Abstract
Hepatocellular carcinoma (HCC) is among the most lethal and prevalent cancers in the human population. The initiation and progression of HCC is closely associated with chronic liver inflammation. Recent research indicates that nonhomologous end joining (NHEJ), one of the DNA repair mechanisms, autophagy and senescence are all involved in the pathogenesis of HCC induced by carcinogens or oxidative stress. DNA repair proteins including XRCC6/KU70 and XRCC5/KU80 are the critical NHEJ factors that play pivotal roles in genome-maintenance issues such as DNA replication and repair, telomere maintenance and chromosomal instability. Our studies indicate that a deficiency of toll-like receptor 4 (TLR4)-mediated immune activities results in a decreased expression of XRCC5 and XRCC6 in response to insult by the carcinogen diethylnitrosamine (DEN). This effect causes a failure in DNA repair, and promotes the transformation of precancerous hepatocytes and HCC development. Ectopic expression of XRCC6 protects against HCC initiation and progression by restoring the cellular senescent response and activation of immune networks, which induces an effective autophagic degradation, removes the accumulated reactive oxygen species (ROS), decreases DNA damage, attenuates proliferation, and promotes programmed cell death in TLR4-deficient livers. Our work indicates that repairing DNA damage by XRCC6 reverses TLR4-deficiency-worsened HCC development via restoring immunity to support senescence and autophagy in liver cells.
Keywords: DNA damage repair, hepatocellular carcinoma, KU70, selective autophagy, senescence
The DNA double-strand break (DSB) repair pathways are an indispensable system for eukaryote genetic integrity. DSB damage may be introduced into the genome during physiological processes such as lymphocyte development, meiosis and DNA replication, as well as during pathological processes such as viral infection, oncogenic agents, metabolic stress and cellular transformation. Therefore, accumulation of DNA DSB damage in cells can lead to oncogenic chromosomal translocations and eventually to cancer. The DNA DSB repair pathways include homologous recombination and nonhomologous end joining, the latter of which is a major repair pathway in human cells. In addition, Ku proteins are the critical NHEJ factors that regulate the DNA NHEJ DSB pathway choice. Ku proteins include XRCC5 and XRCC6 that can form a heterodimer binding to DNA double-strand break ends to participate in the NHEJ pathway of DNA repair. In addition to its role in NHEJ, Ku proteins are involved in various genome-maintenance processes such as DNA replication and repair, telomere maintenance and chromosomal stability. Ku proteins not only recognize DSB ends and recruit other factors that process ends, but also have a direct role in end-processing steps. The deficiency of DNA repair Ku proteins causes an irreversible genomic instability and malignant transformation, which is responsible for multiple cancer diseases such as pancreatic cancer, breast cancer, HCC and immunodeficiency.
The DNA damage-repair signaling and immune responses can regulate each other and both are the front lines to resist DNA damage caused by pathogen- or damage-associated molecular patterns (DAMPs). On the one hand, the DNA damage-repair signaling takes part in various kinds of immune regulations: viral infection, B cell development, antibody class conversion, immunocyte activation, induction of senescence and autophagy. On the other hand, immune response-mediated senescence and autophagy play a critical role in regulating the initiation and progression of cancers through interacting with the DNA damage-repair signaling. For example, we have recently found that activation of TLR4 can reduce tumor metastasis via STAT1-IFNG signaling activation of autophagy. In addition, genetic or pharmacological inhibition of TLR4 activity causes an aggravated DNA damage, accumulation of ROS, suppression of senescence and autophagy in response to DEN insult due to an impaired DNA repair protein kinase complex XRCC5-XRCC6-ATM-PRKDC/DNA-PKcs, which eventually leads to HCC development and progression. Indeed, recent studies indicate that the DNA damage-repair signaling regulates senescent and autophagic activity. For instance, PRKDC can induce autophagic cell death to promote the efficacy of radiotherapy and chemotherapy. The activation of PARP1 is a key to elevate autophagy by exhausting NAD+ and ATP. Also, reducing the expression or activation of TP53 can promote formation of autophagic vacuoles and death in cancer cells. In our current study, we find that TLR4 mutation causes a decrease in the expression of DNA repair proteins XRCC5-XRCC6 in the DEN-injured livers. It is the suppressed expression of XRCC5-XRCC6 that leads to a persistent DNA damage and ROS-endoplasmic reticulum stress in TLR4mut liver. We find that XRCC6 indirectly induces autophagy by increasing the expression or activity of the DNA-dependent repair kinase complex ATM-PRKDC as well as PARP1 and TP53, which together lead to apoptosis of precancerous hepatocytes and HCC suppression. Thus, the DNA damage-repair signaling regulates autophagy by influencing the autophagic and senescent signal pathways.
