Towards new therapies for disorders of fear and anxiety
By recent estimates, 28% of the U.S. population suffers from some form of anxiety-related disorders during their lifetime [1]. These illnesses result in numerous adverse effects that extend from the impoverishment of the individual’s quality of life to the high financial cost incurred to treat them. Understanding the functioning of the nervous system in a healthy and affected state will aid in the development of treatments for these disorders [2]. While the eventual goal of such investigation is to ameliorate these conditions in humans, a productive and complementary strategy that can pave the way for intervention in humans uses animal models, wherein the etiology and treatment of anxiety-related disorders is studied [3]. Focusing on anxiety- and fear-related disorders, this brief review attempts to synergize the existing state of knowledge in terms of their neural underpinnings, currently used therapeutic intervention, and potential treatments that hold promise for the future using studies that focus on both animals and humans.
Anxiety and Fear: Operational Definitions
While the physical and psychological manifestations of anxiety and fear appear to share commonalities, they are in fact two independent entities that merit operational definitions. Anxiety is characterized as a state of being that arises from general and non-specific stimuli that are perceived as being potentially threatening in the future. This perception often results in an apprehensive mood accompanied by increased arousal and vigilance, which when taken to an extreme, persist for extended periods of time. In contrast, fear is stimulated by specific stimuli and results in active defensive responses that gradually subside when the specific stimulus is no longer present [4]. Clinically, fear can be thought of as mirroring the response to a specific cue (eg. the fear of snakes), while anxiety is a more long-lasting phenomenon that is not specific to overt cues.
Anxiety in laboratory rodents is often measured using crude behavioral assays such as the Elevated Plus Maze, while fear to specific cues is modeled by employing Pavlovian conditioning to cues such as tones or lights [5]. Recently, nuanced experimental paradigms that more faithfully represent the aforementioned operational distinctions between anxiety and fear have been employed in rats. Anxiety can be modeled using both light- and dark-enhanced startle paradigms, context conditioning, and by exploiting the unpredictability of aversive events (such as mild shock) [6,7]. In contrast, fear can be elicited and measured using paradigms that include but are not limited to cue-specific conditioning, and verbal threat in humans [8]. Further discussion of details about how best to model anxiety and fear in rodents and humans is outside the scope of this review, and there are other excellent comprehensive reviews [9–12]. The need to operationally define anxiety vs fear arises due to the fact that these two behavioral states are mediated by shared as well as independent sub-systems in the brain, and can be treated with independent therapeutic strategies [13].
