transport out of the endoplasmic reticulum. Traffic. 2012;13:1508–1521. doi: 10.1111/j.1600-0854.2012.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Hollis T, Svinth M, Day P, Monzingo AF, Milne GW, Robertus JD. Structure-based identification of a ricin inhibitor. J. Mol. Biol. 1997;266:1043–1049. doi: 10.1006/jmbi.1996.0865. [DOI] [PubMed] [Google Scholar]
- Yermakova A, Mantis NJ. Protective immunity to ricin toxin conferred by antibodies against the toxin's binding subunit (RTB) Vaccine. 2011;29:7925–7935. doi: 10.1016/j.vaccine.2011.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermakova A, Vance DJ, Mantis NJ. Sub-Domains of Ricin's B Subunit as Targets of Toxin Neutralizing and Non-Neutralizing Monoclonal Antibodies. PLoS ONE. 2012;7:e44317. doi: 10.1371/journal.pone.0044317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Chen CC, Zhang MS, Wu HC. Disruption of the Golgi apparatus by brefeldin A inhibits the cytotoxicity of ricin, modeccin, and Pseudomonas toxin. Exp. Cell Res. 1991;192:389–395. doi: 10.1016/0014-4827(91)90056-z. [DOI] [PubMed] [Google Scholar]
- Zhu S, Romano PR, Wek RC. Ribosome targeting of PKR is mediated by two double-stranded RNA-binding domains and facilitates in vivo phosphorylation of eukaryotic initiation factor-2. J. Biol. Chem. 1997;272:14434–14441. doi: 10.1074/jbc.272.22.14434. [DOI] [PubMed] [Google Scholar]
- Zinker S, Warner JR. The ribosomal proteins of Saccharomyces cerevisiae. Phosphorylated and exchangeable proteins. J. Biol. Chem. 1976;251:1799–1807. [PubMed] [Google Scholar]