Abstract
Flies expressing the most common Parkinson disease (PD)-related mutation, LRRK2-G2019S, in their dopaminergic neurons show loss of visual function and degeneration of the retina, including mitochondrial abnormalities, apoptosis and autophagy. Since the photoreceptors that degenerate are not dopaminergic, this demonstrates nonautonomous degeneration, and a spread of pathology. This provides a model consistent with Braak’s hypothesis on progressive PD. The loss of visual function is specific for the G2019S mutation, implying the cause is its increased kinase activity, and is enhanced by increased neuronal activity. These data suggest novel explanations for the variability in animal models of PD. The specificity of visual loss to G2019S, coupled with the differences in neural firing rate, provide an explanation for the variability between people with PD in visual tests.
Keywords: Parkinson disease, LRRK2, Drosophila, vision, photoreceptors, electroretinogram, neurodegeneration, dopaminergic neurons
The discovery of genes associated with Parkinson disease offers the hope that genetic animal models would provide revolutionary advances, through novel insights into the molecular pathology and the mechanisms of cell death. Many mouse models have been disappointing, and failed to capture the essential features of PD. However, work with that most tractable of genetic organisms, the fruit fly, has provided a range of insights. For example, several PD-related genes (park/parkin, Pink1 and Lrrk/LRRK2) belong to a common pathway. Again, fly models have emphasized mitochondrial dysfunction and aberrant fission/fusion, leading to novel therapeutic approaches. We have now extended the contribution made by Drosophila, showing that expression of the common PD-related mutation (Lrrk-G2019S) in dopaminergic neurons leads to spread of degeneration from neuron to neuron, due to the increased kinase activity of the G2019S mutant protein, and that this spread is exacerbated by increasing demands on neuronal activity.
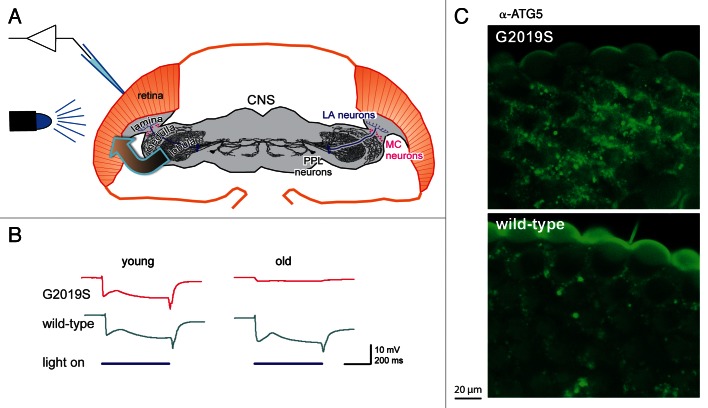
It was known that some people with PD had reported deficits in their sight, including contrast adaptation, and that both mammals and flies had dopaminergic neurons and receptors in their visual system. As loss of dopaminergic neurons is characteristic of PD, we therefore exposed flies to a 500-ms flash of light and recorded their response using electroretinograms (ERGs, Fig. 1A). We used the powerful GAL4-UAS system to express either the normal (wild-type) or the mutant (G2019S) form of human LRRK2, in dopaminergic neurons. Up until 10 d, both wild-type and G2019S flies respond consistently, but by 28 d the G2019S response has nearly flat-lined, while the wild-type flies still respond fully (Fig. 1B). The almost complete loss of ERG response indicates that the photoreceptors are no longer sensitive to light. The loss of photoreceptor function is accompanied by signs of degeneration throughout the visual lobes of the brain, including dilation and disorganization of the mitochondria in the G2019S photoreceptors, and stronger staining of the G2019S photoreceptors by markers of apoptosis and autophagy (Fig. 1C).
