Abstract
During development in the thymus, each T lymphocyte is equipped with one, essentially unique, T cell receptor (TCR)-specificity. Due to its random nature, this process inevitably also leads to the emergence of potentially dangerous T lymphocytes that may recognize ‘self.’ Nevertheless, autoimmune tissue destruction, the cause of diseases such as multiple sclerosis and diabetes, is the exception rather than the rule. This state of immunological self-tolerance is to a large degree based upon a process called ‘negative selection’: prior to joining the circulating lymphocyte pool, immature T cells test their receptor on self-antigens within the thymic microenvironment, and TCR engagement at this immature stage elicits an apoptotic suicide program. We now find evidence that macroautophagy supports the tolerogenic presentation of self-antigens in the thymus.
Keywords: thymus, central tolerance, MHC class II, antigen presentation, promiscuous gene expression, thymic epithelium
Intuitively, one may assume that negative selection is unlikely to cover T cells specific for self-antigens whose expression is confined to peripheral tissues, because these antigens might be ‘invisible’ to developing T cells in the thymus. Surprisingly, however, the range of self-antigens that are actually expressed in the thymus covers an unexpectedly large fraction of the genome. This phenomenon, called ‘promiscuous gene expression,’ is confined to a rare stromal cell type, medullary thymic epithelial cells (mTECs). It is at least in part dependent on the autoimmune regulator (AIRE) protein, which, by an as yet poorly understood mechanism, generates ‘transcriptional noise’ that broadens the scope of central tolerance.
How do T cells ‘see’ self-antigens promiscuously expressed by mTECs? T cells recognize proteolytically processed peptides, so-called epitopes, which can be embedded in one of two types of major histocompatibility complex (MHC) molecules on the cell surface. MHC class I (MHC I) molecules present peptides generated through proteasomal degradation of cytoplasmic proteins, whereas MHC II molecules carry peptides derived from lysosomal processing of endocytosed proteins. Thus, as a ‘textbook’ principle, MHC I-bound peptides represent a cell’s cytoplasmic protein content, whereas MHC II-bound peptides represent the extracellular milieu. By inference, one would not be surprised if mTECs efficiently presented ‘promiscuously expressed’ gene products on MHC I for negative selection of CD8 T cells (which recognize MHC I antigens), but such antigens would be unlikely to be loaded onto MHC II for CD4 T cell tolerance. Remarkably though, mTECs seem to have evolved means to violate these classical rules of MHC loading. Thus, despite abundant expression of MHC II on the surface, mTECs are extremely inefficient in loading exogenous antigens onto MHC II. This led us to hypothesize that, instead of shuttling exogenous material into the MHC II loading machinery, TECs may preferentially use an unconventional pathway of ‘endogenous’ MHC II presentation.
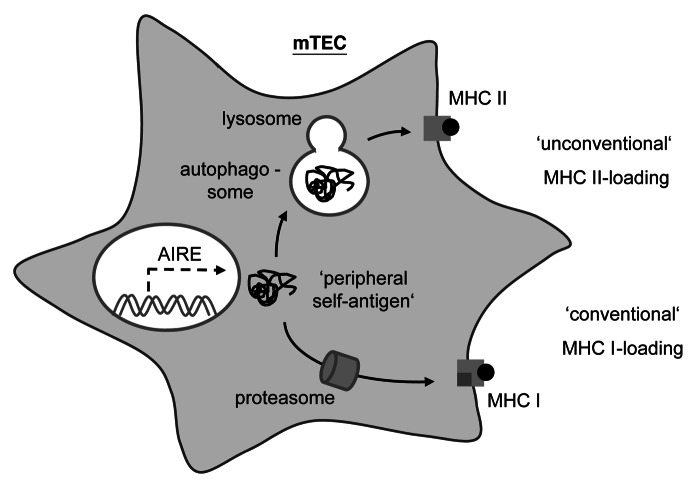
Macroautophagy turned out to be an attractive candidate mechanism for ‘endogenous’ MHC II loading in TECs. Autophagosomes deliver their content to endosomal/lysosomal compartments for proteolytic degradation. The same applies to endocytic compartments derived from ingestion of extracellular material ultimately destined for loading onto MHC II. Consequently, an intersection of autophagy with the MHC II loading pathway is conceivable (Fig. 1). Indeed, evidence from cell culture models indicates that macroautophagy can shuttle intracellular antigens onto MHC II. Intriguingly, autophagy displays a curiously high constitutive activity in TECs, whereas in essentially all other tissues it reaches comparable levels only upon nutrient deprivation.