Anxiety and fear in the brain-from the connectome to the epigenome
Neural circuitry underlying anxiety and fear
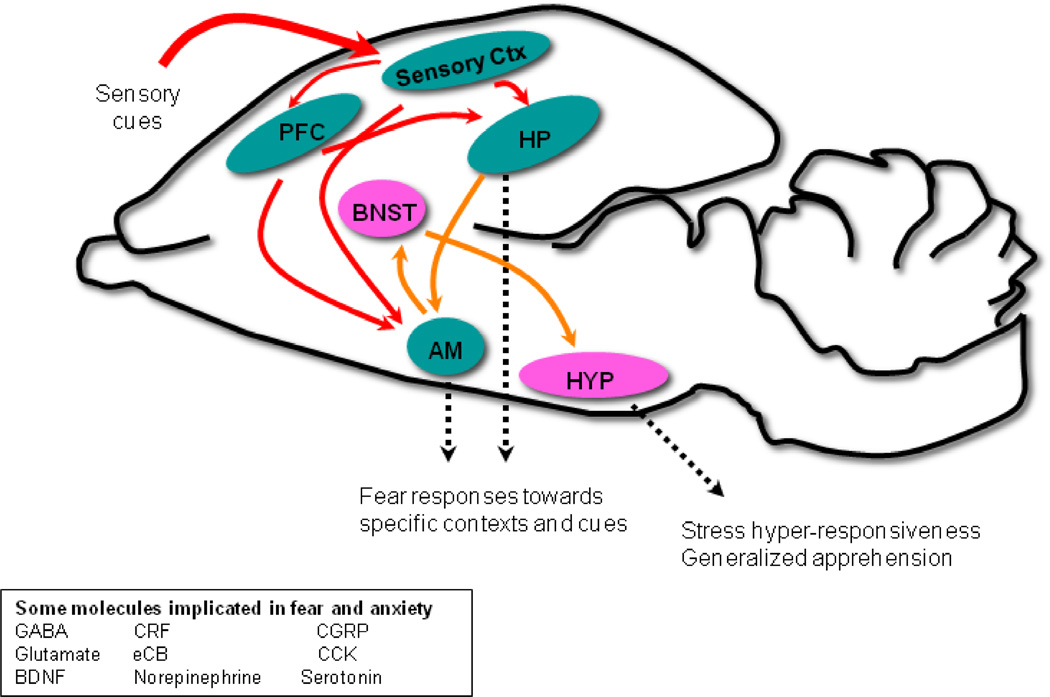
As with any neuropsychiatric disorder, a constellation of brain regions underlie anxiety- and fear-like states [14••, 15•, 16–18]. At the core of this is thought to be the “extended amydgala” which includes the central (CeA) and medial (MeA) nuclei of the amygdala and the bed nucleus of the stria terminalis (BNST) (Figure 1). The extended amygdala is responsive to afferent input from the basolateral amygdala (BLA), and cortical regions such as the insular cortex. Divisions of the prefrontal cortex (PFC)- medial PFC (mPFC) and infralimbic PFC (iPFC) are pivotal sites of consolidation and extinction of fear [19–21]. A review of this nature would not do justice to the intricacies of the neural circuitry underlying anxiety and fear, and we recommend [22,23] for those interested in the same.
Figure 1. Structural and Molecular Pathways Involved in Fear and Anxiety.
A schematic mammalian brain is shown in which prefrontal cortex (PFC) and sensory cortex interact with hippocampal (HP), bed nucleus of the stria terminalis (BNST), amygdala (AM) and hypothalamic (HYP) circuits to mediate stress, anxiety, and fear responses.
Molecular underpinnings of anxiety and fear
One of the most exciting observations in understanding the translation of mammalian fear to human anxiety- and fear-related disorders is the shared conservation of the ‘fear reflex’ across all mammals (Figure 2). Seeing that the triggering of anxious and fearful behavior are stimuli that are perceived as stressful and threatening, it should come as no surprise that Corticotropin Releasing Factor (CRF) at the apex of the Hypothalamic-Pituitary-Adrenal axis plays a pivotal role in the manifestation of these states [24,25]. The successful treatment of anxiety- and fear-related disorders using benzodiazepines and D-cycloserine make a compelling case for the involvement of neurotransmitters such as GABA and glutamate in these behavioral states [26–29]. Similarly neuromodulators such as serotonin, dopamine, and norepinephrine are involved, based on the use of antidepressant medication to treat anxious and fearful phenotypes. Small molecules like calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), and the endocannabinoid system are receiving recent attention for their roles in these disorders [30–32]. We reference [33,34] for a more exhaustive treatment of the molecules implicated in anxiety and fear.
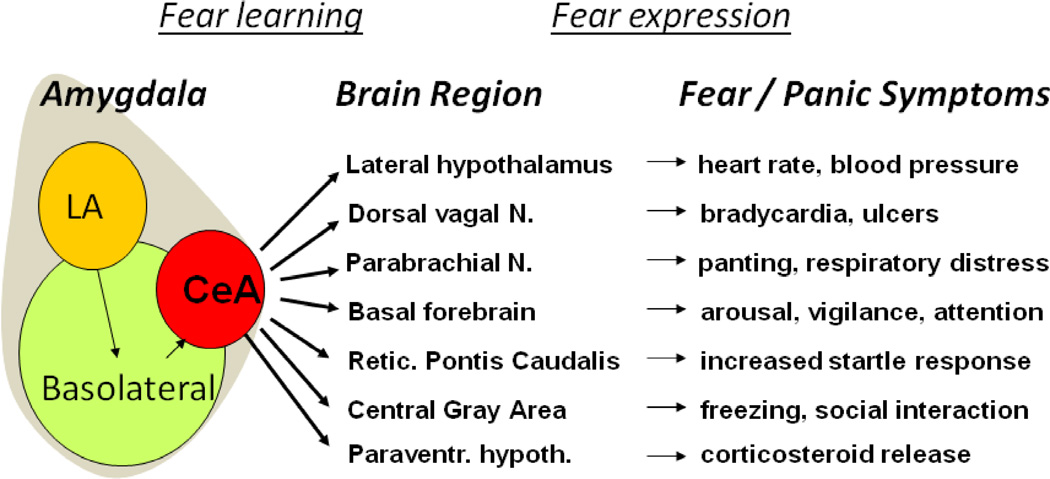
Figure 2. The Hard-wired ‘Fear Reflex’ Underlies the Symptoms of Fear and Panic Responses.
