Abstract
Objective
To examine gonadal protective properties of granulocyte colony-stimulating factor (G-CSF) alone or in combination with stem cell factor (SCF) in female mice treated with high-dose alkylating chemotherapy.
Design
Experimental laboratory animal study.
Setting
Tertiary care academic hospital and research institute.
Animal(s)
Six- and 8-week-old C57Bl/6 female mice.
Intervention(s)
Adult female mice were treated with [1] cyclophosphamide and busulfan (CTx), [2] CTx + G-CSF/SCF, [3] CTx + G-CSF, or [4] normal saline and dimethyl sulfoxide (DMSO; vehicle control).
Main Outcome Measure(s)
Follicle counts, microvessel density, cellular response to DNA damage, and litter production.
Result(s)
G-CSF ± SCF increased microvessel density and decreased follicle loss in CTx-treated female mice compared with CTx-only treated female mice. Mice administered CTx alone exhibited premature ovarian insufficiency, with only 28% of mice producing two litters. However, 100% of mice receiving CTx with G-CSF + SCF, and 80% of mice receiving CTx + G-CSF alone produced at least three litters and 20% of mice in each group produced five litters.
Conclusion(s)
Treatment of mice with G-CSF decreases chemotherapy-induced ovarian follicle loss and extends time to premature ovarian insufficiency in female mice. Further studies are needed to validate these preclinical results in humans and compare efficacy with the established GnRH analogue treatments.
Keywords: Granulocyte colony-stimulating factor, chemotherapy, fertility preservation
Recent improvements in cancer treatment and surveillance have resulted in decreased mortality rates and longer survival. Between 1990 and 2008 all cancer-related mortality in women decreased by 15.3% (1). In addition, there are currently 7.2 million female cancer survivors in the United States, 360,000 of whom are 40 years or younger (2). Providers contributing to the care of girls and women of reproductive age diagnosed with cancer are currently concerned not only with achievement of a disease-free status, but also with preservation of the best possible quality of life after cancer treatment. Young female patients undergoing chemotherapy or radiation as a part of cancer therapy may suffer significant quality of life impairment secondary to early menopause and infertility.
It has been traditionally accepted that females are born with a finite number of ovarian follicles and the pool of primordial follicles subsequently undergoes depletion through atresia and maturation that results in infertility and menopause. The effect of cancer treatment on ovarian function is related to the decline of the number of primordial follicles as well as a reduction in the number of larger maturing follicles (3). Alkylating agents are most commonly implicated in causing ovarian damage and female infertility (4, 5). Busulfan and cyclophosphamide are common alkylating chemotherapeutic agents most frequently used in conditioning regimens for allogeneic and autologous hematopoietic stem cell transplantation in humans (6) and are associated with a high risk of premature ovarian insufficiency (7, 8). Therefore, investigation of strategies to protect ovaries from the damaging effects of chemotherapy is an active area of research (9–14). The mechanisms of oocyte damage by chemotherapeutics are still inadequately characterized, but may include DNA damage in follicles (15, 16) and/or damage to the ovarian microvasculature (17).
The ovary is dependent on cyclic microvascular remodeling that occurs throughout the menstrual/estrous cycle (18). This is relevant, as proliferating endothelial cells may be susceptible to chemotherapy-induced damage. Previous studies by Meirow and colleagues (17) in the human ovary have shown that chemotherapy results in blood vessel damage in the ovary, with subsequent loss of the follicles in the segment supplied by that vessel.
Recently, Lee and co-workers (9) demonstrated that bone marrow transplantation either 1 week or 2 months after high-dose cyclophosphamide and busulfan chemotherapy treatment resulted in preservation of fertility in chemotherapy-treated female mice. The protective mechanism of bone marrow transplantation on the ovary was considered to be either a direct effect on the cells responsible for oogenesis or an influence on the microenvironment that supports the oocytes (9). In the present study, we used the same chemotherapy treatment regimen and tested the hypothesis that stimulating endogenous bone marrow with granulocyte colony-stimulating factor (G-CSF) would similarly preserve ovarian follicles and fertility in mice treated with high-dose cyclophosphamide and busulfan chemotherapy.
It was recently reported that G-CSF in combination with vascular endothelial growth factor protects primordial follicles in ovaries grafted after excision and freezing in mice (19). These factors may play a crucial role in preventing or minimizing ischemia-induced follicle loss. The G-CSF receptors are expressed by endothelial cells (20, 21), and G-CSF alone, or in combination with vascular endothelial growth factor, has been shown to promote neovascularization after brain, kidney, heart, and limb ischemia (22–25). However, the use of vascular endothelial growth factor in the setting of cancer diagnosis could potentially be problematic because of its proven proliferative effect on the cancer vasculature (26). Studies in mice with mutations at the stem cell factor (SCF) and c-kit (SCF receptor) gene loci have demonstrated that activity of this growth factor–receptor complex is critical for normal gametogenesis, and SCF is known to exert potent antiapoptotic effects in germ cells (27, 28). In vivo, the combination of G-CSF and SCF has been shown to increase the number of bone marrow-derived endothelial cells in different organs after ischemia (29). The G-CSF has an established safety profile and is successfully used in cancer patients for prevention of chemotherapy-induced neutropenia without decreasing the efficacy of chemotherapeutic agents (30, 31).
In the present study, we produced a preclinical mouse model of premature ovarian insufficiency by treatment with cyclophosphamide and busulfan chemotherapy, as previously described (9). We used this model to test the hypothesis that G-CSF treatment alone or in combination with SCF protects ovaries from chemotherapy damage and preserves fertility. To test this hypothesis, we examined the effects of G-CSF on ovarian follicle number, DNA damage response, microvascular density, and fertility of chemotherapy treated mice. To our knowledge this is the first study to evaluate the gonadal protective properties of G-CSF ± SCF in chemotherapy treated female mice.
