Abstract
Following an internal contamination event, the transport of actinide and lanthanide metal ions through the body is facilitated by endogenous ligands such as the human iron-transport protein transferrin (Tf). The recognition of resulting metallo-transferrin complexes (M2Tf) by the cognate transferrin receptor (TfR) is therefore a critical step for cellular uptake of these metal ions. A high performance liquid chromatography-based method has been used to probe the binding of M2Tf with TfR, yielding a direct measurement of the successive thermodynamic constants that correspond to the dissociation of TfR(M2Tf)2 and TfR(M2Tf) complexes for Fe3+, Ga3+, La3+, Nd3+, Gd3+, Yb3+, Lu3+, 232Th4+, 238UO22+, and 242Pu4+. Important features of this method are (i) its ability to distinguish both 1:1 and 1:2 complexes formed between the receptor and the metal-bound transferrin, and (ii) the requirement for very small amounts of each binding partner (<1 nmol of protein per assay). Consistent with previous reports, the strongest receptor affinity is found for Fe2Tf (Kd1 = 5 nM and Kd2 = 20 nM), while the lowest affinity was measured for Pu2Tf (Kd1 = 0.28 µM and Kd2 = 1.8 µM) binding to the TfR. Other toxic metal ions such as ThIV and UVI, when bound to Tf, are well recognized by the TfR. Under the described experimental conditions, the relative stabilities of TfR:(MxTf)y adducts follow the order Fe3+ >> Th4+ □ UO22+ □ Cm3+ > Ln3+ □ Ga3+ >>> Yb3+ □ Pu4+. This study substantiates a role for Tf in binding lanthanide fission products and actinides, and transporting them into cells by receptor mediated endocytosis.
Introduction
The increasing use of nuclear materials in the civilian industry and defense sectors over the past 70 years has resulted in persistent environmental and health issues, since a large inventory of radionuclides, including lanthanide (Ln) fission products and actinides (An), are generated and released during these activities.1 While the scenarios that could lead to human exposure are numerous (terrorism, nuclear power plant accident, war, accidental wound or inhalation for researchers and nuclear workers),2 the molecular processes involved in contamination with Ln or An are still poorly understood.3 Although Ln Fission products and An do not play any role in essential biochemical reactions, they can bind to endogenous chelators, such as metal-binding proteins, to form complexes that are potentially involved in the translocation of the metal ions.4, 5 Identifying these biological ligands and studying their properties as Ln/An complexes is critical to unraveling the mechanisms of uptake, transport and intracellular storage of toxic radioactive f-elements.
Among the many biochemical targets for Ln and An, the mammalian iron transporter transferrin (Tf) is of importance and interest.6 Normally, this protein shuttles iron as Fe3+ in the blood stream between sites of uptake, utilization and storage. Tf is a glycoprotein that consists of 679 amino acids folded into two homologous lobes (N-lobe and C-lobe) connected by a short bridge.7 The sequences of the two lobes are 40% identical and each lobe can hold a single ferric ion in a binding pocket, in which two tyrosine residues, one histidine, and one aspartate come together to bind the iron in the presence of a bidentate synergistic carbonate anion.8 The two iron-binding pockets of Tf are similar but not identical, each creating an octahedral environment suitable to stabilize Fe3+ and other metal ions.9 Metal-free apo-transferrin (apoTf) has an open binding cleft, and binding to Fe3+ triggers cleft closure to yield either of two monoferric species FeCTf and FeNTf (where a single Fe3+ is bound to the C-lobe or the N-lobe, respectively), and or the diferric species Fe2Tf. The concentration of Tf in a normal individual is around 30 µM, but only ca. 30% of the protein is saturated with Fe3+. A reported distribution is: 27% Fe2Tf, 23% FeNTf, 10% FeCTf and 40% apoTf.10 Tf can therefore potentially transport up to approximately 34 µM of alternative metals including transition metals or f-block metals, acquired as a result of internal contamination. Several studies have reported the binding of Tf to metals other than iron, including a number of therapeutic metals (Ti4+, VO2+, Cr3+, Ru3+, Bi3+), radio-therapeutic agents (Ga3+, In3+) and toxic metals (Al3+, Zn2+, Ln3+, Tl3+, Th4+, UO22+, Np4+, Pu4+, Am3+, Cm3+).5
Once bound to Tf, Fe3+ can be transported inside cells, through recognition by the cognate Tf receptor, TfR, which is a type II transmembrane homodimeric receptor with a butterfly shape (Fig. 1).11 TfR is located on the extracellular surface of virtually all actively dividing cells and is able to bind apoTf and Fe2Tf reversibly (Kd ~ 1 µM to 1 nM), with a 1:2 stoichiometry (one Tf unit binding to each monomer of TfR).12 Complexes formed between Fe2Tf and TfR undergo endocytosis and subsequently release iron inside cells by a pH-dependent mechanism.13 The TfR:apoTf complexes are then shuttled back to the cellular surface where Tf can acquire new ferric ions and start a new cycle.12 Though tightly controlled for iron homeostasis, TfR-mediated endocytosis has also been discussed as a possible mechanism for effective cellular uptake of exogenous metal ions.14–17 Probing the TfR recognition of Tf loaded with Ln or An ions is therefore crucial to understand the cellular acquisition pathways of such toxic metals and to potentially develop new preventive strategies against internal Ln and An contamination. The specific recognition of metal-bound Tf (MxTf, x = 1 or 2) by TfR has been studied extensively for the diferric species Fe2Tf.13, 18–21 Other Tf complexes formed with the metal centers Bi3+, Co3+, Ga3+, UO22+ and Pu4+ have been probed by kinetic measurements or bioassays.15, 22, 23 The methods employed in such studies generally allow the determination of the overall affinity of the receptor for the metal-protein complex but not the sequential dissociation constants corresponding to the stepwise binding of a single Tf molecule per TfR monomer unit. In addition, previously described methods require a significant amount of materials, which is a prohibitive constraint for studies with radioactive elements.
