Abstract
Senna is a pod or leaf of Senna alexandrina P. Mill and is used as a stimulant laxative. In the large intestine, bacterial enzymes break sennosides and release rhein-9-anthrone, the active form for the laxative effect. To determine potential toxic effects of senna, a 5-week dose range finding study in the C57BL/6N mouse and a 40-week toxicology and carcinogenesis study in the C3B6.129F1-Trp53tm1Brd N12 haploinsufficient (p53+/−) mouse were conducted. In the 5-week study, C57BL/6N mice were exposed up to 10,000 ppm senna in feed. Increased incidences of epithelial hyperplasia of the cecum and colon were observed in males and females exposed to 5,000 or 10,000 ppm senna. These intestinal lesions were not considered to be of sufficient severity to cause mortality and, thus, in the p53+/− mouse 40-week study, the high dose of 10,000 ppm was selected. Significant increases in the incidences of epithelial hyperplasia of the colon and cecum were observed at 10,000 ppm in p53(+/−) males and females, and the incidence of hyperplasia of the colon was significantly increased at 3,000 ppm in females. In conclusion, the large intestine was the major target of senna-induced toxicity in both wild-type and the p53+/− mouse model. There was no neoplastic change, when senna was administered to p53 +/− mouse.
Keywords: Senna, Trp53, carcinogenesis, toxicity, laxative, large intestine
Introduction
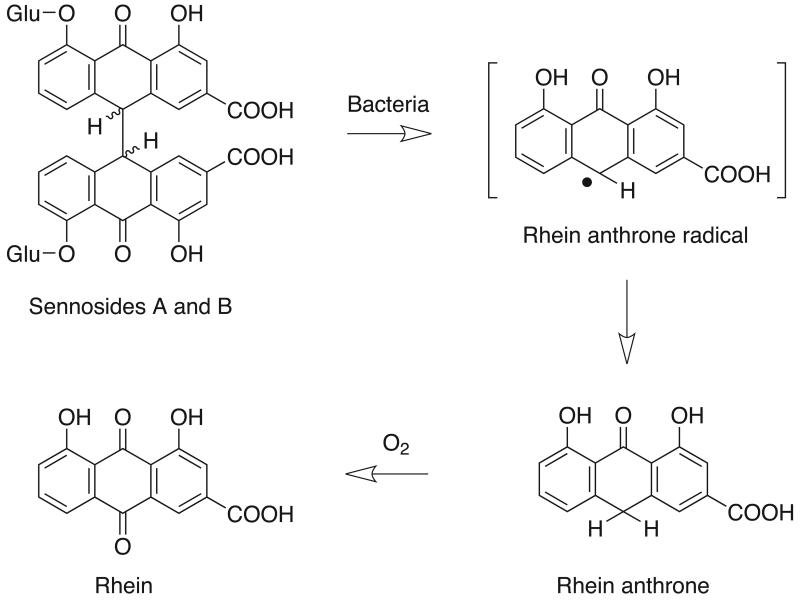
Senna is a pod or leaf of Senna alexandrina P. Mill (Leguminosae) (Blumenthal, 2000) and is available as over-the-counter laxatives and tea. It is widely used as a stimulant laxative for children and adults, including pregnant women (Smitherman et al., 2005, Shah et al., 2008, Moussally et al., 2009). In addition, a high dose of senna (3~13 times higher than recommended dose) is used for cleansing the bowel in preparation for a colonoscopy or surgery (Kleibeuker et al., 1995, Occhipinti and Di Palma, 2009). The senna plant contains a multitude of chemical components including dianthrones, anthraquinones, naphthalene derivatives, and flavonoids (Franz, 1993). The pharmacological activity of senna is associated with the formation of rhein-9-anthrone (rhein anthrone) from sennosides (Figure 1). The sennosides are not readily absorbed from the mammalian gut, due to the presence of a β-glycosidic bond but once they reach the large intestine they are deconjugated and reduced to the active form (rhein anthrone) by microflora (Lemli and Lemmens, 1980, Dreessen et al., 1981). The laxative effect is driven by increasing peristalsis and reduced absorption of water and electrolytes (Leng-Peschlow, 1986).
Figure 1.
Reduction of sennosides A and B to pharmacologically-active rhein anthrone by gut microflora and oxidation to rhein (Adapted from Lemli and Lemmons, 1980 and Dressen et al., 1981). Glu=glucose
Most of the published genotoxicity studies with senna reported negative results, but some positive results for senna extracts were reported in bacterial gene mutation test systems as well as in mammalian cells in vitro (Brusick and Mengs, 1997). In vivo, results of a single study assessing chromosomal damage following treatment with senna extract were negative (Mengs et al., 1999). Sennosides, which are major constituents of senna, were generally negative in in vitro bacterial mutagenicity tests (Mengs, 1988, Sandnes et al., 1992, Heidemann et al., 1993, Heidemann et al., 1996) and in vitro mammalian cell mutagenicity and chromosomal damage assays (Mengs, 1988, Sandnes et al., 1992, Heidemann et al., 1993). Results of chromosomal damage tests in mice yielded mixed results, with one study showing no effect in mice after treatment with mixed sennosides (Mengs, 1988, Heidemann et al., 1993) and another showing a small dose-related (P<0.05) increase in structural chromosomal aberrations in bone marrow cells of mice treated with sennoside B (Mukhopadhyay et al., 1998). Conflicting results in genotoxicity test with rhein, a component as well as a metabolite of senna, were observed in vitro as well as in vivo (Westendorf et al., 1990, Sandnes et al., 1992, Heidemann et al., 1993, Mengs and Heidemann, 1993, Mukhopadhyay et al., 1998, Makena and Chung, 2007). Components of certain senna products, particularly the emodin and aloe-emodin compounds, have been shown to be genotoxic in vitro (Brown and Dietrich, 1979, Westendorf et al., 1990, Kawasaki et al., 1992, Sandnes et al., 1992, Heidemann et al., 1993, Mueller et al., 1998, Nesslany et al., 2009).