Neural connectivity between the Central Amygdala outputs (CeA) to brainstem and other subcortical areas activates a known and increasingly well-understood series of pathways which mediate the differential fear reflex patterns that are experienced as a fear or panic-attack in humans with anxiety and fear-related disorders. (modified from [80])
Genetic contribution to anxiety and fear
win studies suggest that the heritable risk of anxiety-related disorders is on the same order of magnitude as Depression and other moderately heritable psychiatric disorders [35–37]. The field of anxiety genetics is in the process of gathering sufficiently large sample sizes for properly powered, hypothesis-neutral, genome-wide association studies of anxiety and its specific subtypes. However, several ongoing large studies of traumatized civilian and military cohorts are beginning to reveal hopeful results that await replication [38,39]. Several studies have investigated the genetic basis of these disorders mostly using SNP analysis of candidate genes. For example, the BDNF (Val66Met) SNP has been associated with anxiety and fear-learning in both humans and rodents [40••, 41]. A recent study from our laboratory emphasized a role for the PACAP-PAC1 receptor in PTSD in females but not males [42••]. The observation that the SNP in the PAC1 receptor most strongly associated with PTSD symptoms resides in a predicted Estrogen Response Element (ERE) points to one potential mechanism for the predominance of PTSD in women. This finding was recently replicated in a separate analysis of physiological responses in children of traumatized patients [43], as well as in a separate cohort examining PTSD symptoms interacting with level of trauma exposure [44]. Notably, a separate independent study [45] conducted with sample populations completely different from those used in [42] did not find an association between the PAC1 gene and PTSD. Such experimental discrepancies emphasize how parameters like population samples, demographics, trauma exposure, and social conditions of the population profoundly influence genome-wide association studies (GWAS). Findings such as these caution us to interpret these GWAS studies strictly within the context of the sample population studied. Some other candidate genes implicated in anxiety- and fear-related disorders are FKBP5, COMT, CCK, and enes associated with the serotonergic system [reviewed in 46]. Given the complexity of anxiety- and fear-related disorders, it would be naïve to assume that a single or only a handful of genes might be involved in their etiology.
An epigenetic basis for anxiety and fear
Epigenetic marking of the genome that includes modification of histone proteins and DNA methylation, as well as non-coding RNA based gene targeting, all allow for post-transcriptional regulation of gene expression [47]. Post-natal stress in rats alters the epigenetic signature of the BDNF gene with this effect appearing to persist in subsequent generations [48•]. Noncoding RNAs such as miRNA have been shown to be involved in the extinction of learned fear in rodent models [49•]. While the importance of such epigenetic regulation of gene expression was first elucidated for its role in cancer, more studies are implicating this mechanism in neuropsychiatric disorders [50].
While the information provided thus far does not even begin to delve into the complexities of anxiety-and fear-related disorders, it serves as a skeleton for us to describe how currently used treatments for these disorders, as well as future treatments target biological entities that span from the connectome to the epigenome.