Fig. 1.
Human transferrin receptor 1 bound by two diferric transferrin molecules (PDB ID: 1SUV).11
Here, we report a relatively simple method based on size-exclusion high performance liquid chromatography (HPLC) that allows discrimination, at a micro-molar scale, between metal-free apoTf, unbound receptor TfR, and the two receptor:metallo-protein complexes TfR:(M2Tf) and TfR:(M2Tf)2. Hence, we are able to determine the two sequential dissociation constants Kd1 and Kd2 corresponding to the stepwise binding of M2Tf by TfR for several metal ions, including alpha and neutron emitters: Fe3+, Ga3+, La3+, Nd3+, Gd3+, Yb3+, Lu3+, 232Th4+, 238UO22+, and 242Pu4+. The thermodynamic constants obtained for each investigated receptor:metallo-Tf complex highlight the feasibility of Tf-mediated cellular acquisition of exogenous Ln and An metal ions. This study also underscores the link between thermodynamic stability determined in vitro and in vivo biokinetic distribution patterns for Ln and An metals. Finally the physical chemistry approach described in this manuscript could easily be implemented to probe other binding pairs.
Experimental
General
All chemicals were obtained from commercial suppliers and were used as received. All solutions were prepared using deionized water purified with a Millipore Milli-Q reverse osmosis cartridge system. All thermodynamic measurements were conducted at room temperature (unless otherwise indicated). The pH of the solutions was adjusted using either concentrated HCl or NaOH. All proteins were stored in the dark at 4°C between experiments. All experiments were carried out with freshly prepared 100 mM ammonium bicarbonate (NH4HCO3) at pH 7.4.
Metal stock solutions
Stock Ln3+ solutions were prepared by dissolving about 10 mg of metal chloride hexahydrate in 1 mL of 0.1 M HCl, to prevent hydrolysis, and adjustment of the volume to 10 mL with water. The La3+ solution was prepared following the same procedure, but with La(NO3)3·6H2O as the starting salt. A Ga3+ standard solution (995.0 ppm) was purchased from Sigma Aldrich. The FeNTA stock solution was prepared by combining 10.23 mg of Fe(NO3)3·9H2O and 11.32 mg of nitrilotriacetic acid (NTA) in 1 mL of 6 M HCl, raising the pH to 1.5, and adjusting the volume to 10 mL with water. The stock solution of 232ThNTA22− was prepared from ThCl4·8H2O, following the same procedure. The stock 238UO22+ solution was prepared by dissolving 3.94 mg of UO2(NO3)2·6H2O in 1.5 mL of 0.1 M HCl. A 2 mM working solution of 242PuNTA22− was prepared from a 113 mM stock solution of 242Pu4+ in 1 M HNO3.
Protein solutions
Human apoTf (9.57 mg, 0.12 µmol, 98 % iron-free, Sigma-Aldrich) was dissolved in 1 ml and desalted by passing through a freshly equilibrated Sephadex G-25 column (PD-10 column, GE Healthcare) to prepare apoTf stock solutions. The concentration of apoTf in each stock was determined by measuring the absorbance at 278 nm (ε = 92,300 M−1 cm−1).24 To prepare solutions of metal-loaded Tf, 100 µL of an apoTf stock solution and 10 µL of a metal stock solution (2.5 to 4 equivalents) were mixed with 390 µL of fresh buffer. Absorbance at 245 nm was measured until the equilibrium was reached (at least 300 min), and excess metal was then removed by passing the solution through a freshly equilibrated Sephadex G-25M column (PD-10 column, GE Healthcare). The final MxTf concentration was determined by UV-Visible spectroscopy.24 Recombinant His-Tagged TfR was produced and purified from a BHK cell expression system. This preparation is described elsewhere.18, 21 Solutions of TfR were stored at 4°C in 100 mM NH4HCO3, pH 7.4. The TfR:(MxTf)y complexes where prepared by mixing aliquots of a 4.92 µM TfR stock solution with MxTf solutions and buffer. Solutions were incubated at room temperature for at least 80 min to allow equilibration.