Toxicity of senna has been reported in literature. Sub-acute exposure to senna (10% senna in diet) resulted in signs of liver and kidney toxicity in rats (Al-Yahya et al., 2002). In a sub-chronic study, reversible hyperplastic changes in the forestomach and the large intestine have been observed in rats (Mengs et al., 2004). In human, case studies of hepatitis and allergic reactions associated with excessive use of senna have been reported (Beuers et al., 1991, Marks et al., 1991, Helin and Makinen-Kiljunen, 1996, Spiller et al., 2003, Seybold et al., 2004, Vanderperren et al., 2005). Sennosides increased colonic epithelial cell proliferation in humans (Kleibeuker et al., 1995, van Gorkom et al., 2000).
Studies reported in the literature suggest that there may be an association between laxative use and colon cancer in humans (Siegers et al., 1993b, Satia et al., 2009). For example, findings from an epidemiology study revealed that more patients with gastrointestinal cancer were senna users than patients without cancer and patients without gastrointestinal disease (Boyd and Doll, 1954). However, the relationship between senna use and colon cancer has not been clearly demonstrated. Experimental animal models have not been able to resolve this association between senna use and cancer. Several studies showed senna or sennosides did not affect incidences of neoplastic lesions (Lyden-Sokolowski et al., 1993, Siegers et al., 1993a, Borrelli et al., 2005, Mitchell et al., 2006). However, in literature senna extract or sennosides have been shown to increase tumor formation (Mereto et al., 1996, Mascolo et al., 1999).
The p53 tumor suppressor gene suppresses cancer in both humans and mice. The p53 protein is critical to cell cycle control, DNA repair and apoptosis, etc., and is often mutated or lost in human and rodent cancers (Soussi and Beroud, 2001, Zuckerman et al., 2009). The haploinsufficient Trp53 tumor suppressor gene mouse model heterozygous for a wildtype and null (+/−) Trp53 alleles (Donehower et al., 1992, Donehower et al., 1995) was used in these studies. This Trp53 protein haploinsufficient mouse model has been extensively tested as a short-term cancer bioassay mouse model (Tennant et al., 1995, Dunnick et al., 1997, French et al., 2001a, French et al., 2001b, Pritchard et al., 2003, French, 2004) based upon the observation that mice with only a single wildtype Trp53 allele show a significant decrease in the time required for genotoxic carcinogen-induced tumors to develop. These tumors are often associated with either a mutation and/or a loss of heterozygosity of the remaining wildtype Trp53 allele. Few to no sporadic tumors occur in concurrent or historical study control groups in this model, which allows tests to be conducted with fewer animals and direct analysis of the target wildtype Trp53 allele to test for genotoxicity in vivo as a mode of action. Genetically-altered mouse models have been shown to be useful for detecting carcinogenic activity of genotoxic chemicals (Pritchard et al., 2003), which is the basis for use of an alternative mouse model. In addition, the B6129-Trp53tm1Brd haploinsufiicient model has tested positive for colorectal tumors induced by azoxymethane and dimethylhydrazine (Lubet et al., 2000, Hu et al., 2005, Mladenova et al., 2011).
Laxative use is widespread in the United States and there is concern about potential toxicity from long-term use, including commonly used products containing senna (U. S. Food and Drug Administration, 1999). Additional characterization of the toxic potential of senna is needed for risk assessment in humans. Therefore, we conducted toxicological and carcinogenicity studies of senna in the C3B6.129F1-Trp53tm1Brd haploinsufficient mouse model.
Materials and Methods
Chemicals
Senna was obtained from Madaus AG (Koln, Germany) in one lot (1999000). Senna was identified by chromatographic comparisons to purchased reference standards using two high-performance liquid chromatography with ultraviolet detection (HPLC/UV) analytical systems. Weight percentages of active components were estimated: 0.7% sennoside A, 1.3% sennoside B, 0.06% sennidin A, and 0.03% sennidin B. The senna was sterilized by exposure to cobalt-60 ionizing radiation. Comparison of the irradiated material with the non-irradiated material indicated that all 10 major peaks present in the non-irradiated material were present in the irradiated material at a relative concentration of ≥ 90.7%. Examination of the chromatograms before and after irradiation indicated no differences in significant peaks.
Animals
Male and female C57BL/6NTac mice as well as male and female C3B6.129F1-Trp53tm1Brd N12 haploinsufficient (p53 +/−) mice, heterozygous for a wildtype and null allele, were obtained from Taconic Farms, Inc. (Germantown, NY). Animals were quarantined for at least 6 days. Mice were housed individually. NTP-2000 diet (Zeigler Brothers, Inc. Gardners, PA) and water were available for ad libitum consumption. The care of animals in this study was according to NIH procedures as described in the “The U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals” (NIH, 2002).
An outcross between C3H/HeNTac (C3, Taconic Model P53N12-M) female mice homozygous for the wildtype Trp53 allele and the congenic C57BL/6.129Sv-Trp53tm1Brd N12 (abbreviated B6.129-Trp53tm1Brd) N12 (Model P53N12-M) backcross generation males (homozygous for the Trp53 null allele) produced congenic C3B6.129F1-Trp53Tm1Brd N12 progeny, Taconic Laboratory Animals and Sciences (Germantown, NY). The N12 backcross generation was selected in order to reduce tumor penetrance variability observed in the N5 generation model used in the ILSI/HESI alternative mouse model studies (unpublished result/John E. French, NIEHS/NTP). Trp53 null mutation was introduced by homologous recombination in AB1 murine embryonic stem cells, which were derived from a black agouti 129Sv inbred mouse. By targeted insertion of a polII neo cassette, an engineered null mutation was induced as a result of the deletion of a 450-base pair gene fragment from the Trp53 gene that included 106 nucleotides of exon 5 and approximately 350 nucleotides of intron 4 that eliminated both mRNA and p53 protein expression from this allele (Donehower et al., 1992, Donehower et al., 1995).
Five-week study
Groups of 5 male and 5 female C57BL/6NTac mice at 6-7 weeks of age were used. NTP – 2000 diets containing 0, 625, 1,250, 2,500, 5,000, or 10,000 ppm senna were provided for ad libitum consumption for 29 days. Feed consumption was recorded weekly by animal. Clinical findings were recorded weekly. The animals were weighed initially, weekly, and at the end of the study. At the end of the study, carbon dioxide was used to euthanize animals. At necropsy, organ weights were recorded for the heart, right kidney, liver, lung, right testis, and thymus. Histopathologic examinations were performed on all mice.