Figure 1. Thymic epithelial cells use macroautophagy to shuttle intracellular antigens into the MHC II loading pathway. In mTECs, the AIRE protein promotes the ‘promiscuous expression’ of peripheral tissue-antigens. Cytoplasmic self-antigens in mTECs are loaded onto MHC I for CD8 T cell tolerance via the ‘conventional’ MHC I pathway that involves the proteasomal degradation of substrates and the subsequent delivery of peptides into the endoplasmic reticulum. However, these cytoplasmic antigens may also be loaded onto MHC II for CD4 T cell tolerance through an ‘unconventional’ MHC II pathway, whereby subsequent to being engulfed by an autophagosome, these substrates are proteolytically processed by lysosomal proteases prior to reaching MHC II loading compartments.
In order to test the role of autophagy in TECs for CD4 T cell selection, we genetically interfered with autophagy specifically in TECs through grafting of Atg5-deficient thymi into athymic nude mice. The immune system of these chimeric mice is subsequently filled with T cells ‘educated’ on autophagy-deficient TECs. Consistent with a defect in T-cell selection, this resulted in manifestations of immune-mediated tissue destruction. However, it remained unclear as to how far autoimmunity was truly driven by the escape of otherwise ‘vetoed’ T cell specificities from negative selection, or rather by lymphopenia due to impaired thymic output, whereby these factors were not necessarily mutually exclusive.
More recently, we set out to visualize whether T-cell specificities that are otherwise negatively selected through recognition of self-antigens expressed and presented by mTECs in fact escape from central tolerance when autophagy is abolished. In a first series of experiments, we followed the fate of T cells that recognize the model antigen pigeon cytochrome c/CYCS (PCC). It turned out that when PCC was expressed in its native form as a mitochondrial self-antigen in TECs, direct presentation on MHC II for negative selection of specific CD4 T cells indeed required an intact macroautophagy pathway. Remarkably, when PCC was engineered to be expressed as a membrane protein, direct presentation by TECs and negative selection were independent of autophagy, suggesting that the subcellular distribution of an antigen influences whether or not its direct presentation depends upon autophagy. Conceivably, mitophagy may promote the direct presentation of mitochondrial PCC, whereas PCC when expressed as a type II membrane protein may gain access to MHC II in an autophagy-independent manner through an intersection of its biosynthetic pathway with that of MHC II.
In a second set of experiments, we used a novel model system in which ‘the same’ mTEC-specific antigen was deliberately targeted for autophagosomal degradation in one setting but not in a second setting. This was achieved through fusion of a model antigen to either ‘native’ LC3 or to a mutated LC3 in which position 120 was changed from glycine to alanine. Because anchoring of LC3 to the autophagosomal membrane requires the covalent attachment of phosphatidylethanolamine to glycine 120, we hypothesized that the ‘native’ fusion protein would efficiently be targeted to autophagosomes and thereby reach the MHC II pathway, whereas the mutated version would not behave in this manner. Indeed, when expressed at identical levels specifically in mTECs, direct presentation of the autophagosomally targeted model antigen and negative selection of specific CD4 T cells turned out to be substantially more efficient as compared with the mutant form. Taken together, our observations indicate that the immune system co-opts an evolutionarily ancient cellular housekeeping and catabolic process in order to ‘boost’ the tolerogenic presentation of self-antigens within the thymic microenvironment.
Glossary
Abbreviations:
- MHC
major histocompatibility complex
- mTECs
medullary thymic epithelial cells
- PCC
pigeon cytochrome c
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/24374