HPLC TfR binding assay
All High Pressure Liquid Chromatography runs were performed on an Agilent 1200 Series LC module, by injection of protein solutions through a gel filtration ZORBAX GF-250 (4.6 × 250 mm, 4 µm, 4.6 × 250 mm) column kept at 25°C. An isocratic method with a 0.1 mL min−1 flow of 100 mM NH4HCO3, pH 7.4, was applied to elute the proteins and protein complexes. Detection of the different species was achieved by UV-Visible absorption (multi wavelength detector tuned at 210, 240, 278, 320 and 440 nm). Samples were prepared by dilution of a stock solution of TfR in 100 mM NH4HCO3 buffer at pH 7.4, to reach a concentration of 0.95 µM. An increasing amount of equilibrated MxTf was added to a constant amount of TfR, leading to a MxTf:TfR ratio varying from 0.2 to more than 2.4. Freshly prepared buffer was added to reach a volume of 20 µL and HPLC injections of 9 µL were performed after a minimum equilibration time of 80 min. For each sample two independent injections at 8 hours interval and two independent data treatments were performed. Each titration contained at least 12 samples. HPLC chromatograms were fitted for species evaluation with a maximum of 4 peaks, each built with a Gaussian function to reach the maximum peak value and with a Lorentzian function to include peak shape in the fits. Equilibrium dissociation constants (Kd1 and Kd2) were then determined by nonlinear regression analysis of peak areas vs. protein concentration, using a two-site binding model as implemented in the refinement program DynaFit.25
Results and discussion
TfR affinity measurements and analyses
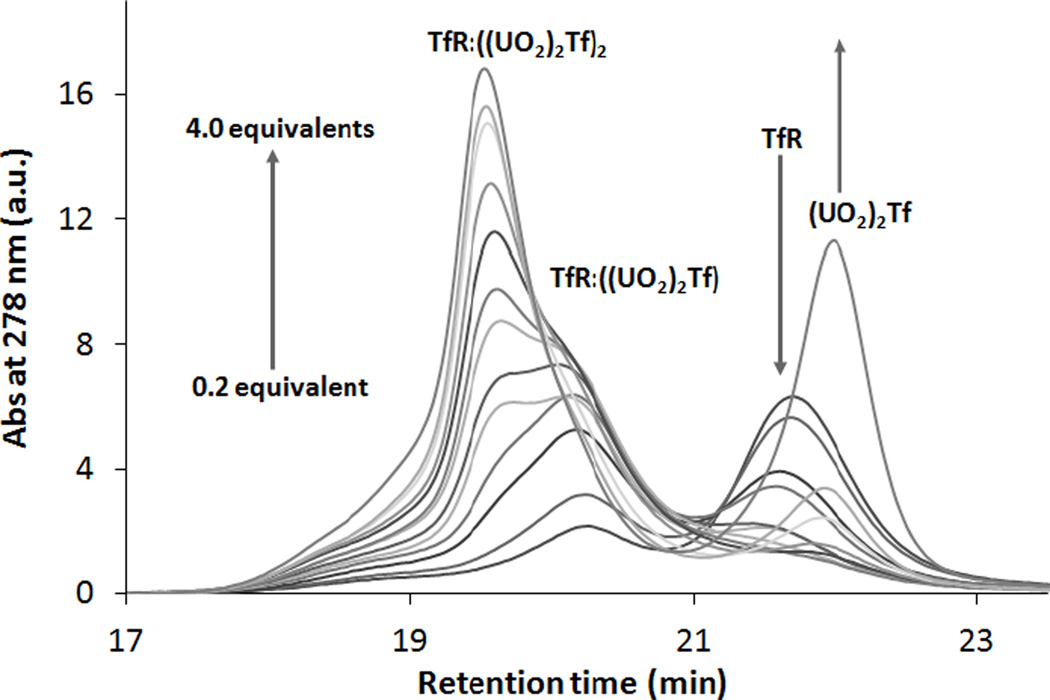
The thermodynamic parameters associated with the recognition of metal-substituted M2Tf by TfR were determined by monitoring the formation of TfR:(M2Tf), TfR:(M2Tf)2, and M2Tf, as well as the disappearance of free TfR, in batch titrations of TfR containing 0 to 2.4 equivalents of M2Tf. For each sample, gel filtration HPLC analysis (100 mM NH4HCO3, 0.1 mL min−1 flow rate, pH 7.4) allowed the separation of these four species based on their different molecular weights, the larger complexes eluting with shorter retention times. UV-Visible spectroscopy was used to detect the largest complex TfR:(M2Tf)2, with a molecular weight of 350 kDa, at a 19.8 min retention time, while the 270 kDa TfR:(M2Tf) was observed at 20.2 min, the 190 kDa TfR at 21.8 min, and the 80 kDa M2Tf around 22.1 min (Fig. 2). As the ratio M2Tf:TfR increased in each titration, the peaks corresponding to TfR:(M2Tf), TfR:(M2Tf)2 and free M2Tf appeared, while the intensity of the peak corresponding to free TfR decreased. The determination of the dissociation constants was performed by fitting each chromatogram with a maximum of four peaks corresponding to the four species potentially present in the system (Fig. 3): unbound M2Tf, unbound TfR, TfR:(M2Tf), and TfR:(M2Tf)2. Subsequently, the evolution of the peak area of each species was fitted versus the ratio M2Tf:TfR, allowing the determination of the stepwise dissociation constants Kd1 and Kd2. A small tailing was observed at very short retention times on the chromatograms (Fig. 2, 3, and S1), probably as a result of aggregation between TfR molecules forming very high molecular weight adducts; this hump was ignored in the fitting process since the stoichiometry of such adducts is unknown under the described experimental conditions. The binding assay was performed with apoTf as well as Tf bound to transition metals (Fe3+, Ga3+), and metals from the Ln series (La3+, Nd3+, Gd3+, Yb3+, Lu3+) and the An series (232Th4+, 238UO22+, and 242Pu4+). Two dissociation constants, Kd1 and Kd2, corresponding to the sequential formation of the 1:1 adduct TfR:(M2Tf) and the 1:2 adduct TfR:(M2Tf)2, are reported for each metal ion in Table 1.
Fig. 2.
Evolution of chromatograms upon the addition of (UO2)2Tf to TfR. [TfR] = 0.95 µM, [NH4HCO3] = 100 mM, pH = 7.4, 25°C. Volume injected: 9 µL.
Fig. 3.
Difference of TfR affinity for a) Fe2Tf, b) 242Pu2Tf, c) 232Th2Tf, and d) 238(UO2)2Tf. Chromatograms obtained for samples containing 0.95 µM TfR and 2.28 µM M2Tf (100 mM NH4HCO3, pH = 7.4), and eluted at a 0.1 mL min−1 flow rate. Raw data are shown as dotted lines and fits as solid lines. Chromatograms were deconvoluted for the following species: TfR:(M2Tf)2 (~19.5 min, diamonds), TfR:(M2Tf) (~20.2 min, dashed line), M2Tf (~22.1 min, dot-dashed line).
Table 1.
