Abstract
We have elucidated a novel mechanism through which the autophagy-specific class III phosphatidylinositol 3-kinase (PtdIns3K) complex can be recruited to the PAS in mammalian cells, through the interaction between BECN1 and the vacuole membrane protein 1 (VMP1), an integral autophagosomal membrane protein. This interaction involves the binding between the C-terminal 20 amino acids of the VMP1 hydrophilic domain, which we have named the VMP1 autophagy-related domain (VMP1-AtgD), and the BH3 domain of BECN1. The association between these two proteins allows the formation of the autophagy-specific PtdIns3K complex, which activity favors the generation of phosphatidylinositol-3-phosphate (PtdIns3P) and the subsequent association of the autophagy-related (ATG) proteins, including ATG16L1, with the phagophore membranes. Therefore, VMP1 regulates the PtdIns3K activity on the phagophore membrane through its interaction with BECN1. Our data provide a novel model describing one of the key steps in phagophore assembly site (PAS) formation and autophagy regulation, and positions VMP1 as a new interactor of the autophagy-specific PtdIns3K complex in mammalian cells.
Keywords: VMP1, BECN1, phosphatidylinositol 3-kinase, autophagy, pancreatitis
Macroautophagy (hereafter autophagy) is a process essential to maintain cell and organismal homeostasis. Since the discovery of the yeast Atg proteins, autophagosome formation has been dissected at the molecular level but a lot of questions about this pathway remain unanswered. In mammalian cells, the sequential association of at least a subset of the ATG proteins leads to the assembly of the PAS. PAS formation also requires PtdIns3P generation, and it is thought that this lipid is present in specialized subdomains of the endoplasmic reticulum (ER) termed omegasomes, the site where the PAS is assembled and autophagosomes are generated. Among the key mediators initiating autophagosome formation, there is a set of evolutionarily conserved ATG gene products including the autophagy-specific PtdIns3K complex (composed of BECN1 (yeast Vps30/Atg6), PIK3C3/VPS34, PIK3R4/p150 and ATG14) and two ubiquitin-like conjugation systems leading to the formation of the ATG12–ATG5-ATG16L1 complex and the LC3 (yeast Atg8)–phosphatidylethanolamine conjugate (i.e., LC3-II). A second group of ATG proteins, which does not have orthologs in yeast, has also recently emerged and appears to play a key role in regulating autophagy in higher eukaryotes. One of these proteins is VMP1, whose expression triggers autophagy in mammalian cells even under nutrient-rich conditions. Conversely, autophagy is completely blocked in the absence of VMP1. Importantly, VMP1 is also expressed early during the onset of several pathologies, including diabetes mellitus, pancreatitis and pancreatic cancer. VMP1 expression is induced by mutated KRAS in cancer cell lines and by the hyperstimulation of the Gq-coupled receptors for cholecystokinin, a pancreatic secretagogue, during acute pancreatitis. In line with these observations, the tissue-specific transgenic-expression of VMP1 in vivo prevents the disease onset in a mouse model for acute pancreatitis. In addition to having a role in triggering autophagy in pathological situations, VMP1 is also required for the biogenesis of autophagosomes in mammalian cells in all conditions, underscoring its upstream regulatory function in autophagy.
BECN1 is a haploinsufficient tumor suppressor and an effector of autophagy. BECN1 is a subunit of various class III PtdIns3K complexes, the action of which is antagonized by BCL2. BECN1 contains a BH3 domain that mediates its binding to BCL2. This interaction negatively regulates autophagy by interfering with the assembly and activity of the autophagy-specific PtdIns3K complex. Critically, the induction of autophagy by VMP1 expression requires the interaction between the VMP1-AtgD and the BECN1-BH3 domains. This event allows the localization of the PtdIns3K complex, a key positive regulator of autophagy, at the site where autophagosomes are generated. BECN1 binding to VMP1 also concomitantly and synergistically promotes its dissociation from BCL2, an autophagy inhibitor, driving BECN1 into the autophagic pathway.
VMP1 is part of at least a pool of the autophagy-specific PtdIns3K complexes, which regulates autophagy induction in mammalian cells under numerous conditions. To the best of our knowledge VMP1 is the only transmembrane ATG protein with no homolog in yeast and other lower eukaryotes. Our data have revealed that the interaction between VMP1 and BECN1 requires the BECN1-BH3 domain. Recent findings provide biochemical evidence that BECN1 is present in distinct BECN1-containing PtdIns3K complexes. Each complex has a protein core consisting of BECN1, PIK3C3 and PIK3R4 and one or more specific interactors including ATG14, UVRAG, KIAA0226/Rubicon and SH3GLB1/Bif-1. Remarkably, unlike VMP1, any of these molecules interacts with the BH3 domain of BECN1. Interestingly, the yeast homolog of BECN1, Vps30/Atg6, does not possess a BH3 motif. Binding between BCL2 and BECN1 inhibits autophagy, and, accordingly, the dissociation of BCL2 from BECN1 is an important regulatory event that induces this pathway. Since the expression of VMP1 leads to the dissociation of the BCL2-BECN1 complex, VMP1 through the interaction with the BH3 domain of BECN1 regulates initial steps of autophagy in mammalian cells.