Forty-week study
Groups of 25 male and 25 female P53 +/− mice at 6-8 weeks of age were used. NTP-2000 diets containing 0, 100, 300, 1,000, 3,000 or 10,000 ppm senna were provided for ad libitum consumption for 40 weeks. Feed consumption was recorded weekly by animal. Clinical findings were recorded weekly. The animals were weighed initially, weekly, and at the end of the study. At the end of the study, carbon dioxide was used to euthanize animals. At necropsy, all organs were fixed in 10% neutral buffered formalin, processed, and stained with hematoxylin and eosin (H&E) for histological examination.
Pathology
Hyperplasia of the large intestinal mucosa was graded similarly in the 5-week and 40-week studies. In the colon, normal crypt depth in controls was up to 175 microns. Colonic hyperplasia was graded based upon crypt depth, as follows: in minimal (grade 1) hyperplasia, crypt depth was approximately 200 microns (from basement membrane to surface of crypt epithelium, measured where an entire crypt was in longitudinal section), but only focally or multifocally; in mild (grade 2) hyperplasia, crypt depth was ≥ 200 microns over much of the circumference, and was focally approximately 300 to 350 microns; in moderate (grade 3) hyperplasia almost the entire circumference of the section examined was involved with crypts 300 to 350 microns deep. The same criteria were used for the cecum, but due to the naturally thicker mucosa found in the rectum, severity grading for the rectum was done more subjectively.
Statistical analysis
The Fisher exact test was used to determine statistical differences in incidences of lesions between treatment groups.
Results
5-week study in C57BL/6NTac mice
A 5-week study was designed to investigate toxicological effects of senna in C57BL/6NTac mice. All mice survived to the end of the study. Final mean body weights of all exposed groups were within 5% of the controls. Feed consumption by exposed groups was similar to that by the controls. Concentrations of 625, 1,250, 2,500, 5,000, and 10,000 ppm senna in feed resulted in average daily doses of 115, 245, 490, 975, and 2,075 mg senna/kg body weight to males and 160, 310, 625, 1,190, and 2,570 mg/kg to females. There were no apparent treatment-related clinical findings. Significant decreases in absolute heart weights occurred in all exposed groups of males. Relative heart weights were significantly decreased in 625, 5,000, and 10,000 ppm males.
Exposure to senna resulted in epithelial hyperplasia of the cecum and colon in males and of the cecum, colon, and rectum in females (Table 1 and Figure 2). A no-observed-effect level (NOEL) could not be determined for the male mice because the 625 ppm group had two mice with minimal hyperplasia of the cecum, and one animal in the 1,250 ppm group had hyperplasia of the cecum and colon. The NOEL for female mice was 1,250 ppm, based upon the lack of lesions in that group and the presence of two animals in the 2,500 ppm group with cecal hyperplasia and one with hyperplasia of the colon. The hyperplasia was characterized by a thickening of the mucosa by increased depth of the crypts (Figure 2). This was due to increased numbers of epithelial cells, which were small, with basophilic cytoplasm and round to oval nuclei, which became more vesicular toward the luminal surface of the glands. Goblet cells were interspersed within these cells, but did not constitute an increased proportion of the epithelial cells in the colon or cecum. Mitotic figures were numerous.
Table 1.
Final mean body weight, and incidences of hyperplasia in the large intestine in C57BL/6NTac mice in the 5-week feed study of senna.
| Dose (ppm) |
Final weight relative to controls (%) |
Heart Weight | Cecum |
Colon |
Rectum |
|||
|---|---|---|---|---|---|---|---|---|
| Epithelial hyperplasia |
Epithelial hyperplasia |
Epithelial hyperplasia |
||||||
| Male | ||||||||
| 0 | 0.15±0.01 | 0a | 0 | |||||
| 625 | 99 | 0.13±0.00& | 2 | (1.0)b | 0 | |||
| 1,250 | 96 | 0.13±0.01& | 1 | (1.0) | 1 | (1.0) | ||
| 2,500 | 99 | 0.13±0.01& | 1 | (1.0) | 1 | (1.0) | ||
| 5,000 | 99 | 0.13±0.01& | 3 | (1.0) | 4* | (1.3) | ||
| 10,000 | 96 | 0.12±0.00& | 5** | (1.8) | 5** | (2.0) | ||
|
| ||||||||
| Female | ||||||||
| 0 | 0.12±0.00 | 0 | 0 | 0 | ||||
| 625 | 95 | 0.12±0.01 | 0 | 0 | 0 | |||
| 1,250 | 99 | 0.12±0.00 | 0 | 0 | 0 | |||
| 2,500 | 100 | 0.13±0.01 | 2 | (1.0) | 1 | (1.0) | 0 | |
| 5,000 | 94 | 0.13±0.01 | 4* | (1.0) | 5** | (1.4) | 0 | |
| 10,000 | 96 | 0.11±0.00 | 5** | (1.8) | 5** | (3.0) | 3 | (1.0) |
N=5
Significantly different (P≤0.01) from the control group by William’s or Dunnett’s test
Significantly different (P≤0.05) from the control group by the Fisher exact test
P≤0.01
Number of animals with lesion
Average severity grade of lesions in affected animals: 1=minimal, 2=mild, 3=moderate, 4=marked
Figure 2.
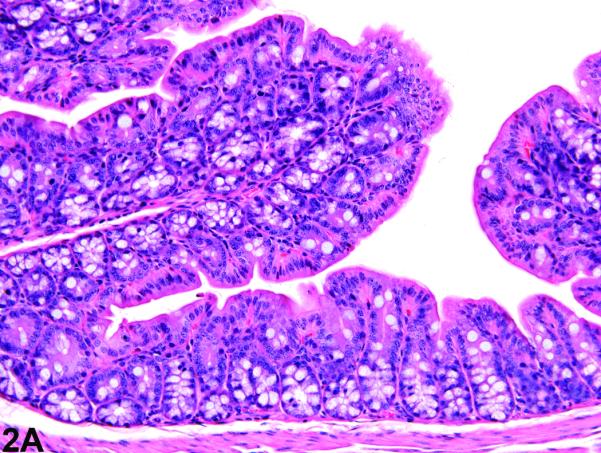
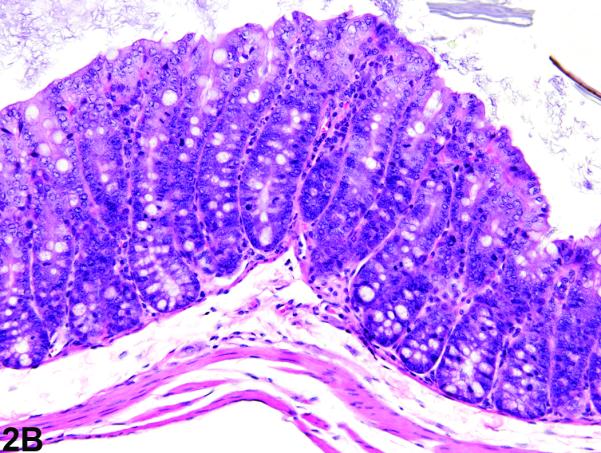
Colon from the 5 week study of senna. A, a section of colon from a control female mouse. 25X, H&E. B, a section of colon with moderate hyperplasia from a female mouse exposed to 10,000 ppm senna. The depths of the crypts and the number of epithelial cells are increased, as evidenced by crowding, increased basophilia, and increased mitotic figures. 25X, H&E.