Therapeutic interventions for anxiety- and fear-related disorders
Brain stimulation and optogenetics
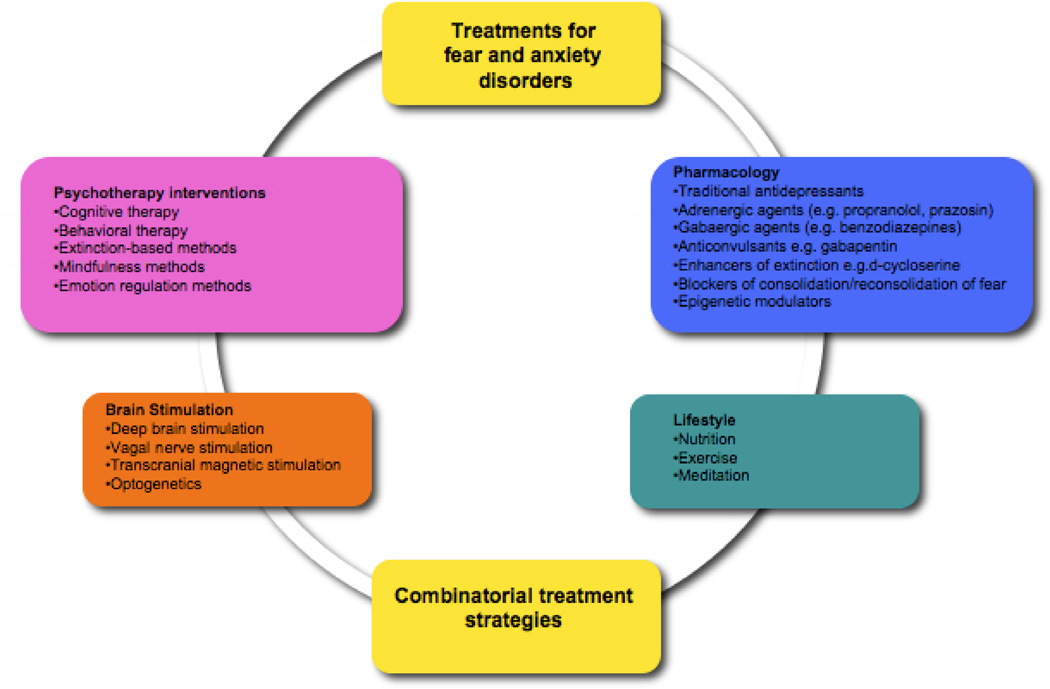
There are many approaches ranging from current therapy and psychopharmacological treatments to future combination and brain-stimulation approaches that hold promise in the treatment of anxiety (Figure 3). Imaging studies have provided evidence to suggest that alteration of brain activity and structure within and between neural circuits plays a critical role in the manifestation of neuropsychiatric disorders [51–53]. Manipulating this altered brain activity using diverse techniques is proving to be a useful therapeutic strategy. Electroconvulsive therapy (ECT), vagal nerve stimulation (VNS), transcranial magnetic stimulation (TMS), and deep brain stimulation (DBS) are some of the strategies used to manipulate neural circuit function [54••]. While they have historically been used in neurological disorders and to ameliorate depressive-like symptoms in Major Depressive Disorder (MDD), more recent studies emphasize their role in treating anxiety and fear [reviewed by 55••]. However, the main efficacy of these treatments in most of these studies lies in their reversing of co-morbid depression. Most recently more is becoming known about their reversal of the specific symptoms such as hyper-vigilance and arousal associated with anxiety, and cue-specific fear as in PTSD using rodent models [56]. Our current understanding of the mechanisms of action of these treatments speaks to their effect on the firing patterns of neuromodulatory systems in the brain such as the serotonergic and noradrenergic pathways [57]. While more challenging to specifically manipulate, future use of stimulation techniques would do well to affect the balance between excitatory (eg. glutamatergic) and inhibitory (eg. GABergic) drive within specific regions of the brain, which some think is the root cause of anxiety and fear [58].
Figure 3. Current and Promising New Approaches to Treatments for Anxiety and Fear-Related Disorders.
Currently utilized treatments include antidepressants (targeting serotonin, norepinephrine, and dopamine monoaminergic pathways), GABA-acting benzodiazepines, and beta-adrenergic receptor blockers, as well as cognitive-behavioral therapies. However, all of these treatments have limited efficacy, and new direct and combined treatments specifically targeting known neural pathways underlying fear and anxiety are on the horizon.
The recent use of controlling the firing of specific cell populations using light-based manipulation of ion channels termed “optogenetics” provides a glimpse into what the future manipulation and understanding of neural activity in neuropsychiatric illness might look like [59••]. From a basic science perspective, such approaches will give us a firmer appreciation for the specific molecular and neural circuitry involved in anxiety and fear. Further, it may allow us to refine brain coordinates that ought to be stimulated using the more traditionally used stimulation techniques. Treatments using an optogenetic strategy would first necessitate some manner by which to insert into specific brain regions ion channels that could eventually be manipulated by light. In this direction, new advances in bio-safety and bio-engineering ought to make this a reality in the future. For example, manipulation of neural firing using optogenetic methods has already shown to be effective in rodent and non-human primate models of anxiety, fear-learning and depression [60•, 61–63]. More specifically, stimulation of Basolateral Amygdala fibers that project to the Central Amygdala reduced anxiety-like behavior in mice [61]. Such optogenetic approaches allow for more targeted manipulation of neural activity and could potentially reduce side-effects that might arise from the general non-targeted manipulation of activity that is a concern of the currently used stimulation strategies.