Figure 1. Flies expressing the most common PD-related mutation, Lrrk-G2019S, in their dopaminergic neurons show loss of visual response and degeneration of the retina. (A) Schematic of the experiment: G2019S or the wild-type human LRRK2 was expressed in the dopaminergic neurons (LA, MC and PPL neurons). Electrical recordings were made of the response of the photoreceptors in the eye to flashes of blue light (electroretinograms). Note that the transgene is expressed in one class of neurons, dopaminergic neurons, and the response measured in another, the histaminergic photoreceptors (arrow). (B) In young flies, the ERG trace consistently shows a sustained deflection, indicating photoreceptor activity, as long as the light is on. Old G2019S flies show a much-reduced response, showing failure of the photoreceptors. (C) The loss of function is accompanied by neurodegeneration, exemplified here by increased abundance of the autophagy marker ATG5, in the outermost layer of the photoreceptors of the G2019S flies. (Fig. modified based on those in the original publication).
The first key development is that our data potentially model the “spreading pathology” model of PD championed by Braak. This arises from the fact that fly photoreceptors are not dopaminergic, but histaminergic, yet it was in the dopaminergic neurons that the transgene is expressed, and in the photoreceptors that the visual function and degeneration are assessed. Thus, in this model of PD (unlike previous experimental systems), the loss of function and neuronal degeneration is not cell autonomous, but requires cell–cell transmission. When G2019S is expressed pan-neuronally, the loss of visual function is much less than when it is expressed in just the dopaminergic neurons, suggesting that an asymmetry between adjacent cells in G2019S expression enhances the spread of the degeneration. Cell–cell transmission of another PD-related protein, SNCA/α-synuclein, has been implicated in analysis of fetal grafts implanted in PD patients, in graft-host transmission in animal models and in cell culture. It has always been said that flies have no SNCA homolog, but other possible signals include the transmitter dopamine, growth factors or cytokines. Like SNCA, LRRK2 is secreted in exosomes, for example from the kidney, so another possibility is transmission by LRRK2-G2019S itself.
A second key point is that the model is highly specific to G2019S: mutations at other points along the human LRRK2 or Drosophila Lrrk gene do not induce loss of visual response. These include mutations affecting the GTPase domain of LRRK2. Equally, the kinase dead, G2019S-K1906M form, of LRRK2, does not induce degeneration. This high degree of specificity makes the model very suitable for “first in vivo” drug testing. It also provides an explanation for the varying data from visual tests on PD patients: the differences in visual dysfunction between patients may reflect the fact that only some of them carry the LRRK2-G2019S mutation. At the moment this remains a hypothesis, as most data on the visual responses of people with PD was obtained before the genetic era.
A third important aspect of this report is the finding that increasing the neural activity in the visual system (either by keeping the flies in a flashing “disco” chamber, or by genetically downregulating voltage-gated potassium channels) accelerates the loss of visual function. Neuronal function (action potentials, transmitter release and recycling) is energetically demanding, and the brain already consumes up to 20% of resting metabolism. Additional neural activity will lead to an increased demand for ATP, as membrane pumps are activated to maintain intracellular levels of signaling cations and transmitters. This demand for ATP will provide extra load on the mitochondria, leading to oxidative stress generation, apoptosis and autophagy. The accelerated decline of vision in flies constantly adapting their eyes to new light intensities led Hindle et al. to suggest a new explanation for discrepancies in dopaminergic neuron loss between fly models of PD. Previous explanations had focused on variation in microscopy or food; now it may be essential to take differences in energy demand (e.g., from changing illumination) into consideration. Furthermore, increasing the activity of the visual system of mammalian models may make their phenotype stronger and more consistent. Finally, in human populations, the penetrance of the G2019S disease ranges from 25–50% at age 70, and it may be that part of this variability derives from differences in neuronal energy demand.
Acknowledgment
This work was supported by Parkinson’s UK (grant number K-1007), the Wellcome Trust (grant number 097829/Z/11/A). We are grateful to Sean Sweeney for his comments on the manuscript.
Glossary
Abbreviations:
- ERGs
electroretinograms
- PD
Parkinson disease
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/24397