40-week study in p53+/− mice
A 40-week study was conducted to investigate the toxic and/or carcinogenic potential of senna in p53+/− mice. In the 5 week study in C57BL/6NTac mice, no effect on survival and body weights was observed. In addition, intestinal lesions were not considered to be of a sufficient severity to cause mortality in a 40-week study in p53+/− mice. Therefore, the high dose of 10,000 ppm was selected. Due to increased sensitivity in p53+/− mice and increased duration of exposure, a broader range of exposure (0, 100, 300, 1,000, 3,000, 10,000 ppm) was selected for the 40 week study.
Survival of all exposed groups was similar to that of the control groups. Mean body weights of exposed male and female mice were within 10% of those of the controls throughout the study. Feed consumption by exposed mice was generally similar to that by the controls throughout the study. Concentrations of 100, 300, 1,000, 3,000, and 10,000 ppm in feed resulted in average daily doses of 12, 36, 120, 365, and 1,260 mg/kg to males and 14, 42, 140, 435, and 1,520 mg/kg to females. There were no apparent exposure-related clinical findings. The absolute and relative liver weights of 10,000 ppm males were significantly less than those of the controls.
Significant increases in the incidences of epithelial hyperplasia of the colon and cecum occurred in 10,000 ppm males and females, and the incidence of hyperplasia of the colon was significantly increased in 3,000 ppm females (Table 2, Figures 3 and 4). Three 3,000 ppm males had epithelial hyperplasia of the colon and single occurrences of epithelial hyperplasia of the rectum were recorded in the 10,000 ppm males and females; although lacking statistical significance, these lesions are considered biologically noteworthy. The changes were characterized by a thickening of the mucosa by a lengthening of the crypts (Figure 3 and 4). This was due to increased numbers of epithelial cells that appeared crowded together. These cells were small and had basophilic cytoplasm and round to oval nuclei, which became more vesicular toward the luminal surface of the glands. Goblet cells were interspersed with these cells, but did not constitute an increased proportion of the epithelial cells in the colon or cecum. Mitotic figures were numerous. The cecum was usually less severely affected than the colon.
Table 2.
Incidences of hyperplasia in the large intestine in heterozygous F1 p53+/− mice in the 40-week feed study of senna.
| Dose (ppm) |
Survivala | Final weight relative to controls (%) |
Cecum | Colon | Rectum | |||
|---|---|---|---|---|---|---|---|---|
| Epithelial hyperplasia |
Epithelial hyperplasia |
Epithelial hyperplasia |
||||||
| Male | ||||||||
| 0 | 25/25 | 0/25b | 0/25 | 0/24 | ||||
| 100 | 24/25 | 101 | 0/25 | 0/25 | 0/25 | |||
| 300 | 25/25 | 104 | 0/25 | 0/25 | 0/25 | |||
| 1,000 | 25/25 | 98 | 0/25 | 0/25 | 0/25 | |||
| 3,000 | 23/25 | 101 | 0/23 | 3/24 | (1.3) | 0/24 | ||
| 10,000 | 24/25 | 95 | 22/25* | (1.4)c | 25/25* | (2.8) | 1/25 | (2.0) |
|
| ||||||||
| Female | ||||||||
| 0 | 23/25 | 0/25 | 0/25 | 0/25 | ||||
| 100 | 23/25 | 99 | 0/25 | 0/25 | 0/25 | |||
| 300 | 23/25 | 96 | 0/25 | 0/25 | 0/25 | |||
| 1,000 | 22/25 | 99 | 0/25 | 0/25 | 0/25 | |||
| 3,000 | 22/25 | 97 | 0/25 | 7/25* | (1.0) | 0/25 | ||
| 10,000 | 24/25 | 93 | 19/25* | (1.3) | 25/25* | (2.7) | 1/25 | (1.0) |
Significantly different (P≤0.01) from the control group by the Fisher exact test
Number of animals surviving at 40 weeks/number initially in group
Number of animals with lesion
Average severity grade of lesions in affected animals: 1=minimal, 2=mild, 3=moderate, 4=marked
Figure 3.