MATERIALS AND METHODS
Animals
All studies were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and Magee-Womens Research Institute (assurance #A3654-01) in accordance with the National Academies of Science Guide for Care and Use of Laboratory animals. Young adult inbred C57BL/6 female mice were purchased from Jackson Laboratories.
G-CSF, SCF, and Chemotherapy Treatments
Fifty-two 6-week-old mice were divided into four groups (n = 13 mice per group). The first group received five daily IP injections of normal saline, which is the carrier for G-CSF and SCF and one-time IP injection of cyclophosphamide (100 mg/kg; cyclophosphamide monohydrate; Sigma-Aldrich; Cat# 29875) and busulfan (12 mg/kg; busulfan; Sigma-Aldrich; Cat# 150606) (CTx). This treatment regimen was chosen because it is nonlethal (does not require bone marrow transplantation), but causes infertility in female mice (9). A similar regimen called little Bu-Cy (busulfan 16 mg/kg, cyclophosphamide 120 mg/kg) is used for conditioning before autologous and allogenic stem cell transplantation in humans (32, 33) and causes infertility in women (32).
The second group received five daily IP injections of G-CSF (50 μg/kg/d; Neupogen; Amgen Ltd.) and SCF (100 μg/ kg/d; R&D Systems) with a single injection of CTx on day 3. The third group received five daily IP injections of G-CSF with single injection of CTx on day 3. The fourth group (vehicle control) received five daily IP injections of normal saline with a single IP injection of 50% dimethyl sulfoxide (DMSO), which is the carrier for busulfan on day 3. An SCF-only group was not assigned as the main objective of this study was to assess the efficacy of G-CSF. G-CSF is an agent that is already used in clinical medicine for the treatment of febrile neutropenia in patients with cancer. SCF is an agent that is not used in humans, but has been used in previous animal studies. The G-CSF/SCF group was assigned to evaluate whether adding an additional growth factor would have any benefit over G-CSF alone. The volume of injection was adjusted for the lack of SCF in the G-CSF-only group. Ovaries were analyzed 48 hours or 21 days after treatment to assess the immediate effects on DNA damage response (48 hours) and the sustained effects on follicles and blood vessels (21 days).
Follicle Counts
Differential follicle counts were performed to assess the effect of the chemotherapy on ovarian follicles and to evaluate protective effects of the G-CSF and SCF. Ovaries were collected 48 hours (n = 3 mice per group) and 21 days (n = 10 mice per group) after CTx treatment and fixed in a solution containing 0.34 N glacial acetic acid, 10% formalin, and 28% ethanol, and embedded in paraffin. They were serially sectioned (8-μm sections) and stained with picric acid/methyl blue and Weigert’s hematoxylin. The number of resting (primordial) and early-growing (primary and secondary) follicles per ovary was determined as detailed previously elsewhere (34). Briefly, differential follicle counts were calculated after counting every fifth section. The obtained number was then multiplied by five for every type of follicle (primordial, early-growing) to estimate the total number of follicles per ovary. All counts were performed by an examiner blinded to the experimental groups. Primordial follicle counts were also performed by an independent examiner by counting Lhx8-positve follicles in 10 nonconsecutive sections from the center of each ovary. Lhx8 is a member of the LIM-homeobox transcription factor family and preferentially expressed in oocytes and germ cells within the mouse ovary. Lhx8 transcripts localize to oocytes of germ cell clusters and primordial, primary, and antral follicles in the mouse ovary. Lhx8 is critical in early follicle formation and oocyte differentiation (35).
Immunohistochemistry
Immunohistochemical staining of ovaries for Lhx8, phospho-gamma H2AX, and platelet endothelial cell adhesion molecule (PECAM1/CD31) were performed to quantify primordial follicles, cellular response to DNA damage, and blood vessel density, respectively. Endothelial cell marker PECAM1/ CD31 is a membrane protein that mediates cell-to-cell adhesion and is reliably detected in vascular endothelial cells in the mouse ovaries (18).
Ovaries were dissected 48 hours (for phospho-gamma H2AX only) or 21 days after CTx treatment (for Lhx8 and PECAM1/CD-31) and fixed in buffered 4% paraformaldehyde followed by paraffin embedding. Ovaries were serially sectioned (6-μm sections). The standard fluorescent technique was performed for CD-31 antibody and colorimetric for Lhx8 and phospho-gamma H2AX antibody, according to the instructions of the manufacturer. Antigen retrieval was achieved in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20 at pH 6.0). Sections were incubated overnight in the primary antibody at 4°C as follows: anti-Lhx8 1:200 (36), anti-CD-31 1:100 (PECAM-1; Santa Cruz Biotechnologies), anti-phospho-gamma H2AX (Ser139) 1:100 (#9718; Cell Signaling Technology Inc.). Sections were then washed in phosphate-buffered saline (PBS) and primary antibody immunoreactivity was revealed by incubation with secondary antibodies (1:200) for 45–60 minutes at room temperature: biotinylated goat anti-guinea pig IgG (Vector Laboratories, Inc.) for Lhx8, biotinylated donkey anti-rabbit IgG (sc-2089; Santa Cruz Biotechnology Inc.) for phospho-gamma H2AX and donkey anti-rabbit Alexa 488 antibody (Invitrogen) for CD-31. Antigen specificity was confirmed with negative controls in which tissue sections were prepared as described but with isotype control instead of primary antibody. For colorimetric immunohistochemistry (LhX8 and phospho-gamma H2AX) ABC reagent (VECTASTAIN ABC Kit [Standard*], Vector Laboratories, Inc.) was used to amplify signal intensity and 3, 3′-diaminobenzidine solution (DAB peroxidase substrate kit, Vector Laboratories, Inc.) was used to visualize horseradish peroxidase as a brown precipitate. Immunofluorescent staining (PECAM1/CD31) was visualized using an epifluorescent microscope and a fluorescein isothiocyanate (FITC)/tetramethyl rhodamine isothiocyanate (TRITC) filter cube, which helps distinguish specific fluorescence (green) from epifluorescence (red) and provides contrast to visualize tissue architecture.