Pharmacological intervention
The symptoms of anxiety and fear can be treated in animals and humans to varying degrees of success via administration of benzodiazepines, common antidepressant treatments, and a suite of molecules like buspirone and propanolol that affect serotonergic, dopaminergic and noradrenergic neuromodulation [eg. 26, 64,65]. In several instances chronic treatment is required for their therapeutic efficacy to come to the fore. With respect to reducing fear toward specific stimuli, recent success in both rodent and human models was achieved after the administration of a partial NMDA-receptor agonist, D-cycloserine (DCS) but only after the subjects had also been exposed to an extinction training regimen [27,66,67] (see combinatorial treatment regimens below). Looking to the future, the use of small molecules that cross the blood-brain-barrier and target end-points such as BDNF signaling hold promise [27,28,41,68]. Also, manipulating the epigenetic landscape in anxious and fearful states using drugs that interfere with histone modifications or DNA methylation (eg. HAT inhibitors, HDAC inhibitors, DNMT inhibitors) may provide a fruitful therapeutic strategy [69•, 70]. For example, in mice, overexpression of Histone DeAcetylase2 (HDAC2) has been shown to impair memory processes, with these impairments being reversed after administration of HDAC inhibitors [71].
Cognitive Behavioral Therapy (CBT) and Extinction-based intervention
Most of the success in treating anxiety- and fear-related disorders comes from behavioral therapies that typically involve some form of extinction-based methodology [72]. This could take the form of exposing the subject to the stimulus that triggers fear in the absence of the negative outcome, or talking about the general state of anxiousness. Another behavioral approach taps into the reconsolidation theory of memory that posits that every time a memory or trigger is recalled, it becomes labile and reconsolidated. Behavioral or pharmacological interventions at this crucial timepoint have been shown to interfere with this reconsolidation [73••, 74•, 75••, 76]. In this direction, virtual reality environments have been especially useful in exposing subjects to environmental triggers [77]. While their mechanisms of action are unknown, the efficacy of lifestyle interventions like diet, exercise, and meditation will undoubtedly come to bear as efficient therapeutic strategies in the treatment of anxiety and fear [78,79].
Combinatorial treatment regimens
Given the complexity that underlies disorders like anxiety and fear, it is unlikely that one single form of intervention will be completely successful. What will more likely be needed are treatment regimens that act across several levels. Support for this comes from studies like the one wherein subjects with an intense fear of heights reported less fear after being subjected to a combinatorial treatment regimen that was comprised of virtual-reality extinction-based therapy and DCS administration [27,28,67], as well as the potential mechanisms of combined reconsolidation and extinction [74•, 75••]. With the plethora of potential treatments listed above, the permutations and possibilities for combinatorial treatment are large indeed.
In summary, while a lot has been understood about anxiety and fear from animal models and human case studies, we are only beginning to peel away the layers of complexity associated with these orders. As this happens, we will not only understand new neural mechanisms that go awry in these disorders, but also gain new molecular, anatomical, and behavioral foci that can be targeted with advances in drug design, and technology. Until then a multi-pronged approach that combines basic research in animals, with early diagnosis and intervention in humans will enable these disorders to be treated in a more efficient manner. We believe that due to the translational understanding of fear, which is the basis of anxiety-related disorders, this set of psychiatric syndromes will be the earliest understood at a mechanistic level among common psychiatric illnesses. With current rapid progress in understanding of neural mechanisms of fear and emotion regulation, the future of new and promising approaches is indeed bright.
Highlights.