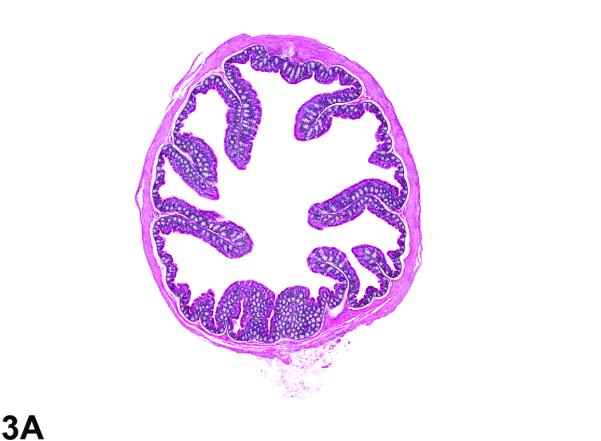
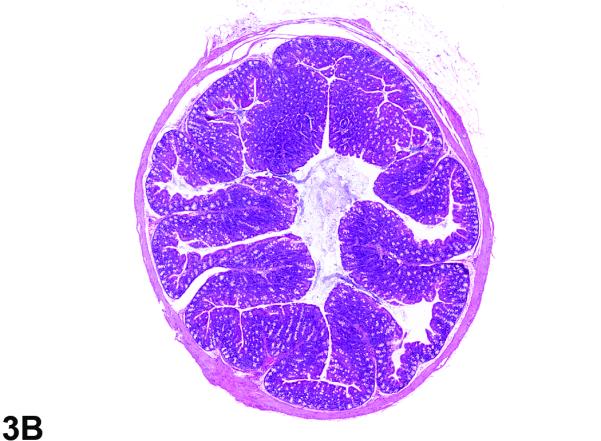
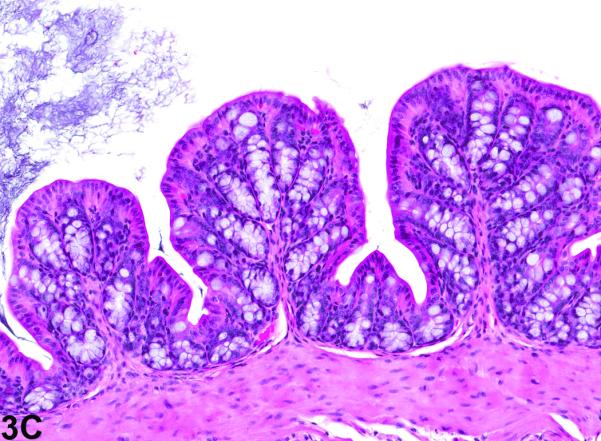
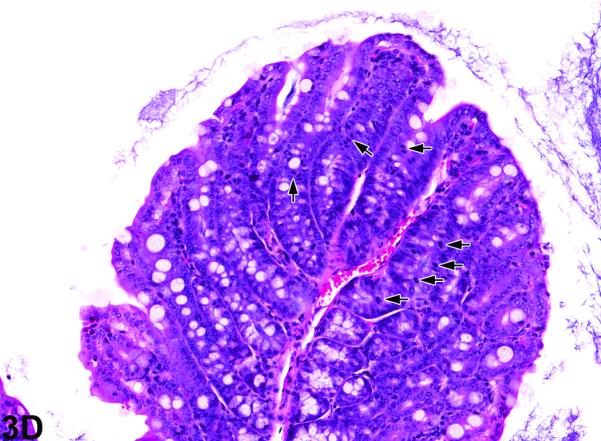
Colon from the 40-week study of senna. A, a cross section of the colon from a control male mouse. 3X, H&E. B, a cross section of the colon from a male F1 p53+/− mouse exposed to 10,000 ppm. There is moderate epithelial hyperplasia, with diffuse thickening of the epithelium. 3X, H&E. C, Higher magnification of colonic epithelium from a control male mouse. 20X, H&E. D, a section of colon from a male mouse exposed to 10,000 ppm senna. The epithelium is thickened as evidenced by increased distance from the luminal surface to the base of the crypts. Epithelial cells are increased in number and are basophilic and crowded, the cells lack the orderly arrangement seen in the control mouse, and there are numerous mitotic figures (arrows). 20X, H&E.
Figure 4.
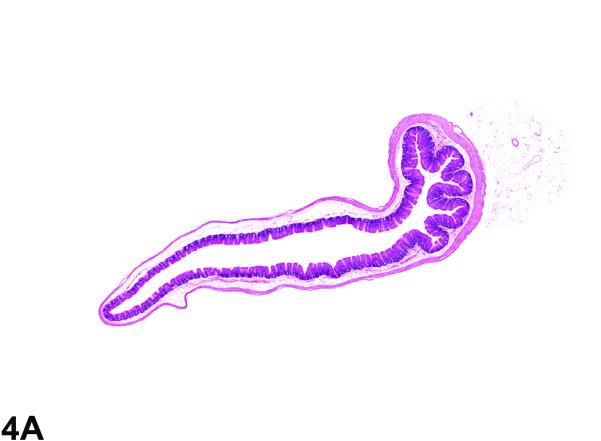
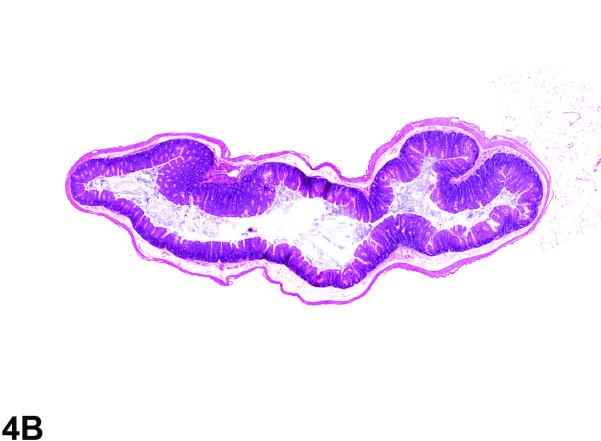
Cecum from the 40-week study of senna. A, Low magnification of the cecum from a control male. 2.3X, H&E. B, cecum from a male mouse exposed to 10,000 ppm senna. There is moderate hyperplasia; the epithelium of the cecum is diffusely thickened in the treated animal when compared to the control animal. 2.3X, H&E.
Osteosarcomas occurred in 1,000 and 3,000 ppm males but not in the control group; the incidences in these groups were within the historical control range (Table 3). In 1,000 ppm females, the incidence of osteosarcoma exceeded the historical control range by one tumor, but was not significantly greater than the incidence in the concurrent control group. Osteosarcomas in the mice were found in various sites, including femur (1 male and 1 female), maxilla (4 males and 2 females), rib (1 female), tibia (1 female), vertebra (1 male and 4 females), and unspecified (1 female). The incidence of hepatocellular adenoma in 300 ppm males was increased, which exceeded the historical control range, but not significantly (Table 3). The biological relevance of these findings is unknown.
Table 3.