Quantification of Phospho-gamma H2AX-positive Follicles
Phospho-gamma H2AX staining and quantification was performed to assess the DNA damage response in ovarian follicles after chemotherapy exposure as well as the protective effects of G-CSF ± SCF. Phospho-gamma H2AX-positive follicles were quantified 48 hours after exposure to chemotherapy under light microscope (×600 magnification). Follicles were considered phospho-gamma H2AX-positive if either the oocyte alone, oocyte and granulosa cells (GCs), or oocyte negative, but >50% of GCs were positive. The number >50% of positive GCs was chosen arbitrarily. Data presented are only from follicles with the nucleolus in the plane. Six to eight sections were counted per mouse and there were three mice per treatment group. All counts were performed by an examiner blinded to the experimental groups.
Quantification of Microvessels
Microvessel density was assessed to address the effect of chemotherapy on ovarian microvasculature as well as the protective effects of G-CSF ± SCF. The PECAM1/CD31-positive vessels were evaluated using an epifluorescent microscope. Only cross-sections of microvessels with a clearly identifiable lumen were counted. Microvessel density calculation was conducted by a single examiner who was blinded to treatment group to avoid interobserver variation. Microvessel counts were obtained from every 15th 6-μm section (×600 magnification). Images of each ovarian section were captured with a Spot RT camera (diagnostic Instruments, Inc.) and the area of each section was circumscribed manually in Adobe Photoshop (Adobe Systems) and quantified. The number of microvessels was divided by the area size (in millimeters squared) for each section to determine the microvessel density. Three ovaries per treatment group from different mice were used for statistical analysis.
Breeding Trials
Breeding trials were performed to assess the effect of chemotherapy on litter production as well as the potential protective effects of G-CSF ± SCF. Eight-week-old female mice were divided into four groups (n = 5–7 mice per group) and treated as described previously. Four weeks after completion of CTx treatment, female mice were housed with untreated adult males of proven fertility at a 1:1 ratio. Males were randomly rotated among cages after each breeding. The number of litters, the number of pups per litter, and the birth weight of each pup were recorded from five successive breedings conducted during a period of 6 months.
Statistical Analysis
All statistical tests were performed using GraphPad Prism statistical software (GraphPad Software, Inc.) and R package lme4, Geepack, and DoBy (http://cran.r-project.org/). Data were analyzed by one-way analysis of variance (ANOVA) for each type of follicle (i.e., primordial, early-growing), phospho- gamma H2AX-positive follicles, and microvessel density. This was followed by Tukey multiple comparison test for adjustment. Wald’s test for generalized estimating equation method and generalized linear mixed model method was used to compare litter production rates between groups over time. A P<.05 was chosen to indicate statistically significant differences.
RESULTS
Follicle Counts
Initial follicle counts were performed on serial sections stained with picric acid/methyl blue and Weigert’s hematoxylin as described in the Materials and Methods section. There were no significant differences 48 hours after chemotherapy exposure in the number of primordial or early-growing (primary and secondary) follicles between vehicle, CTx- only, CTx + G-CSF/SCF, and CTx + G-CSF groups (n = 3 mice per group; P>.05; Supplemental Table 1, available online). However, relative to vehicle-treated controls on day 21 (1,235 ± 125 primordial follicles and 942 ± 48 early-growing follicles per ovary), both primordial and early-growing follicles were nearly depleted in CTx-only treated mice (30 ± 10 primordial and 85 ± 17 early-growing follicles/ovary, respectively; P<.0001). Mice injected with CTx + G-CSF/SCF (270 ± 22 follicles/ovary), as well as with CTx + G-CSF alone (210 ± 30 follicles/ovary), had a significantly greater number of primordial follicles compared with mice treated with CTx alone (P<.0001; Fig. 1A). Similarly, the loss of early-growing follicles was partially ameliorated in mice injected with CTx + G-CSF and SCF (175 ± 23 follicles per ovary; P<.01) and CTx + G-CSF alone (151 ± 26 follicles per ovary; P<.05) compared with mice treated with CTx alone (85 ± 17 follicles per ovary; Fig. 1B). Similar treatment effects were obtained by quantifying Lhx8-positive primordial follicles, as described in the Materials and Methods section (Supplemental Table 2, available online). Representative images of Lhx8-stained sections from each experimental group 21 days after CTx treatment are presented in Figure 1C–1F.
FIGURE 1.
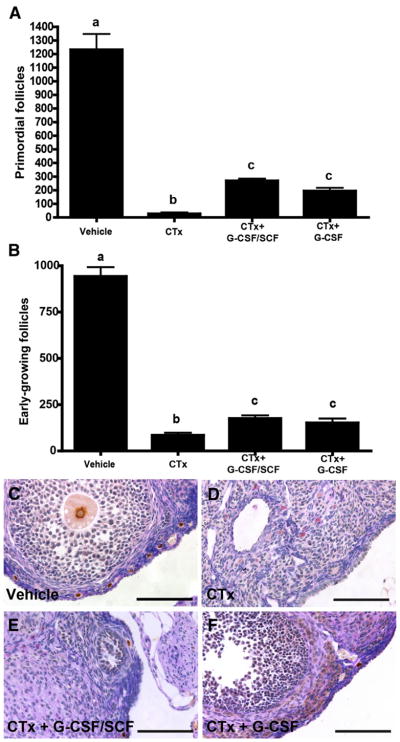
Granulocyte colony-stimulating factor ± stem cell factor (G-CSF ± SCF) maintains primordial and early-growing follicle numbers in high-dose alkylating chemotherapy treated mice. Follicle numbers were assessed 21 days after cyclophosphamide and busulfan (CTx) treatment. Graphs represent the number of primordial (A) and early-growing (B) follicles per ovary in different treatment groups. Bars represent mean ± SEM (n = 10 mice per group; one ovary per mouse; different letters above bars indicate statistically significant differences) (P<.05). LhX8 staining indicates multiple primordial follicles in vehicle-treated controls (C), no primordial follicles in CTx-only treated ovaries (D), and few primordial follicles in CTx + G-CSF/SCF (E) and CTx + G-CSF alone (F) groups. Scale bars = 100 μm. CTx = cyclophosphamide/busulfan; CTx + G-CSF/SCF = cyclophosphamide/busulfan + granulocyte colony-stimulating factor/stem cell factor; CTx + G-CSF = cyclophosphamide/busulfan + granulocyte colony-stimulating factor.