We highlight new therapies for fear and anxiety from the connectome to epigenome
Optogenetic approaches will potentially be useful to treat fear and anxiety
Drugs that modify the epigenetic landscape hold therapeutic promise
Lifestyle interventions (e.g. diet, exercise, meditation) should be considered
Combinatorial approaches hold the most promise to alleviate fear and anxiety
Acknowledgments
This work was supported through NIH ((MH071537 (KJR), MH096764(KJR)) as well as the Burroughs Wellcome Fund. Support was received from the NIH/National Center for Research Resources base grant P51RR000165 to Yerkes National Primate Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Ressler is a founding member of Extinction Pharmaceuticals/Therapade Technologies to develop D-cycloserine, a generically available compound, for use to augment the effectiveness of psychotherapy. He has received no equity or income from this relationship within the last 3 years.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 3.Kas MJ, Krishnan V, Gould TD, Collier DA, Olivier B, Lesch KP, Domenici E, Fuchs E, Gross C, Castren E. Advances in multidisciplinary and cross-species approaches to examine the neurobiology of psychiatric disorders. Eur Neuropsychopharmacol. 2011;21:532–544. doi: 10.1016/j.euroneuro.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 5.Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 7.Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- 8.Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 9.Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- 10.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Kalueff AV, Wheaton M, Murphy DL. What's wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav Brain Res. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- 13.Risbrough VB, Geyer MA. Anxiogenic treatments do not increase fear-potentiated startle in mice. Biol Psychiatry. 2005;57:33–43. doi: 10.1016/j.biopsych.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 14. LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. •• An exhaustive analysis of how to think about the neural basis of emotion.
- 15. Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. • An important review that presents operational definitions of anxiety and fear, while encapsulating the various methods to model them in an empirical setting in humans and animals.
- 16.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 17.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 18.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 19.Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010;107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 22.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology (Berl) 1986;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- 25.Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci U S A. 2012;109:16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 27.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Bontempo A, Panza KE, Bloch MH. D-cycloserine augmentation of behavioral therapy for the treatment of anxiety disorders: a meta-analysis. J Clin Psychiatry. 2012;73:533–537. doi: 10.4088/JCP.11r07356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 30.Bowers ME, Choi DC, Ressler KJ. Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiol Behav. 2012 doi: 10.1016/j.physbeh.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruehle S, Rey AA, Remmers F, Lutz B. The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol. 2012;26:23–39. doi: 10.1177/0269881111408958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J Neurosci. 2011;31:1802–1810. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu LJ, Kim SS, Zhuo M. Molecular targets of anxiety: from membrane to nucleus. Neurochem Res. 2008;33:1925–1932. doi: 10.1007/s11064-008-9679-8. [DOI] [PubMed] [Google Scholar]
- 35.Wolf EJ, Miller MW, Krueger RF, Lyons MJ, Tsuang MT, Koenen KC. Posttraumatic stress disorder and the genetic structure of comorbidity. J Abnorm Psychol. 2010;119:320–330. doi: 10.1037/a0019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 37.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 38.Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, Uddin M, Wildman D, Galea S, Koenen KC, Miller MW. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornelis MC, Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Curr Psychiatry Rep. 2010;12:313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxietyrelated behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. •• An example of translational research wherein a SNP in the BDNF gene was shown to be important for anxiety behavior in humans and rodents. Such an approach is gaining traction as a viable method to investigate the genetic basis of neuropsychiatric disorders.
- 41.Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. •• While the hormonal contribution to the predominance of PTSD in women is well known, this study provides a genetic framework within which to investigate this sex bias. The authors find a SNP within an Estrogen Response Element (ERE) of the Pac1 receptor gene to be associated with PTSD-like symptoms in females but not males.
- 43.Jovanovic T, Norrholm SD, Davis J, Mercer KB, Almli L, Nelson A, Cross D, Smith A, Ressler KJ, Bradley B. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uddin M, Chang SC, Zhang C, Ressler K, Mercer KB, Galea S, Keyes KM, McLaughlin KA, Wildman DE, Aiello AE, Koenen KC. Adcyap1r1 Genotype, Post Traumatic Stress Disorder, and Depression among women exposed to childhood maltreatment. Depress Anxiety. 2012 doi: 10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang SC, Xie P, Anton RF, De Vivo I, Farrer LA, Kranzler HR, Oslin D, Purcell SM, Roberts AL, Smoller JW, Uddin M, Gelernter J, Koenen KC. No association between ADCYAP1R1 and post-traumatic stress disorder in two independent samples. Mol Psychiatry. 2012;17:239–241. doi: 10.1038/mp.2011.118. [DOI] [PubMed] [Google Scholar]
- 46.Norrholm SD, Ressler KJ. Genetics of anxiety and trauma-related disorders. Neuroscience. 2009;164:272–287. doi: 10.1016/j.neuroscience.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graff J, Mansuy IM. Epigenetic codes in cognition and behaviour. Behav Brain Res. 2008;192:70–87. doi: 10.1016/j.bbr.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 48. Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. • With the importance of epigenetics on a variety of neuropsychiatric conditions becoming apparent, this study illustrates how the post-natal environment can profoundly affect following generations via epigenetic mechanisms.