Incidences of osteosarcoma and hepatocellular adenoma in heterozygous F1p53+/− mice in the 40-week feed study of senna
| Dose (ppm) |
Osteosarcoma | Hepatocellular Adenoma |
|---|---|---|
| Male | ||
| 0 | 0a | 1c |
| 100 | 0 | 0 |
| 300 | 0 | 5 |
| 1,000 | 3 | 0 |
| 3,000 | 2 | 2 |
| 10,000 | 0 | 1 |
|
| ||
| Female | ||
| 0 | 2b | 0 |
| 100 | 2 | 0 |
| 300 | 1 | 0 |
| 1,000 | 3 | 0 |
| 3,000 | 0 | 0 |
| 10,000 | 2 | 0 |
N=25
historical incidence for control groups in 9-month heterozygous F1 p53+/− mouse studies:5/77, range 0-12%
historical incidence for control groups in 9-month heterozygous F1 p53+/− mouse studies:3/76, range 0-8%
historical incidence for control groups in 9-month heterozygous F1 p53+/− mouse studies:5/76, range 4-11.5%
Discussion
The daily senna exposure concentrations in the current studies overlapped with daily exposures in humans taking senna in laxative preparations. In the 40-week study, based on the percent total weight values for sennosides A and B (approximately 2% of senna by weight), the sennosides A and B consumption was approximately 0.24, 0.72, 2.4, 7.3, or 25.4 mg/kg or 0.72, 2.2, 7.2, 21.9, or 76.1 mg/m3 (on a body surface area basis) per day (Freireich et al., 1966). Senna consumption by females was similar to that by males. For a typical over-the-counter senna laxative preparation taken twice daily, the amount of sennosides consumed is approximately 1.4 mg/kg per day or 51.8 mg/m3 (body surface area basis) per day (Novartis, 2010). In some cases, laxatives may be taken in greater than recommended amounts (Cance et al., 2005, Bryant-Waugh et al., 2006, Mond et al., 2006, Tozzi et al., 2006).
Senna had a laxative effect in rats receiving daily doses of 300 mg/kg or greater by oral gavage for 13 consecutive weeks (Mengs et al., 2004). Also, senna extract or sennosides had laxative effect in rats (Lyden-Sokolowski et al., 1993, Toyoda et al., 1994, Mascolo et al., 1999). However, in the current studies no laxative effect was observed at comparable or higher doses in mice, possibly indicating species-dependent differences in sensitivity or kinetics in formation of rhein anthrone, primarily responsible for the purgative activity of senna (Leng-Peschlow, 1992, Yamauchi et al., 1993). Additionally, a vehicle effect cannot be ruled out due to possible interaction of sennoside-derived species with the feed particles. Rhein anthrone is formed by intestinal bacteria at the site of action and is purportedly metabolized through a free radical (Lemli and Lemmens, 1980, Dreessen et al., 1981, Lemli, 1988), a possible mechanism in the formation of the intestinal hyperplastic response observed at the high dose in the current studies.
Significant decrease in heart weights was observed from 5 week study. However, no gross lesions were found from heart tissues. In 40-week study, there was no change in heart weights or no treatment related lesions in the heart. Therefore, decrease in heart weight was not considered treatment related. In the 40-week study, there were changes recorded in the liver weights (reduction in the mean absolute and relative liver weight in the 10,000 ppm males and an increase in the relative liver weight in the 10,000 ppm females), but the incidences of chronic active inflammation, or any other lesion in the liver, could not explain the liver weight changes.
There were increases in the incidences of osteosarcoma in 1,000 and 3,000 males. However, the incidences were within historical control range. Increased incidence of osteosarcoma in 1,000 females was not significantly increased but greater than historical control range. Increased incidence of hepatocellular adenoma in 300 ppm males exceeded the historical control range but not significantly. Therefore, increased incidences of osteosarcoma and hepatocellular adenoma were not considered as treatment related finding.
Treatment-related epithelial hyperplasia in the large intestine occurred in both the 5-week senna study in C57BL/6NTac mice and in the 40-week senna study in p53+/− mice. These results are consistent with other rodent studies. In rats receiving up to 0.05% sennoside A in feed for 7 days, a dose dependent increase in proliferation index in the large intestine (Cecum and colorectum) was observed (Toyoda et al., 1994). However, in contrast to our results, inflammatory changes (neutrophilic leukocytes infiltration and crypt abscess formation) were observed in that study. In rats receiving up to 1500 mg/kg senna for 13 weeks, minimal to slight hyperplastic changes in the mucosa of the large intestine in rats receiving more than 100 mg/kg of senna were observed. These hyperplastic lesions were not observed after 8 weeks of recovery period (Mengs et al., 2004). Also, in rats receiving 25, 100, or 300 mg/kg senna for 2 years, dose-dependent, minimal to slight mucosal epithelial hyperplasia of the large intestine (colon and cecum) was observed (Mitchell et al., 2006). In addition, in human, 2 mg/kg sennoside A and B administration increased the proliferative index in the large intestine (cecum, colon and rectum) (van Gorkom et al., 2000).
In agreement with our results, exposure to senna, senna extract or sennosides to rodents in previous studies was not associated with tumor formation. Mitchell et al. (Mitchell et al., 2006) conducted a 2-year oral gavage study of senna in Sprague-Dawley rats (0, 25, 100, or 300 mg/kg per day). There were no treatment-related carcinogenic effects in any target organ and there was only a slight intestinal hyperplasia in the treated groups in that study. In another 2-year study in Sprague-Dawley rats administered a senna extract (reported to contain up to 35% to 42% sennosides) in drinking water (to deliver 0, 5, 15, or 25 mg/kg per day of the test article), no intestinal lesions were observed in treated or control groups (Lyden-Sokolowski et al., 1993). Also, administration of 30 or 60 mg/kg senna extract by oral gavage (six times per week) for 110 weeks in male Wistar rats did not induce aberrant crypt foci or tumors (Borrelli et al., 2005). Mascolo et al. (Mascolo et al., 1999) showed that a laxative-producing dose of senna pod extract (10 mg/kg) administered to male Wistar rats for 13 to 28 weeks did not promote the formation of tumors induced by azoxymethane. In addition, a diet containing 0.03% sennoside (86% sennosides) fed to male NMRI mice for 20 weeks with concurrent administration of dimethylhydrazine (20 mg/kg, subcutaneous) for 10 weeks did not promote formation of colorectal tumors (Siegers et al., 1993a). The numbers and multiplicities of aberrant crypt foci were not affected in dimethylhydrazine-treated male Sprague-Dawley rats fed a 0.1% sennoside-containing diet (Mereto et al., 1996).
Results of NTP bacterial mutagenicity tests with senna and some of its constituents (sennosides, rhein, chrysophanic acid) were mixed, with some samples giving negative results and others, including rhein and the senna sample used in the 40-week mouse study giving positive results (NTP, 2011). Despite the observed activity with some of these compounds in vitro, results of the in vivo mouse peripheral blood micronucleus test with senna were negative, a result that is consistent with the results of the 40-week bioassay (NTP, 2011).