We identify a crucial mediator responsible for regulating the autophagy-specific PtdIns3K complex activity on the phagophore. We propose that VMP1 is a new player in the activation of the autophagy-specific PtdIns3K complex, providing a novel explanation about how this complex controls autophagosome formation. Although further studies are needed to demonstrate the requirement of VMP1 in omegasome formation, our results are consistent with the findings of Itakura and coworkers reporting that VMP1 colocalizes with ATG14 at the PAS. We show that VMP1 interacts with both BECN1 and PIK3C3 (and presumably also with PIK3R4), but BECN1 fails to associate with PIK3C3 when VMP1 lacks its autophagy-related domain. The VMP1-BECN1 direct interaction promotes PtdIns3P generation on phagophore membranes. We find a remarkable colocalization between LC3 and the PdtIns3P-probe 2xFYVE upon rapamycin treatment and VMP1 expression. Moreover, endogenous VMP1 expression is required for the docking of the PtdIns3K complex onto phagophore membranes since the 2xFYVE probe does not colocalize with these intermediates when VMP1 expression is silenced under rapamycin treatment. VMP1ΔAtgD mutant, which is not able to interact with BECN1, fails to rescue rapamycin-treated cells from VMP1 downregulation, further supporting the notion that the VMP1-BECN1 interaction is required for the correct localization of PtdIns3K onto the phagophore membrane during mammalian autophagy (Fig. 1).
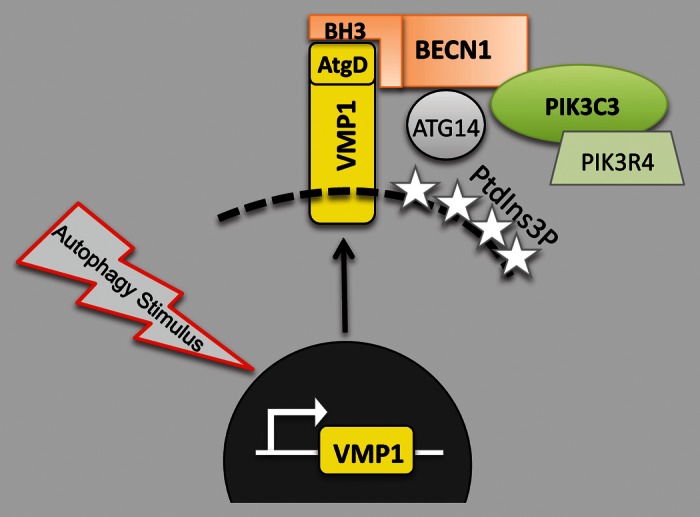
Figure 1. Schematic representation showing the role of VMP1-BECN1 interaction in the induction of autophagy. Autophagy stimulus induces VMP1 expression. The VMP1-AtgD interacts with the BECN1 BH3 domain partitioning BECN1 to the autophagic pathway. This interaction promotes the recruitment of the autophagy-specific PtdIns3K complex to the PAS. The activation of the PtdIns3K complex generates the PtdIns3P necessary to recruit the rest of the ATG machinery, leading to autophagosome formation.
In mammalian cells, the ATG12–ATG5-ATG16L1 conjugate is involved in determining the site of LC3 lipidation. We have shown that the VMP1-BECN1 interaction triggers the membrane association of the ATG12–ATG5-ATG16L1 complex. Thus, VMP1 promotes the generation of PtdIns3P, and the subsequent localization of ATG16L1 and LC3 to the PAS during autophagosome formation. Therefore, the VMP1-BECN1 interaction acts upstream of the ATG12–ATG5-ATG16L1 complex during the generation of an autophagosome. This notion is indirectly supported by the observation of VMP1-BECN1 accumulation in dots of large size when ATG5 is silenced in VMP1-expressing cells.
Together, our data show that VMP1 is a key regulator of the early steps of autophagosome formation in mammalian cells. These characteristics collectively point at VMP1 as a unique ATG transmembrane protein that positively regulates autophagy induction in several human diseases. Our findings set the basis for future studies on the regulation of autophagy in pathological situations and highlight VMP1 as a biomarker and also as a target for therapeutic interventions.
Acknowledgments
The authors are grateful to Dr. Fulvio Reggiori for his insightful comments. M.I.V. and A.R. are supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT-PICT01627-M.I.V. and ANPCyT-PICT0411-A.R.), the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-PIP2527-M.I.V.) and the University of Buenos Aires (UBA-UBACyT 2011-M.I.V.). M.I.M. is a CONICET doctoral fellow.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/24390