Binding constants of metal-loaded transferrin and its soluble receptor 1 obtained by HPLC measurement in NH4HCO3 buffer at pH 7.4.
| Metal ion in MxTf | Kd1 (nM)a | Kd2 (nM)a | Log K1b | Log K2b | Log β2c |
|---|---|---|---|---|---|
| La3+ | 42 ± 3 | 916 ± 60 | 7.38 ± 0.03 | 6.04 ± 0.03 | 13.42 ± 0.06 |
| Nd3+ | 110 ± 79 | 332 ± 235 | 6.96 ± 0.24 | 6.48 ± 0.23 | 13.44 ± 0.57 |
| Gd3+ | 231 ± 21 | 709 ± 846 | 6.64 ± 0.04 | 6.15 ± 0.34 | 12.79 ± 0.38 |
| Yb3+ | 267 ± 59 | 1119 ± 69 | 6.57 ± 0.09 | 5.95 ± 0.03 | 12.52 ± 0.12 |
| Lu3+ | 98 ± 1 | 588 ± 221 | 7.01 ± 0.01 | 6.23 ± 0.14 | 13.24 ± 0.15 |
| Th4+ | 65 ± 29 | 250 ± 89 | 7.19 ± 0.16 | 6.60 ± 0.13 | 13.79 ± 0.29 |
| UO22+ | 49 ± 16 | 140 ± 15 | 7.31 ± 0.13 | 6.86 ± 0.04 | 14.17 ± 0.17 |
| Cm3+ | 74 ± 26d | 153 ± 77d | 7.13 ± 0.13 | 6.82 ± 0.18 | 13.95 ± 0.31 |
| Pu4+ | 282 ± 112 | 1766 ± 281 | 6.55 ± 0.14 | 5.75 ± 0.06 | 12.30 ± 0.20 |
| FeCCmNTf | 42d, e | 32d, e | 7.38 | 7.49 | 14.87 |
| Fe3+ | 5.1 ± 1 | 20 ± 5 | 8.30 ± 0.06 | 7.69 ± 0.09 | 15.99 ± 0.15 |
| apoTf | 130 ± 2 | 439 ± 383 | 6.89 ± 0.01 | 6.36 ± 0.27 | 13.25 ± 0.28 |
| Ga3+ | 91 ± 12 | 800 ± 532 | 7.04 ± 0.05 | 6.10 ± 0.22 | 13.14 ± 0.27 |
Uncertainties on Kd values are calculated from two independent titrations and two independent data treatments.
LogKx is the stepwise binding constant derived from Kdx as follows: Kx = 1/Kdx; uncertainties on log Kx values are calculated by error propagation.
Log β2 is the overall binding constant and is calculated as follows: β2= K1 × K2.
Previously reported.
Value based on a single experimental titration.
Based on these dissociation constants, TfR speciation was then calculated for all investigated metals, with the following fixed concentrations [TfR] = 1 µM and [M2Tf] = 1 or 2 µM; the percentage of free TfR in solution under those conditions is depicted in Fig. 4. This method of data examination underlines the influence of the ratio between M2Tf and TfR in the experimental setting. While working with stoichiometric amounts of M2Tf and TfR, which is often the case in reported bio-assays, a significant amount of free receptor can still be measured since competition between the 1:1 and 1:2 adducts takes place and the formation of the 1:2 adduct TfR:(M2Tf)2 requires two M2Tf molecules. In contrast, adding up to 2 or more equivalents of M2Tf in experimental titrations, as is the case with the present method, better reflects the real affinity of the probed metal-bound Tf species towards its receptor. At a 1:2 TfR:M2Tf ratio, Fe2Tf binds up to 99.5% of the available receptor, leaving only 0.5% of TfR free in solution, while at a 1:1 ratio, up to 25% of the available TfR remains free in solution. The HPLC-based analysis method presented here therefore illustrates that recognition of a metal-bound Tf by its receptor depends on the total amount of Tf and TfR present in the blood system as well as on the local concentrations of both TfR and M2Tf at the cell surface for example.
Fig. 4.
Percentage of free Tf receptor in solution in the presence of different metal-bound Tf. Top: [TfR] = 1 µM, [M2Tf] = 1 µM. Bottom: [TfR] = 1 µM, [M2Tf] = 2 µM. Speciation calculated using the simulation program HySS26 with the stability constants for the adducts TfR:(M2Tf) and TfR:(M2Tf)2 determined in this study.
Competing with Fe for TfR-mediated endocytosis
For metal-free apoTf and all investigated metal-substituted Tf, the dissociation constant of the 1:2 complex TfR:(MxTf)2 (0 ≤ x ≤ 2) is significantly higher than the dissociation constant of the 1:1 complex TfR:(MxTf) (Table 1). This difference in affinity suggests negative cooperativity in the binding of the second apoTf or M2Tf molecule. As expected, the strongest affinity towards TfR was observed for the diferric protein Fe2Tf, with stepwise Kd values of 5 nM and 20 nM for TfR:(Fe2Tf) and TfR:(Fe2Tf)2, respectively. These values are well within the range of constants based on bioassays and previously reported for the binding of Fe2Tf by TfR.19, 20 They also indicate stronger TfR recognition than in the case of metal-free apoTf (Kd1 = 130 nM and Kd2 = 439 nM) and confirm that metal binding triggers closure of the Tf lobes to promote subsequent binding to the receptor. The stability of the 1:1 complex involving Fe3+, TfR:(Fe2Tf), is about 10-fold stronger than for any other metal-substituted Tf and 5-fold higher than that of its 1:2 counterpart TfR:(Fe2Tf)2. The high stability of the ferric complexes is due to the specificity of the soluble TfR for Fe2Tf, which is the naturally metal-loaded Tf present in the blood system. Such selectivity means that at similar concentrations there would be no competition between Fe2Tf and LnxTf or AnxTf for TfR binding. This conclusion remains valid from a thermodynamic point of view but does not exclude a kinetic competition, as suggested by Hémadi et al.23 with the uranyl-substituted species (UO2)2Tf. It should be noted that the uptake of foreign metal-loaded Tf by TfR also depends on the amount of foreign metal present in the blood system as demonstrated by Stradling et al. with Th uptake studies.27 Furthermore, since only ca. 30 % of human serum Tf is saturated with Fe3+,18 a significant amount of toxic metal could still potentially be transported by Tf at any given time, as a bis-substituted species M2Tf or as one of the mixed species MCFeNTf and FeCMNTf.