In contrast to our result, a diarrhea-producing dose (100 mg/kg, by gavage) of senna pod extract administered for 13 weeks increased the incidence and multiplicity of tumors induced by azoxymethane (Mascolo et al., 1999). In addition, Sprague-Dawley rats fed a diarrhea-inducing level of sennoside in the diet (0.2%) exhibited a significantly increased number of crypts/foci compared to controls (Mereto et al., 1996). However, oral administration of 30 or 60 mg/kg senna pod extract for 2 years to male Wistar rats treated with the initiating agent azoxymethane (7.5 mg/kg, intraperitoneal) decreased the formation of aberrant crypt foci and colon tumors compared to animals that received azoxymethane alone (Borrelli et al., 2005).
In conclusion, the major effect of senna administered in feed to mice was a dose-related increase in the incidence of intestinal epithelial hyperplasia. There was no neoplastic change in this experimental situation, when senna was administered to p53 +/− mouse ; however, it remains to be determined if the observance of senna-induced intestinal hyperplasia in rodents indicates potential risk to senna-exposed humans.
Acknowldgements
This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). However, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government. We greatly acknowledge the expert review by Ms. Kristine Witt, and Dr. Arun Pandiri. The in life phase of the study was conducted under NIEHS contracts N01-ES-65406 and N01-ES-55536. The authors would like to thank Drs. Milton Hejtmancik, Diane Gerken, Daphne Vasconcelos and other staffs at Battelle Memorial Institute for helping the conduct of these studies.
Footnotes
Conflict of Interest The authors declare no conflicts of interest.
References
- Al-Yahya MA, Al-Farhan AH, Adam SE. Toxicological interactions of Cassia senna and Nerium oleander in the diet of rats. Am J Chin Med. 2002;30:579–587. doi: 10.1142/S0192415X02000521. [DOI] [PubMed] [Google Scholar]
- Beuers U, Spengler U, Pape GR. Hepatitis after chronic abuse of senna. Lancet. 1991;337:372–373. doi: 10.1016/0140-6736(91)91012-j. [DOI] [PubMed] [Google Scholar]
- Blumenthal M. Herbal Medicine;Expanded Commission E Monographs. 2000:341–345. [Google Scholar]
- Borrelli F, Capasso R, Aviello G, Di Carlo G, Izzo AA, Mascolo N, Capasso F. Senna and the formation of aberrant crypt foci and tumors in rats treated with azoxymethane. Phytomedicine. 2005;12:501–505. doi: 10.1016/j.phymed.2003.10.008. discussion 505. [DOI] [PubMed] [Google Scholar]
- Boyd JT, Doll R. Gastro-intestinal cancer and the use of liquid paraffin. Br J Cancer. 1954;8:231–237. doi: 10.1038/bjc.1954.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Dietrich PS. Mutagenicity of anthraquinone and benzanthrone derivatives in the Salmonella/microsome test: activation of anthraquinone glycosides by enzymic extracts of rat cecal bacteria. Mutat Res. 1979;66:9–24. doi: 10.1016/0165-1218(79)90003-x. [DOI] [PubMed] [Google Scholar]
- Brusick D, Mengs U. Assessment of the genotoxic risk from laxative senna products. Environ Mol Mutagen. 1997;29:1–9. doi: 10.1002/(sici)1098-2280(1997)29:1<1::aid-em1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Bryant-Waugh R, Turner H, East P, Gamble C, Mehta R. Misuse of laxatives among adult outpatients with eating disorders: prevalence and profiles. Int J Eat Disord. 2006;39:404–409. doi: 10.1002/eat.20267. [DOI] [PubMed] [Google Scholar]
- Cance JD, Ashley OS, Penne MA. Unhealthy weight control behaviors and MDMA (Ecstasy) use among adolescent females. J Adolesc Health. 2005;37:409. doi: 10.1016/j.jadohealth.2004.11.122. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr., Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Vogel H, McArthur MJ, Montgomery CA, Jr., Park SH, Thompson T, Ford RJ, Bradley A. Effects of genetic background on tumorigenesis in p53-deficient mice. Mol Carcinog. 1995;14:16–22. doi: 10.1002/mc.2940140105. [DOI] [PubMed] [Google Scholar]
- Dreessen M, Eyssen H, Lemli J. The metabolism of sennosides A and B by the intestinal microflora: in vitro and in vivo studies on the rat and the mouse. J Pharm Pharmacol. 1981;33:679–681. doi: 10.1111/j.2042-7158.1981.tb13903.x. [DOI] [PubMed] [Google Scholar]
- Dunnick JK, Hardisty JF, Herbert RA, Seely JC, Furedi-Machacek EM, Foley JF, Lacks GD, Stasiewicz S, French JE. Phenolphthalein induces thymic lymphomas accompanied by loss of the p53 wild type allele in heterozygous p53-deficient (+/−) mice. Toxicol Pathol. 1997;25:533–540. doi: 10.1177/019262339702500601. [DOI] [PubMed] [Google Scholar]
- Franz G. The senna drug and its chemistry. Pharmacology. 1993;47(Suppl 1):2–6. doi: 10.1159/000139654. [DOI] [PubMed] [Google Scholar]
- Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- French J, Storer RD, Donehower LA. The nature of the heterozygous Trp53 knockout model for identification of mutagenic carcinogens. Toxicol Pathol. 2001a;29(Suppl):24–29. doi: 10.1080/019262301753178456. [DOI] [PubMed] [Google Scholar]
- French JE. Identification and characterization of potential human carcinogens using B6.129tm1Trp53 heterozygous null mice and loss of heterozygosity at the Trp53 locus. IARC Sci Publ. 2004:271–287. [PubMed] [Google Scholar]