Follicular DNA Damage Response: Phospho-gamma H2AX Staining
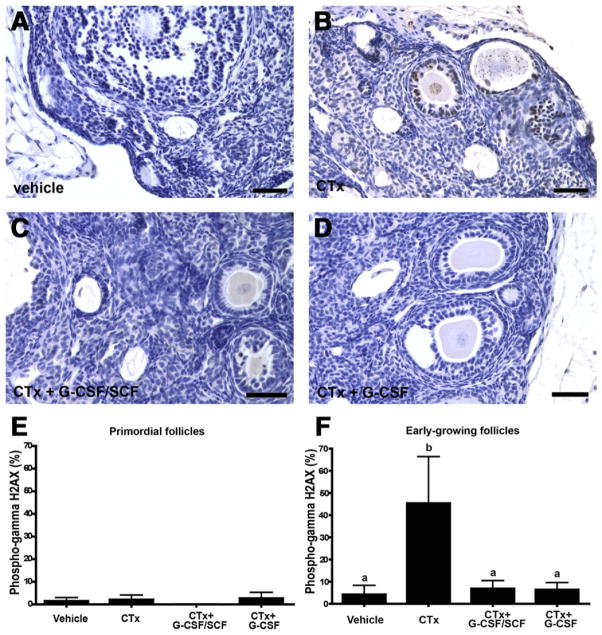
DNA damage response was assessed by counting phospho-gamma H2AX positive follicles 48 hours after exposure to chemotherapy (Fig. 2). Phospho-gamma H2AX is a marker for cellular response to DNA damage because it is required for assembly of DNA repair proteins to sites with damaged chromatin (37).
FIGURE 2.
Granulocyte colony-stimulating factor ± stem cell factor (G-CSF ± SCF) treatment decreases chemotherapy-induced gamma H2AX phosphorylation in early-growing follicles, the earliest cellular response to DNA damage. Representative panels from (A) vehicle controls, (B) cyclophosphamide and busulfan (CTx), (C) CTx + G-CSF/SCF, (D) CTx + G-CSF are shown. The percent of primordial (E) and early-growing (F) follicles exhibiting phospho-gamma H2AX staining. Bars are mean ± SEM; n = 3 mice per group; one ovary per mouse. Different letters above bars represent statistical significance (P<.05). Scale bars = 50 μm. CTx = cyclophosphamide/busulfan; CTx + G-CSF/SCF = cyclophosphamide/busulfan + granulocyte colony-stimulating factor/stem cell factor; CTx + G-CSF = cyclophosphamide/busulfan + granulocyte colony-stimulating factor.
There was no difference among treatment groups in the number of phospho-gamma H2AX positive primordial follicles (Fig. 2E; P>.05). The average number of primordial follicles was 1.5 ± 1.5 in vehicle-treated controls, 2.1 ± 1.7 in the CTx-only group, 0 in the CTx + G-CSF/SCF group, and 2.6 ± 2.6 in the CTx + G-CSF groups (Fig. 2E; P>.05). In contrast, phospho-gamma H2AX positive early-growing follicles (Fig. 2F) were significantly increased in the CTx-only treated group (45.3 ± 21.1 follicles) compared with all other groups 48 hours after chemotherapy treatment (P<.01). The number of phospho-gamma H2AX positive early-growing follicles (Fig. 2F) in the CTx + G-CSF/SCF (6.8 ± 3.8 follicles) and CTx + G-CSF (6.3 ± 3.3 follicles) groups were similar to vehicle-treated controls (4.5 ± 3.3 follicles; P>.05).
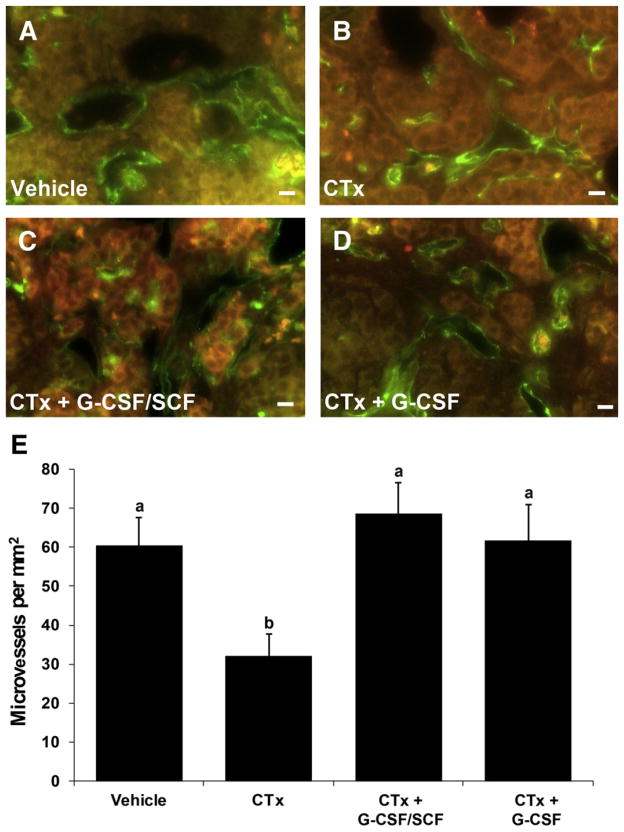
Blood Vessel Density
To assess microvessel density, blood vessels were identified by immunohistochemical staining for PECAM-1/CD31 (Fig. 3A– 3D) and quantified as shown in Figure 3E. Microvessel densities were 60.4 ± 7.1 microvessels/mm2 for vehicle controls (Fig. 3A and E), 32.0 ± 5.7 microvessels/mm2 for CTx (Fig. 3B and E), 68.5 ± 8.0 microvessels/mm2 for CTx + G-CSF/ SCF (Fig. 3C and E), and 61.6 ± 9.4 for CTx + G-CSF (Fig. 3D and E). Microvessel density in the CTx group was significantly reduced compared with all other groups (Fig. 3B and E; P<.05). Microvessel densities in the CTx + G-CSF/ SCF and CTx + G-CSF groups were comparable to vehicle-treated controls (Fig. 3E; P>.05).