TfR recognition of Tf bound to tetravalent metal ions
The lowest affinity towards TfR was measured for Tf loaded with Pu4+, confirming the results of a recently reported qualitative study.15 The biological coordination chemistry of the Pu4+ cation is commonly considered very similar to that of Fe3+ and Tf is known to be a major Pu transporter in the blood, with stability constants (log β11 values) corresponding to the direct complexation of these metal ions by Tf reported as ca. 22 for Pu5 and 21 for Fe.28 However, the dissociation constants determined here for the binding of Pu2Tf by TfR, Kd1 = 0.28 µM and Kd2 = 1.8 µM, indicate that the overall affinity of TfR for Pu2Tf is about 50-fold lower than for Fe2Tf. This result highlights the selectivity of TfR based on the metal-induced Tf conformation: while Tf exhibits very high affinities for Pu4+ and Fe3+, the resulting metal-bound Tf species must display different features that impact TfR recognition, as suggested by recent small-angle X-ray scattering studies.15 Therefore, even though Pu is well complexed by Tf itself, TfR may act as an efficient primary barrier to preclude internalization of Pu2Tf through cellular membranes. Such suggestion is in good agreement with the fact that Pu4+ is poorly taken up by cells such as hepatocytes, in comparison to the fraction of contaminant that deposits in bones as Pu-hydroxyapatite complexes. Fig. 3 and Table 1 also underline the singular behavior of TfR towards Pu2Tf, compared to the other actinide-loaded Tf, specifically, Th2Tf, (UO2)2Tf, and Cm2Tf. In the presence of excess Pu2Tf, TfR forms nearly the same amount of TfR:(Pu2Tf)2 and TfR:(Pu2Tf), whereas the 1:2 complexed species TfR:(M2Tf)2 is predominant with FeIII, and other metals, under similar conditions. To our knowledge, the effect of the particular stoichiometry of the TfR:Tf adduct on the endocytosis process has never been investigated. However, it is possible that endocytosis may require the formation of a 1:2 TfR:Tf adduct, which would in turn suggest that the predominance of the mono-complexed species TfR:(Pu2Tf) is the limiting factor for Pu cellular acquisition. Finally, knowing that TfNFe may represent about a quarter of the total amount of Tf in circulation,10 internalization of Pu as a mixed Tf species such as PuCFeNTf is not excluded, as proposed by Jensen et al.15
The Kd values obtained in the case of Th4+ (65 nM and 250 nM for TfR:(Th2Tf) and TfR:(Th2Tf)2, respectively, Table 1) are much lower than those obtained for Pu4+, and reflect strong recognition of Th2Tf by TfR. While in terms of metal acidity, Fe3+ resembles Pu4+ more than Th4+,29 these dissociation constants suggest that the shape and conformation of Fe2Tf are closer to those of Th2Tf than of Pu2Tf. Such changes in the conformation of Pu2Tf may be the result of the presence of additional coordinating water molecule(s) and carbonate anion(s) in the Tf metal-binding sites (not needed for the complexation of Fe3+ or Th4+), preventing the transferrin from closing completely around Pu4+, and thereby decreasing the affinity for the receptor. Unfortunately, no crystal structures of the carbonated Pu-Tf complexes have been reported yet to confirm this particular hypothesis. However previous EXAFS studies on mixed Pu-Tf-NTA species have shown that additional hydroxide molecules are involved in the metal coordination sphere, which suggests that Pu2Tf might not be in a fully closed conformation30 and may therefore be only partially recognized by TfR.