- 49. Lin Q, Wei W, Coelho CM, Li X, Baker-Andresen D, Dudley K, Ratnu VS, Boskovic Z, Kobor MS, Sun YE, et al. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat Neurosci. 2011;14:1115–1117. doi: 10.1038/nn.2891. • This study is among the first to examine the role of a miRNA in a specific fear-related phenomenon.
- 50.Wang W, Kwon EJ, Tsai LH. MicroRNAs in learning, memory, and neurological diseases. Learn Mem. 2012;19:359–368. doi: 10.1101/lm.026492.112. [DOI] [PubMed] [Google Scholar]
- 51.Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, Ely T, Gutman DA, Ressler KJ. White Matter Integrity in Highly Traumatized Adults With and Without Post-Traumatic Stress Disorder. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 54. Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. •• Using depression as a disorder most commonly treated using a variety of techniques, this review portends the use of these same techniques to treat anxiety disorders.
- 55. Novakovic V, Sher L, Lapidus KA, Mindes J, J AG, Yehuda R. Brain stimulation in posttraumatic stress disorder. Eur J Psychotraumatol. 2011;2 doi: 10.3402/ejpt.v2i0.5609. •• A comprehensive review on how we think about and use brain stimulation techniques for the treatment of PTSD.
- 56.Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci U S A. 2012;109:8764–8769. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 58.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. •• This is among the first papers to utilize an opotogenetic approach to control activity in the nervous system. Subsequent literature has built on this work to make this approach more modular, efficient, and amenable to in vivo use.
- 60. Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14:387–397. doi: 10.1038/nn.2749. • Approaches outlined herein present a glimpse into what the future application of optogenetics in non-human primates and potentially humans might look like.
- 61.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci U S A. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips MA, Langley RW, Bradshaw CM, Szabadi E. The effects of some antidepressant drugs on prepulse inhibition of the acoustic startle (eyeblink) response and the N1/P2 auditory evoked response in man. J Psychopharmacol. 2000;14:40–45. doi: 10.1177/026988110001400105. [DOI] [PubMed] [Google Scholar]
- 65.Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, Marmar CR. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 66.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J. Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 68.Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry. 2011;168:163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. • Most of our knowledge about the role of epigenetics in human disease come from the field of cancer (epi)genomics. Researchers investigating the epigenetic basis of neuropsychiatric disorders and consequent intervention would do well to follow this literature and mimic any therapeutic agents emanating from this field.
- 70.Dekker FJ, Haisma HJ. Histone acetyl transferases as emerging drug targets. Drug Discov Today. 2009;14:942–948. doi: 10.1016/j.drudis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 71.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 73. Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. •• A paper that resurrected the concept of memories being labile once they are retrieved so that their subsequent reconsolidation could be interfered with using protein synthesis inhibitors.
- 74. Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. • These findings in rodents suggest the importance of interfering with reconsolidation of memories to diminish the persistence of fear memories and paved the way for subsequent work in this realm (eg. Schiller et al.).
- 75. Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. •• Among the first manuscripts to affect fear-learning in humans by interfering with reconsolidation.
- 76.Przybyslawski J, Sara SJ. Reconsolidation of memory after its reactivation. Behav Brain Res. 1997;84:241–246. doi: 10.1016/s0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- 77.de Carvalho MR, Freire RC, Nardi AE. Virtual reality as a mechanism for exposure therapy. World J Biol Psychiatry. 2010;11:220–230. doi: 10.3109/15622970802575985. [DOI] [PubMed] [Google Scholar]
- 78.Manger TA, Motta RW. The impact of an exercise program on posttraumatic stress disorder, anxiety, and depression. International Journal of Emergency Mental Health. 2005;7:49–87. [PubMed] [Google Scholar]
- 79.Sarris J, Moylan S, Camfield DA, Pase MP, Mischoulon D, Berk M, Jacka FN, Schweitzer I. Complementary medicine, exercise, meditation, diet, and lifestyle modification for anxiety disorders: a review of current evidence. Evid Based Complement Alternat Med. 2012;809653 doi: 10.1155/2012/809653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]