FIGURE 3.
Granulocyte colony-stimulating factor ± stem cell factor (G-CSF ± SCF) treatment increases microvessel density after chemotherapy treatment. Microvessel density was assessed 21 days after cyclophosphamide and busulfan (CTx) treatment. Examples of immunofluorescent staining for PECAM1/CD31 (green) of vascular endothelial cells in: vehicle controls (A), CTx (B), CTX + G-CSF/SCF (C), CTx + G-CSF (D). Immunofluorescent staining (PECAM1/CD31) was visualized using an epifluorescent microscope and a fluorescein isothiocyanate (FITC)/ tetramethyl rhodamine isothiocyanate (TRITC) filter cube, which helps distinguish specific fluorescence (green) from epifluorescence (red) and provides contrast to visualize tissue architecture. Scale bars = 10 μm. (E) Quantification of microvessel density (microvessels/mm2; mean ± SEM; n = 3 ovaries/group). Different letters above bars represent statistically significant differences (P<.05). CTx = cyclophosphamide/busulfan; CTx + G-CSF/SCF = cyclophosphamide/busulfan + granulocyte colony-stimulating factor/stem cell factor; CTx + G-CSF = cyclophosphamide/ busulfan + granulocyte colony-stimulating factor.
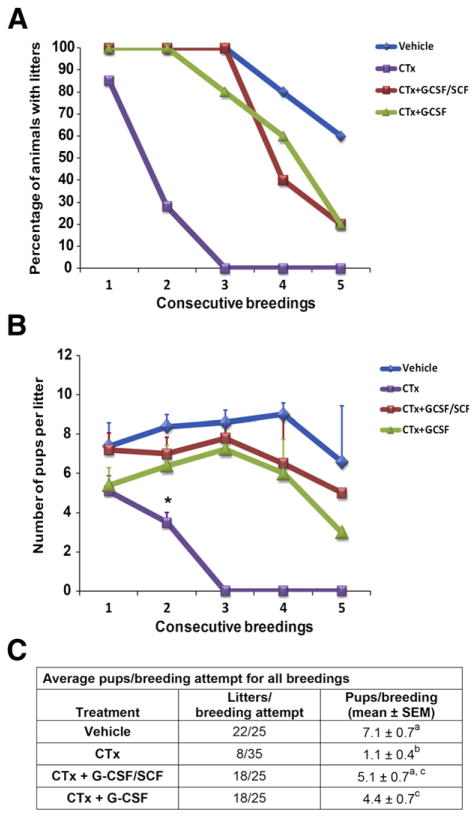
Breeding Trials
To assess the effects of CTx ± growth factors on fertility, mating trials were performed. We found that the litter production rate (percent of mice in each experimental group that produced litters) for all groups, including the vehicle controls, deteriorated significantly over time (Fig. 4A). The deterioration of litter production rate for CTx + G-CSF, CTx + G-CSF/SCF, and vehicle group was significantly slower than for CTx group (P<.05). There was no significant difference between CTx + G-CSF and CTx + G-CSF/SCF (P>.05). During the course of the 6-month breeding trial, all vehicle control females produced three litters, and 60% produced five litters. The CTx-only mice quickly became infertile, with only 28% of mice producing two litters. The CTx-only group did not produce any litters in breedings 3–5 (Fig. 4A). However, 100% of the mice that received CTx + G-CSF/ SCF, and 80% of mice that received CTx + G-CSF produced at least three litters and 20% of mice from each of these groups produced five litters. Pup weights in litters from the first breeding were not different between treatment groups (Supplemental Table 3, available online).
FIGURE 4.
Granulocyte colony-stimulating factor ± stem cell factor (G-CSF ± SCF) treatment extends time to premature ovarian insufficiency in chemotherapy-treated female mice. (A) Percentage of female mice receiving vehicle (n = 5), cyclophosphamide and busulfan (CTx) (n = 7), CTx + G-CSF/SCF (n = 5), and CTx + G-CSF (n = 5) that produced litters in five successive breedings during a 6-month period. (B) Number of pups per litter during the course of the 6-month breeding trial in vehicle controls, CTx, CTx + G-CSF/SCF, and CTx + G-CSF alone. Asterisk denotes significant difference compared with vehicle controls (P<.05). (C) Average number of pups per breeding attempt across all five consecutive breedings. Values with different superscript letters are significantly different (P<.05). CTx = cyclophosphamide/busulfan; CTx + G-CSF = cyclophosphamide/busulfan + granulocyte colony-stimulating factor; CTx + G-CSF/SCF = cyclophosphamide/busulfan + granulocyte colony-stimulating factor/stem cell factor.
The CTx-only group had significantly fewer pups per litter (3.5 ± 0.5 pups per litter; P<.05) in the second breeding than vehicle-treated controls (8.4 ± 0.6 pups per litter) and did not produce any litters in breedings 3–5 (Fig. 4B). With the number of mice used for the breeding study (5–7 mice per treatment group), we did not have sufficient power to detect differences at each individual breeding between vehicle-treated controls, CTx + G-CSF/SCF, and CTx + G-CSF.