Uranyl-Tf: a controversial example
The Kd values obtained for the recognition of (UO2)2Tf by TfR are in the same range as those determined for Th2Tf, again indicating tight binding by the TfR. Notably, they are considerably lower than reported by Hémadi et al. (6 µM).23 This discrepancy can be explained by the fact that data were treated with a monomeric receptor in Hémadi’s study, whereas we clearly observed the formation of two high molecular weight complexes demonstrating that the receptor is present as a dimeric species in our experimental conditions. This observation is supported by work from Cheng et al.11 and Eckenroth et al.,18 in which a dimeric receptor bound to Fe2Tf and apoTf was described. Moreover, our experiments were carried out at 25°C, contrasting to the 37°C conditions in the kinetic experiments, which could partially account for a higher Kd value due to some exothermic effects.20 The presence of micelles from the buffering agent CHAPS (3-[(3-cholamido-propyl)dimethylammonio]-1-propanesulfonate) in working solutions could also have influenced the affinity measurement between M2Tf and TfR since these micelles can mimic the cell membrane. The uptake of UO22+ by Tf itself has already been demonstrated by Montavon et al.,31 with a log β11 of □ 12 and a log β12 of □ 23, and the formation of the 1:2 adduct TfR:((UO2)2Tf)2 with a Kd2 value of 140 nM, as determined here, suggests that uranyl could follow the iron-acquisition pathway under specific conditions. However, a layer of complexity may be added, in that the shuttling of TfR:(MxTf)y species may also depend on the overall shape and properties of the adducts themselves. In other words, while the affinity of TfR for (UO2)2Tf may be high enough to form stable adducts, the shape of the resulting macromolecular entities may not be adequate for cellular incorporation. This additional limiting feature may explain the limited loading of uranyl-Tf in human erythroleukemia K562 cells, as shown in previous studies using flow cytometry analysis.16
Probing differences in the Tf:TfR recognition of trivalent metals
The cations Ga3+ and Fe3+ are closely related and often exhibit similar coordination chemistry. Hence, Ga3+ is a natural choice in probing the features determinant to metal recognition in Fe-specific systems. In addition, the isotope 67Ga is used in nuclear medicine because it attaches to areas of rapid cell division, such as cancer cells, and the Tf:TfR-mediated endocytosis pathway may be involved in this process. Surprisingly, relatively high Kd values were obtained for the formation of TfR adducts of Ga2Tf: Kd1 = 91 nM and Kd2 = 0.8 µM. The affinity of TfR for Ga2Tf is at least 20-times weaker than for Fe2Tf, even if Ga3+ and Fe3+ have identical charges and similar ionic radii.29 Such discrimination might be triggered by the different electronic structures of the metal ions (open d-shell 3d5 for Fe3+ and full d-shell 3d10 for Ga3+), which can affect the interactions of the metals with the Tf binding residues, and could result in different protein conformations. On one hand, the high Kd2 value of 0.8 µM should preclude the formation of TfR:(Ga2Tf)2 and subsequent intracellular uptake of Ga. On the other hand the Kd1 value corresponding to the formation of the TfR:(Ga2Tf) adduct implies that Ga3+ ions may potentially penetrate the cellular membrane via endocytosis of a mixed adduct TfR:(Ga2Tf)(Fe2Tf).
The Ln ions all bind Tf as trivalent metals with similar affinities.32 Along the Ln series, no trend seems to emerge directly from the Kd values determined for the formation of TfR:Ln2Tf adducts. However, when calculating the amount of free receptor in solution in the presence of one and two equivalents of metal-bound Tf, a slight trend appears from La2Tf to Yb2Tf (Fig. 4). As we progress in the Ln series, from La3+ to Yb3+, the speciation pattern shows an increasing amount of free receptor in solution, corresponding to a potential decrease in intracellular uptake activity. More generally, Ln-bound Tf exhibit a lower affinity towards TfR, compared to the An-bound species. Only La2Tf binds TfR as tightly as Th2Tf and (UO2)2Tf (Fig. 4). The Ln2Tf with the lowest affinity measured towards TfR is Yb2Tf. The coordination properties of Yb3+ and Pu4+ are often compared due to their similar ionic radii (87 pm and 86 pm, respectively, for a coordination number of 6).29 Among the 11 metal ions investigated, Tf loaded with Yb3+ or Pu4+ displayed the lowest binding constants for the formation of TfR adducts, even if Yb3+ and Pu4+ both form very stable complexes with Tf itself.32 Even in the presence of 2 equivalents of Yb2Tf or Pu2Tf, more than 15% of TfR remains unbound in solution, indicative of the weak ability of both Yb2Tf and Pu2Tf to compete with Fe2Tf for TfR recognition in physiological conditions.
The TfR-binding HPLC assay reported in this study was originally developed to characterize Cm-Tf complexes, as detailed elsewhere,17 and Cm3+ is the only trivalent An probed under the described conditions. Similar to what was observed with Th4+ and UO22+, the Kd values of 74 nM and 153 nM, corresponding to TfR:(Cm2Tf) and TfR:(Cm2Tf)2,17 respectively, reflect a strong affinity of TfR towards Cm2Tf, as shown in Fig. 3. In addition, the values reported for the mixed Tf species FeCCmNTf (Table 1) fall between those measured for Fe2Tf and Cm2Tf,17 confirming the reliability of this new method. These numbers also corroborate the hypothesis that foreign metals can be recognized by TfR once bound to a non-saturated Tf such as FeCTf, which is present in significant amounts in the blood stream. Comparable results were obtained for Pu4+ in recent studies15 that showed that TfPuCFeN can be recognized by TfR. The cases of Pu4+ and Cm3+ underscore the possibility that mixed metal-substituted FeCMNTf and MCFeNTf species can also take part in a contamination mechanism, knowing that FeCTf and FeNTf collectively represent more than 30% of the total amount of Tf in the blood stream.10
Conclusion
A new method based on size-exclusion HPLC has been used to probe the binding of metal-substituted human Tf by the cognate receptor TfR. In each case, the new method allows the determination of two dissociation constants, corresponding to the stepwise formation of 1:1 and 1:2 TfR:(MxTf)y adducts, and requires only small amounts of material (<1 nmol per titration for both MxTf and TfR). The small amounts of metal ions needed to conduct these assays have allowed us to probe the TfR recognition of Tf loaded with transition metals (Fe3+ and Ga3+), Ln ions (La3+, Nd3+, Gd3+, Yb3+, and Lu3+), as well as radioactive An ions (232Th4+, 238UO22+, 242Pu4+ and 248Cm3+). For each MxTf species investigated and even in the case of apoTf, the first binding constant is higher than the second one suggesting a non-cooperative mechanism. The strongest affinity is recorded for Fe2Tf, with Kd values in good agreement with previously published values. The lowest affinity was found for Tf loaded with two Pu4+ ions, supporting previous cellular uptake studies as well as Pu biokinetic patterns after an internal contamination event. Other actinide ions such as Cm3+, Th4+ and UO22+ are well complexed by Tf and form M2Tf species that are recognized by TfR, with Kd1 values around 60 nM and Kd2 value around 200 nM. While the binding of a metal to human Tf mostly depends on the oxidation state, charge, and size of the metal ion, the recognition of M2Tf by TfR seems to be driven by other factors including shape and conformation of the metal-bound Tf. The stability constants of the TfR:(MxTf)y adducts, under our experimental conditions, follow the order: Fe3+ >> Th4+ □ UO22+ □ Cm3+ > Ln3+ □ Ga3+ >>> Yb3+ □ Pu4+. Based on these thermodynamic constants alone, metal ions that are readily taken up by the liver in vivo33 such as Th4+, UO22+, and Cm3+, would be predicted to follow the TfR:Tf-mediated iron acquisition pathway for cellular incorporation. However, the conformation of the resulting TfR:(MxTf)y adducts may also affect the endocytosis process and this hypothesis would have to be tested by uptake studies with human cell lines.