Indeed, the DNA damage repairing signals play a critical role in the interaction of senescence and autophagy in response to DNA damage stress. In particular, the DNA damage repair signal stimulates a secretion of the senescence-associated secretory proteins including cytokines, chemokines, growth factors and proteases to initiate and support senescence and autophagy in the cells, which form a physiological barrier against carcinogenesis and tumor development. Indeed, we find that restoration of XRCC6 expression in TLR4-deficient mice not only markedly reduces DNA damage, but also restores both TP53-CDKN1A/p21- and CDKN2A/p16-RB1/pRb- dependent cellular senescence, which prevents runaway replication of the damaged hepatocytes. Moreover, the broad-spectrum increase of immune responses causes a significant activation of autophagy as indicated by the elevated expression of LC3-I/-II, BECN1, PIK3C3, and degradation of SQSTM1, and those changes are all associated with an enhanced activity of the TLR4 mediated-p38 MAPK/NFKB and IRF3 signaling. Therefore, senescence and autophagy are all supported by the TLR4-mediated immune response. NFKB can be activated by DNA damage via ATM signaling, and homologous recombination is also involved in a stimulatory role of NFKB in response to dissected distinct DNA DSB damage. Expression of XRCC5-XRCC6 and the DNA repair complex ATM-PRKDC are regulated by NFKB and IRF3 signaling in TLR4-deficient mice. Besides, XRCC6 may function as an intracellular sensor activating immunity and inducing a senescent response against tumorigenesis through interacting with intracellular soluble factors such as IFNL.
Our work reveals that the TLR4-mediated activation of NFKB and IRF3 signaling plays a critical role in the DNA repair pathway and protecting against HCC initiation and progression through upregulation of XRCC6 expression. However, the detailed mechanisms regarding how Ku proteins are regulated by TLR4 still need to be clarified. Our work places TLR4-mediated DNA repair through regulating XRCC6 expression in the intersection of genome instability, senescence and autophagy signaling and integrate the three, which protect against HCC initiation and progression (Fig. 1).
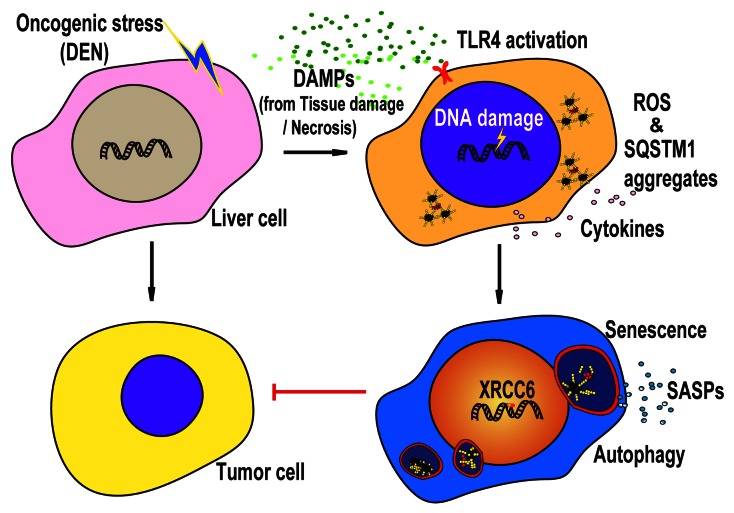
Figure 1. Model of TLR4-mediated DNA repair in the regulation of DEN-induced HCC. The genotoxic carcinogen DEN induces DNA damage and liver injury, which causes the accumulation of ROS and DAMPs released from the injured liver tissue. Accumulated ROS sustain DNA damage and genome instability to lead to hepatocyte transformation, whereas DAMPs interaction with TLR4 stimulates an immune response to liver damage and initiates or supports senescence and autophagy, which can remove accumulated ROS, repair DNA damage and decrease SQSTM1 aggregates. In particular, TLR4-mediated immune responses promote the expression of the DNA repair protein XRCC5-XRCC6 when undergoing DEN insult. XRCC6 can initiate a DNA repair response and rescue cellular senescence and autophagy flux, which defends against HCC development and progression. SASPs, senescence-association secreted proteins.
Glossary
Abbreviations:
- DAMPs
damage-associated molecular patterns
- DEN
diethylnitrosamine
- DSB
double-strand break
- IFN
interferon
- NHEJ
nonhomologous end joining
- ROS
reactive oxygen species
- SASPs
senescence-association secreted proteins
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/24229