Across all five consecutive breedings, vehicle-treated controls produced 22 litters in 25 attempts, with an average of 7.1 ± 0.7 pups per breeding attempt (Fig. 4C). In contrast, CTx-treated mice produced only 8 litters in 35 breeding attempts and produced significantly fewer pups per breeding attempt (1.1 ± 0.4; P<.001) than vehicle-treated controls. The CTx + G-CSF/SCF mice produced 18 litters in 25 breeding attempts and 5.1 ± 0.7 pups per breeding attempt, which was significantly greater than CTx-only mice (P<.001), but not significantly different than vehicle controls (P>.05). Similarly, CTx + G-CSF mice produced 18 litters in 25 breeding attempts and 4.4 ± 0.7 pups per breeding attempt. This was significantly less than vehicle controls (P<.05), but significantly greater than CTx-only mice (P<.01). The number of pups per breeding attempt in the CTx + G-CSF/ SCF (5.1 ± 0.7) and CTx + G-CSF (4.4 ± 0.7) groups were not significantly different (P>.05; Fig. 4C).
The pups derived from the second breeding of the vehicle, CTx + G-CSF/SCF, and CTx + G-CSF groups were kept into adulthood and subsequently bred. There was no difference in the number and gross morphology of the offspring between the groups. The average numbers of pups per litter were 7.2 ± 1.2, 6.2±1.1, and 6.5±1.1 in vehicle controls, CTx + G-CSF/ SCF, and CTx + G-CSF groups (P>.05). There were an insufficient number of second litter females in the CTx-only group to perform next generation breeding.
DISCUSSION
We examined the ovarian protective properties of G-CSF and SCF in a preclinical mouse model of chemotherapy-induced ovarian insufficiency. Treatment with chemotherapy alone caused a dramatic decrease in ovarian follicles, which was associated with decreased ovarian microvascular density, increased DNA damage response in follicles, and rapid fertility loss. In contrast, follicle loss was partially ameliorated in mice receiving G-CSF ± SCF concurrent with chemotherapy treatment. The data were consistent with our hypothesis that treatment with G-CSF ± SCF protects ovaries from chemotherapy damage and preserves fertility. The SCF did not appear to augment the protective effects of G-CSF alone.
Approximately 1 in 46 females in the United States will be diagnosed with cancer between birth and 39 years of age and most will survive (1). The increasing number of girls and reproductive-age women surviving cancer diagnoses has resulted in an increased focus on quality of life after cure. Cancer survivors report that parenthood is important to them and distress over infertility has long-term psychological and relationship implications (38). Despite the importance of the problem, there are few options to preserve fertility (39) and those options are often not feasible in the short window of time between diagnosis and treatment. The only methods for protecting the ovary in situ include fertility-sparing surgery in gynecologic cancers, surgical transposition of the ovary away from the field of radiation, and hormonal protection with GnRH-a (40). Unfortunately, none of the available options are very effective and most include invasive methods. In addition, most methods preserve only future fertility without restoring natural hormonal ovarian function and preventing premature menopause.
Gonadotropin-releasing hormone analogues are used clinically to protect ovaries during oncologic treatment, but the benefit of this approach is subject to debate (13, 41). Furthermore, prolonged use of GnRH-a has been shown to accelerate depletion of the ovarian follicular reserve in a mouse model (42, 43). In addition, GnRH-a therapy is often associated with bothersome side effects, including hot flashes, headaches, memory disorder, insomnia, and loss of bone mineral density (44). The long-term effects of this type of therapy require further assessment and alternative protective strategies need to be developed.
Previous studies demonstrated that sphingosine-1-phosphate, a sphingolipid metabolite, inhibits germ cell apoptosis induced by radiation and chemotherapy and preserves fertility in female mice when injected to the ovarian bursa before the insult (10, 45). These results were recently extended to nonhuman primates by Zelinski and co-workers (11), who demonstrated that a long-acting sphingosine-1-phosphate mimetic, FTY720, protects primate ovaries against radiation-induced damage. Sphingosine-1- phosphate was also recently shown to improve survival of human ovarian xenografts by increasing neoangiogenesis (12). In addition, two recent studies demonstrated follicle protection and long-term fertility preservation in female mice treated with high-dose alkylating chemotherapy followed by bone marrow transplantation (9, 46). These observations led us to hypothesize that stimulation and mobilization of endogenous bone marrow by treatment with G-CSF ± SCF would provide similar protection of ovarian follicles and fertility in mice treated with high-dose chemotherapy. To our knowledge this is the first study to demonstrate the gonadal protective properties of G-CSF ± SCF in chemotherapy-treated female mice.
In the current study we observed significantly higher levels of phospho-gamma H2AX (an indicator of cellular response to DNA damage) in early-growing follicles of ovaries from CTx-treated mice compared to vehicle-treated controls. Phospho-gamma H2AX was observed both in oocytes and GCs. This finding is consistent with previous reports assessing ovarian damage after chemotherapy (15, 16). Soleimani and colleagues (15) reported that doxorubicin caused massive double-stranded DNA (dsDNA) breaks in primordial follicles, oocytes, and GCs as revealed by accumulating phospho-gamma H2AX foci. Furthermore, doxorubicin caused a decrease in ovarian microvascular density as determined by PECAM1/CD31 expression. Petrillo and co-workers (16) observed increase gamma H2AX phosphorylation after exposure of cultured mouse ovaries to phosphoramide mustard, an active metabolite of cyclophosphamide and this was associated with significant losses of primordial and small primary follicles. In the current study, phospho-gamma H2AX staining in early-growing follicles was reduced to the level of vehicle-treated controls when CTx-treated mice were cotreated with G-CSF ± SCF.
In contrast to early-growing follicles, we did not observe a difference between treatment groups in the number of primordial follicles with phospho-gamma H2AX staining. These results may suggest a different mode of protection for primordial follicles or that DNA damage response was simply not detected in primordial follicles at the single time point examined because H2AX phosphorylation is a transient event. Perhaps the cellular DNA damage response is not activated until metabolically inactive primordial follicles are recruited into the growing follicle pool.