This study confirms that even though a considerable portion of human Tf is unsaturated by Fe3+ and thus available to bind a large number of toxic metals, the receptor TfR appears to play a role in the translocation mechanism of exogenous metals by acting as an additional selective barrier for cellular entry. These results also highlight the necessity to establish solution chemistry parameters in order to elucidate in vivo mechanisms. Since FeNTf and FeCTf account for up to 33% of the total Tf in human blood,10 the next step in our in vitro approach would be to investigate further the role of mixed-metal Tf species in the metal-acquisition pathways, as recently discussed for Pu4+ or for Cm3+.15 Finally, in on-going in vivo studies performed in our laboratory (data not yet published), we have noted that contamination with different isotopes of a same element (in our case, U and Am) may result in different body distribution profiles, which suggests that metal acquisition pathways are most likely also driven by kinetic processes.
Supplementary Material
Acknowledgments
Instrument acquisition and method development for the chromatography assays were supported by the National Institutes of Health (R.J.A., RAI087604Z); the experimental work on lanthanide and actinide protein interactions was supported by the Laboratory Directed Research and Development Program at Lawrence Berkeley National Laboratory under Contract No. DE-AC02-05CH11231 (R.J.A.), and by a U. S. Public Service Grant (A.B.M., R01 DK 21739).
Footnotes
Electronic Supplementary Information (ESI) available (Figure S1). See DOI: 10.1039/b000000x/
References
- 1.Albright D, Berkhout F, Walker W. Plutonium and Highly Enriched Uranium 1996: World Inventories, Capabilities, and Policies. New York: Stockholm International Peace Research Institute; 1997. [Google Scholar]
- 2.Cassatt DR, Kaminski JM, Hatchett RJ, DiCarlo AL, Benjamin JM, Maidment BW. Medical Countermeasures against Nuclear Threats: Radionuclide Decorporation Agents. Rad. Res. 2008;170:540–548. doi: 10.1667/rr1485.1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Durbin PW. In: The Chemistry of the Actinide and Transactinide Elements. Morss LR, Edelstein NM, Fuger J, editors. V. Netherlands: Springer; 2006. pp. 3339–3440. [Google Scholar]
- 3.Ansoborlo E, Prat O, Moisy P, Den Auwer C, Guilbaud P, Carriere M, Gouget B, Duffield J, Doizi D, Vercouter T, Moulin C, Moulin V. Actinide Speciation in Relation to Biological Processes. Biochimie. 2006;88:1605–1618. doi: 10.1016/j.biochi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Harris WR, Carrano CJ, Pecoraro VL, Raymond KN. Siderophilin metal coordination. 1. Complexation of thorium by transferrin: structure-function implications. J. Am. Chem. Soc. 1981;103:2231–2237. [Google Scholar]
- 5.Vincent JB, Love S. The binding and transport of alternative metals by transferrin. Biochim. Biophys. Acta. 2012;1820:362–378. doi: 10.1016/j.bbagen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DM. The bioinorganic chemistry of actinides in blood. J. Alloy Compd. 1998;271:6–10. [Google Scholar]
- 7.Wally J. The Crystal Structure of Iron-free Human Serum Transferrin Provides Insight into Inter-lobe Communication and Receptor Binding. J. Biol. Chem. 2006;281:24934–24944. doi: 10.1074/jbc.M604592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker EN. Structure and reactivity of transferrins. Adv. Inorg. Chem. 1994;41:389–463. [Google Scholar]
- 9.Aisen P, Wessling-Resnick M, Leibold EA. Iron metabolism. Curr. Opin. Chem. Biol. 1999;3:200–206. doi: 10.1016/S1367-5931(99)80033-7. [DOI] [PubMed] [Google Scholar]
- 10.Williams J, Moreton K. The distribution of iron between the metal-binding sites of transferrin human serum. Biochem. J. 1980;185:483. doi: 10.1042/bj1850483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y, Zak O, Aisen P, Harrison SC, Waltz T. Structure of the Human Transferrin Receptor-Transferrin Complex. Cell. 2004;116:565–576. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Sun H, Qian ZM. The role of the transferrin–transferrin-receptor system in drug delivery and targeting. Trends Pharmacol. Sci. 2002;23:206–209. doi: 10.1016/s0165-6147(02)01989-2. [DOI] [PubMed] [Google Scholar]
- 13.Steere AN, Byrne SL, Chasteen ND, Mason AB. Kinetics of iron release from transferrin bound to the transferrin receptor at endosomal pH. Biochim. Biophys. Acta. 2012;1820:326–333. doi: 10.1016/j.bbagen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du XL, Zhang TL, Yuan L, Zhao YY, Li RC, Wang K, Yan SC, Zhang L, Sun H, Qian ZM. Complexation of ytterbium to human transferrin and its uptake by K562 cells. Eur. J. Biochem. 2002;269:6082–6090. doi: 10.1046/j.1432-1033.2002.03326.x. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MP, Gorman-Lewis D, Aryal B, Paunesku T, Vogt S, Rickert PG, Seifert S, Lai B, Woloschak GE, Soderholm L. An iron-dependent and transferrin-mediated cellular uptake pathway for plutonium. Nat. Chem. Biol. 2011;7:560–565. doi: 10.1038/nchembio.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidaud C, Gourion-Arsiquaud S, Rollin-Genetet F, Torne-Celer C, Plantevin S, Pible O, Berthomieu C, Quemeneur E. Structural Consequences of Binding of UO22+ to Apotransferrin: Can This Protein Account for Entry of Uranium into Human Cells? Biochemistry. 2007;46:2215–2226. doi: 10.1021/bi061945h. [DOI] [PubMed] [Google Scholar]
- 17.Sturzbecher-Hoehne M, Goujon C, Deblonde GJ-P, Mason AB, Abergel RJ. Sensitizing Curium Luminescence through an Antenna Protein to Investigate Biological Actinide Transport Mechanisms. Submitted. 2012 doi: 10.1021/ja310957f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckenroth BE, Steere AN, Chasteen ND, Everse SJ, Mason AB. How the binding of human transferrin primes the transferrin receptor potentiating iron release at endosomal pH. Proc. Natl. Acad. Sci. 2011;108:13089–13094. doi: 10.1073/pnas.1105786108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianetti AM, Snow PM, Zak O, Bjorkman PJ. Mechanism for Multiple Ligand Recognition by the Human Transferrin Receptor. PLOS Biol. 2003;1:341–350. doi: 10.1371/journal.pbio.0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hemadi M, Kahn PH, Miquel G, El Hage Chahine J-M. Transferrin’s Mechanism of Interaction with Receptor 1. Biochemistry. 2004;43:1736–1745. doi: 10.1021/bi030142g. [DOI] [PubMed] [Google Scholar]
- 20.Mason AB, Byrne SL, Everse SJ, Roberts SE, Chasteen ND, Smith VC, MacGillivray RTA, Kandemir B, Bou-Abdallah FA. A loop in the N-lobe of human serum transferrin is critical for binding to the transferrin receptor as revealed by mutagenesis, iso-thermal titration calorimetry, and epitope mapping. J. Mol. Recognit. 2009;22:521–529. doi: 10.1002/jmr.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason AB, Halbrooks PJ, Larouche JR, Briggs SK, Moffett ML, Ramsey JE, Connolly SA, Smith VC, MacGillivray RTA. Expression, purification, and characterization of authentic monoferric and apo-human serum transferrins. Protein Expres. Purif. 2004;36:318–326. doi: 10.1016/j.pep.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Chick Z, Ha-Duong N-T, Miquel G, El Hage Chahine J-M. Gallium uptake by transferrin and interaction with receptor 1. J. Biol. Inorg. Chem. 2006;12:90–100. doi: 10.1007/s00775-006-0169-7. [DOI] [PubMed] [Google Scholar]; Ha-Duong N-T, Hemadi M, Chick Z, El Hage Chahine J-M. Kinetics and thermodynamics of metal-loaded transferrins: transferrin receptor 1 interactions. Biochem. Soc. Trans. 2008;36:1422–1426. doi: 10.1042/BST0361422. [DOI] [PubMed] [Google Scholar]; Miquel G, Nekaa T, Kahn PH, Hemadi M, El Hage Chahine J-M. Mechanism of Formation of the Complex between Transferrin and Bismuth, and Interaction with Transferrin Receptor 1. Biochemistry. 2004;43:14722–14733. doi: 10.1021/bi048484p. [DOI] [PubMed] [Google Scholar]
- 23.Hemadi M, Ha-Duong N-T, Plantevin S, Vidaud C, El Hage Chahine J-M. Can uranium follow the iron-acquisition pathway? Interaction of uranyl-loaded transferrin with receptor 1. J. Biol. Inorg. Chem. 2009;15:497–504. doi: 10.1007/s00775-009-0618-1. [DOI] [PubMed] [Google Scholar]
- 24.Chasteen ND. Transferrin - A perspective. Adv. Inorg. Chem. 1983;5:201–233. [PubMed] [Google Scholar]
- 25.Kuzmic P. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal. Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- 26.Alderighi L, Gans P, Ienco A, Peters D, Sabatini A, Vacca A. Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999;184:311–318. [Google Scholar]
- 27.Stradling GN, Hodgson SA, Pearce MJ. Recent developments in the decorporation of plutonium, americium and thorium. Radiat. Prot. Dosim. 1998;79:445–448. [Google Scholar]
- 28.Aisen P, Leibman A, Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J. Biol. Chem. 1978;253:1930–1937. [PubMed] [Google Scholar]
- 29.Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A. 1976;32:751–767. [Google Scholar]
- 30.Jeanson A, Ferrand M, Funke H, Hennig C, Moisy P, Solari PL, Vidaud C, Den Auwer C. The role of Transferrin in Actinide(IV) Uptake: Comparison with Iron(III) Chem. Eur. J. 2010;16:1378–1387. doi: 10.1002/chem.200901209. [DOI] [PubMed] [Google Scholar]
- 31.Montavon G, Apostolidis C, Bruchertseifer F, Repinc U, Morgenstern A. Spectroscopic study of the interaction of U(VI) with transferrin and albumin for speciation of U(VI) under blood serum conditions. J. Inorg. Biochem. 2009;103:1609–1616. doi: 10.1016/j.jinorgbio.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Harris WR. In: Less Common Metals in Proteins and Nucleic Acid Probes. Clarke MJ, editor. Springer; 1998. pp. 121–162. [Google Scholar]
- 33.Morss LR, Edelstein NM, Katz JJ. The chemistry of the actinide and transactinide elements. Springer; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.