In addition to activating the cellular DNA damage response, high-dose cyclophosphamide and busulfan chemotherapy caused a significant reduction in ovarian microvessel density compared to vehicle-treated controls. In contrast, microvessel density in mice treated with CTx and G-CSF (with or without SCF) was similar to that seen in vehicle-treated control mice. Additional studies will be required to determine whether the effects of G-CSF ± SCF are mediated directly at the level of ovarian vascular endothelial cells or by indirect mechanisms. Although the specific mechanism of microvessel preservation was not examined in this study, previous reports indicate that G-CSF alone, or in combination with vascular endothelial growth factor, stimulates neoangiogenesis after ischemic injury in different organs (22–25). Therefore, we speculate that G-CSF ± SCF protects ovarian follicles from chemotherapy damage by decreasing chemotherapy-related blood vessel loss and associated ischemia, perhaps by preventing vascular injury and/or stimulating new blood vessel formation.
In addition to decreased evidence of DNA damage response and increased ovarian microvascular density, the follicle reserve in chemotherapy-treated females 3 weeks after G-CSF ± SCF treatment was 10-fold greater than in mice treated with CTx alone. Although follicle numbers were only 15%–20% of that observed in vehicle-treated controls, the reproductive span in most mice treated with CTx and G-CSF ± SCF was significantly greater than mice treated with CTx alone. However, there was a decline in the number of pups per litter over time in the G-CSF-treated group compared with vehicle controls, which may reflect the smaller pool of ovarian follicles available for fertilization in this group.
In summary, based on our results G-CSF ± SCF decreases follicle loss and extends time to premature ovarian insufficiency in mice treated with gonadotoxic chemotherapy. Thus, G-CSF may be a potential fertility preservation agent that can be used during chemotherapy in women. Future studies are necessary to [1] evaluate the optimal timing and dosage of G-CSF relative to chemotherapy, [2] determine its efficacy in primates and humans, and [3] compare the gonadal protective properties of G-CSF with currently established options, such as GnRH-a. More studies are needed to dissect the mechanisms of chemotherapy and radiation-induced gonadal toxicity and reveal potential targets for additional candidate protective agents.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health & Human Development grants HD055475 and HD061289, Magee-Womens Research Institute and Foundation, Richard King Mellon Foundation (K.E.O.) and American Society for Reproductive Medicine Research Grant (M.S.W.).
The authors thank Jennifer Shuttle-worth, B.S., and the Magee-Womens Research Institute Histology Core staff for technical assistance. We are grateful to Tony Battelli and the Magee-Womens Research Institute Lab Animal Research Resource staff for animal care and maintenance.
Footnotes
M.E.S.-W. has nothing to disclose. M.M.M. has nothing to disclose. M.S. has nothing to disclose. J.D. has nothing to disclose. T.C. has nothing to disclose. T.C.K. has nothing to disclose. A.R. has nothing to disclose. K.E.O. has nothing to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Desantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–92. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 4.Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53:727–39. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 5.Donnez J, Martinez-Madrid B, Jadoul P, van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–35. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 6.Litzow MR, Perez WS, Klein JP, Bolwell BJ, Camitta B, Copelan EA, et al. Comparison of outcome following allogeneic bone marrow transplantation with cyclophosphamide-total body irradiation versus busulphan-cyclophosphamide conditioning regimens for acute myelogenous leukaemia in first remission. Br J Haematol. 2002;119:1115–24. doi: 10.1046/j.1365-2141.2002.03973.x. [DOI] [PubMed] [Google Scholar]
- 7.Thibaud E, Rodriguez-Macias K, Trivin C, Esperou H, Michon J, Brauner R. Ovarian function after bone marrow transplantation during childhood. Bone Marrow Transplant. 1998;21:287–90. doi: 10.1038/sj.bmt.1701075. [DOI] [PubMed] [Google Scholar]
- 8.Couto-Silva AC, Trivin C, Thibaud E, Esperou H, Michon J, Brauner R. Factors affecting gonadal function after bone marrow transplantation during childhood. Bone Marrow Transplant. 2001;28:67–75. doi: 10.1038/sj.bmt.1703089. [DOI] [PubMed] [Google Scholar]
- 9.Lee HJ, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM, et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25:3198–204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
- 10.Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–14. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- 11.Zelinski MB, Murphy MK, Lawson MS, Jurisicova A, Pau KY, Toscano NP, et al. In vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female nonhuman primates. Fertil Steril. 2011;95:1440–5. e1–7. doi: 10.1016/j.fertnstert.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6:e19475. doi: 10.1371/journal.pone.0019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clowse ME, Behera MA, Anders CK, Copland S, Coffman CJ, Leppert PC, et al. Ovarian preservation by GnRH agonists during chemotherapy: a meta-analysis. J Womens Health (Larchmt) 2009;18:311–9. doi: 10.1089/jwh.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91:694–7. doi: 10.1016/j.fertnstert.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 15.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY) 2011;3:782–93. doi: 10.18632/aging.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrillo SK, Desmeules P, Truong TQ, Devine PJ. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol Appl Pharmacol. 2011;253:94–102. doi: 10.1016/j.taap.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22:1626–33. doi: 10.1093/humrep/dem027. [DOI] [PubMed] [Google Scholar]
- 18.Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4:18. doi: 10.1186/1477-7827-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skaznik-Wikiel ME, Sharma RK, Selesniemi K, Lee HJ, Tilly JL, Falcone T. Granulocyte colony-stimulating factor in conjunction with vascular endothelial growth factor maintains primordial follicle numbers in transplanted mouse ovaries. Fertil Steril. 2011;95:1405–9. doi: 10.1016/j.fertnstert.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–808. [PubMed] [Google Scholar]
- 21.Kojima H, Otani A, Oishi A, Makiyama Y, Nakagawa S, Yoshimura N. Granulocyte colony-stimulating factor attenuates oxidative stress-induced apoptosis in vascular endothelial cells and exhibits functional and morphologic protective effect in oxygen-induced retinopathy. Blood. 2011;117:1091–100. doi: 10.1182/blood-2010-05-286963. [DOI] [PubMed] [Google Scholar]
- 22.Sacramento CB, da Silva FH, Nardi NB, Yasumura EG, Baptista-Silva JC, Beutel A, et al. Synergistic effect of vascular endothelial growth factor and granulocyte colony-stimulating factor double gene therapy in mouse limb ischemia. J Gene Med. 2010;12:310–9. doi: 10.1002/jgm.1434. [DOI] [PubMed] [Google Scholar]
- 23.Akihama S, Sato K, Satoh S, Tsuchiya N, Kato T, Komatsuda A, et al. Bone marrow-derived cells mobilized by granulocyte-colony stimulating factor facilitate vascular regeneration in mouse kidney after ischemia/reperfusion injury. Tohoku J Exp Med. 2007;213:341–9. doi: 10.1620/tjem.213.341. [DOI] [PubMed] [Google Scholar]
- 24.Sehara Y, Hayashi T, Deguchi K, Zhang H, Tsuchiya A, Yamashita T, et al. Potentiation of neurogenesis and angiogenesis by G-CSF after focal cerebral ischemia in rats. Brain Res. 2007;1151:142–9. doi: 10.1016/j.brainres.2007.01.149. [DOI] [PubMed] [Google Scholar]
- 25.Ieishi K, Nomura M, Kawano T, Fujimoto S, Ikefuji H, Noda Y, et al. The effect of G-CSF in a myocardial ischemia reperfusion model rat. J Med Invest. 2007;54:177–83. doi: 10.2152/jmi.54.177. [DOI] [PubMed] [Google Scholar]
- 26.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Packer AI, Hsu YC, Besmer P, Bachvarova RF. The ligand of the c-kit receptor promotes oocyte growth. Dev Biol. 1994;161:194–205. doi: 10.1006/dbio.1994.1020. [DOI] [PubMed] [Google Scholar]
- 28.Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–71. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- 29.Toth ZE, Leker RR, Shahar T, Pastorino S, Szalayova I, Asemenew B, et al. The combination of granulocyte colony-stimulating factor and stem cell factor significantly increases the number of bone marrow-derived endothelial cells in brains of mice following cerebral ischemia. Blood. 2008;111:5544–52. doi: 10.1182/blood-2007-10-119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geissler K, Koller E, Hubmann E, Niederwieser D, Hinterberger W, Geissler D, et al. Granulocyte colony-stimulating factor as an adjunct to induction chemotherapy for adult acute lymphoblastic leukemia—a randomized phase-III study. Blood. 1997;90:590–6. [PubMed] [Google Scholar]
- 31.Muhonen T, Jantunen I, Pertovaara H, Voutilainen L, Maiche A, Blomqvist C, et al. Prophylactic filgrastim (G-CSF) during mitomycin-C, mitoxantrone, and methotrexate (MMM) treatment for metastatic breast cancer. A randomized study. Am J Clin Oncol. 1996;19:232–4. doi: 10.1097/00000421-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Grigg AP, McLachlan R, Zaja J, Szer J. Reproductive status in long-term bone marrow transplant survivors receiving busulfan-cyclophosphamide (120 mg/kg) Bone Marrow Transplant. 2000;26:1089–95. doi: 10.1038/sj.bmt.1702695. [DOI] [PubMed] [Google Scholar]
- 33.Hassan M, Andersson BS. Role of pharmacogenetics in busulfan/cyclophosphamide conditioning therapy prior to hematopoietic stem cell transplantation. Pharmacogenomics. 2013;14:75–87. doi: 10.2217/pgs.12.185. [DOI] [PubMed] [Google Scholar]
- 34.Tilly JL. Ovarian follicle counts—not as simple as 1, 2, 3. Reprod Biol Endocrinol. 2003;1:11. doi: 10.1186/1477-7827-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, et al. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci U S A. 2006;103:8090–5. doi: 10.1073/pnas.0601083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi Y, Ballow DJ, Xin Y, Rajkovic A. Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol Reprod. 2008;79:442–9. doi: 10.1095/biolreprod.108.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podhorecka M, Skladanowski A, Bozko P. H2AX phosphorylation: its role in dna damage response and cancer therapy. J Nucleic Acids. 2010;2011(Article ID 920161):1–9. doi: 10.4061/2010/920161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer. 2009;53:281–4. doi: 10.1002/pbc.22001. [DOI] [PubMed] [Google Scholar]
- 39.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 40.West ER, Zelinski MB, Kondapalli LA, Gracia C, Chang J, Coutifaris C, et al. Preserving female fertility following cancer treatment: current options and future possibilities. Pediatr Blood Cancer. 2009;53:289–95. doi: 10.1002/pbc.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bedaiwy MA, Abou-Setta AM, Desai N, Hurd W, Starks D, El-Nashar SA, et al. Gonadotropin-releasing hormone analog cotreatment for preservation of ovarian function during gonadotoxic chemotherapy: a systematic review and meta-analysis. Fertil Steril. 2011;95:906–14. e1–4. doi: 10.1016/j.fertnstert.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Maltaris T, Beckmann MW, Mueller A, Hoffmann I, Kohl J, Dittrich R. Significant loss of primordial follicles after prolonged gonadotropin stimulation in xenografts of cryopreserved human ovarian tissue in severe combined immunodeficient mice. Fertil Steril. 2007;87:195–7. doi: 10.1016/j.fertnstert.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 43.Danforth DR, Arbogast LK, Friedman CI. Acute depletion of murine primordial follicle reserve by gonadotropin-releasing hormone antagonists. Fertil Steril. 2005;83:1333–8. doi: 10.1016/j.fertnstert.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 44.Olive DL. Gonadotropin-releasing hormone agonists for endometriosis. N Engl J Med. 2008;359:1136–42. doi: 10.1056/NEJMct0803719. [DOI] [PubMed] [Google Scholar]
- 45.Paris F, Perez GI, Fuks Z, Haimovitz-Friedman A, Nguyen H, Bose M, et al. Sphingosine 1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat Med. 2002;8:901–2. doi: 10.1038/nm0902-901. [DOI] [PubMed] [Google Scholar]
- 46.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.