- French JE, Lacks GD, Trempus C, Dunnick JK, Foley J, Mahler J, Tice RR, Tennant RW. Loss of heterozygosity frequency at the Trp53 locus in p53-deficient (+/−) mouse tumors is carcinogen-and tissue-dependent. Carcinogenesis. 2001b;22:99–106. doi: 10.1093/carcin/22.1.99. [DOI] [PubMed] [Google Scholar]
- Heidemann A, Miltenburger HG, Mengs U. The genotoxicity status of senna. Pharmacology. 1993;47(Suppl 1):178–186. doi: 10.1159/000139857. [DOI] [PubMed] [Google Scholar]
- Heidemann A, Volkner W, Mengs U. Genotoxicity of aloeemodin in vitro and in vivo. Mutat Res. 1996;367:123–133. doi: 10.1016/0165-1218(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Helin T, Makinen-Kiljunen S. Occupational asthma and rhinoconjunctivitis caused by senna. Allergy. 1996;51:181–184. doi: 10.1111/j.1398-9995.1996.tb04584.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Le Leu RK, Young GP. Absence of acute apoptotic response to genotoxic carcinogens in p53-deficient mice is associated with increased susceptibility to azoxymethane-induced colon tumours. Int J Cancer. 2005;115:561–567. doi: 10.1002/ijc.20876. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Goda Y, Yoshihira K. The mutagenic constituents of Rubia tinctorum. Chem Pharm Bull (Tokyo) 1992;40:1504–1509. doi: 10.1248/cpb.40.1504. [DOI] [PubMed] [Google Scholar]
- Kleibeuker JH, Cats A, Zwart N, Mulder NH, Hardonk MJ, de Vries EG. Excessively high cell proliferation in sigmoid colon after an oral purge with anthraquinone glycosides. J Natl Cancer Inst. 1995;87:452–453. doi: 10.1093/jnci/87.6.452. [DOI] [PubMed] [Google Scholar]
- Lemli J. Metabolism of sennosides--an overview. Pharmacology. 1988;36(Suppl 1):126–128. doi: 10.1159/000138431. [DOI] [PubMed] [Google Scholar]
- Lemli J, Lemmens L. Metabolism of sennosides and rhein in the rat. Pharmacology. 1980;20(Suppl 1):50–57. doi: 10.1159/000137398. [DOI] [PubMed] [Google Scholar]
- Leng-Peschlow E. Dual effect of orally administered sennosides on large intestine transit and fluid absorption in the rat. J Pharm Pharmacol. 1986;38:606–610. doi: 10.1111/j.2042-7158.1986.tb03089.x. [DOI] [PubMed] [Google Scholar]
- Leng-Peschlow E. Senna and its rational use. Pharmacology. 1992;44(Suppl 1):1–52. [PubMed] [Google Scholar]
- Lubet RA, Zhang Z, Wiseman RW, You M. Use of p53 transgenic mice in the development of cancer models for multiple purposes. Exp Lung Res. 2000;26:581–593. doi: 10.1080/01902140150216684. [DOI] [PubMed] [Google Scholar]
- Lyden-Sokolowski A, Nilsson A, Sjoberg P. Two-year carcinogenicity study with sennosides in the rat: emphasis on gastro-intestinal alterations. Pharmacology. 1993;47(Suppl 1):209–215. doi: 10.1159/000139860. [DOI] [PubMed] [Google Scholar]
- Makena PS, Chung KT. Effects of various plant polyphenols on bladder carcinogen benzidine-induced mutagenicity. Food Chem Toxicol. 2007;45:1899–1909. doi: 10.1016/j.fct.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Marks GB, Salome CM, Woolcock AJ. Asthma and allergy associated with occupational exposure to ispaghula and senna products in a pharmaceutical work force. Am Rev Respir Dis. 1991;144:1065–1069. doi: 10.1164/ajrccm/144.5.1065. [DOI] [PubMed] [Google Scholar]
- Mascolo N, Mereto E, Borrelli F, Orsi P, Sini D, Izzo AA, Massa B, Boggio M, Capasso F. Does senna extract promote growth of aberrant crypt foci and malignant tumors in rat colon? Dig Dis Sci. 1999;44:2226–2230. doi: 10.1023/a:1026696402212. [DOI] [PubMed] [Google Scholar]
- Mengs U. Toxic effects of sennosides in laboratory animals and in vitro. Pharmacology. 1988;36(Suppl 1):180–187. doi: 10.1159/000138438. [DOI] [PubMed] [Google Scholar]
- Mengs U, Grimminger W, Krumbiegel G, Schuler D, Silber W, Volkner W. No clastogenic activity of a senna extract in the mouse micronucleus assay. Mutat Res. 1999;444:421–426. doi: 10.1016/s1383-5718(99)00115-1. [DOI] [PubMed] [Google Scholar]
- Mengs U, Heidemann A. Genotoxicity of sennosides and rhein in vitro and in vivo. Med Sci Res. 1993;21:749–750. [Google Scholar]
- Mengs U, Mitchell J, McPherson S, Gregson R, Tigner J. A 13-week oral toxicity study of senna in the rat with an 8-week recovery period. Arch Toxicol. 2004;78:269–275. doi: 10.1007/s00204-003-0534-z. [DOI] [PubMed] [Google Scholar]
- Mereto E, Ghia M, Brambilla G. Evaluation of the potential carcinogenic activity of Senna and Cascara glycosides for the rat colon. Cancer Lett. 1996;101:79–83. doi: 10.1016/0304-3835(96)04129-8. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Mengs U, McPherson S, Zijlstra J, Dettmar P, Gregson R, Tigner JC. An oral carcinogenicity and toxicity study of senna (Tinnevelly senna fruits) in the rat. Arch Toxicol. 2006;80:34–44. doi: 10.1007/s00204-005-0021-9. [DOI] [PubMed] [Google Scholar]
- Mladenova D, Daniel JJ, Dahlstrom JE, Bean E, Gupta R, Pickford R, Currey N, Musgrove EA, Kohonen-Corish MR. The NSAID sulindac is chemopreventive in the mouse distal colon but carcinogenic in the proximal colon. Gut. 2011;60:350–360. doi: 10.1136/gut.2010.208314. [DOI] [PubMed] [Google Scholar]
- Mond JM, Hay PJ, Rodgers B, Owen C, Mitchell JE. Correlates of self-induced vomiting and laxative misuse in a community sample of women. J Nerv Ment Dis. 2006;194:40–46. doi: 10.1097/01.nmd.0000195310.38655.19. [DOI] [PubMed] [Google Scholar]
- Moussally K, Oraichi D, Berard A. Herbal products use during pregnancy: prevalence and predictors. Pharmacoepidemiol Drug Saf. 2009;18:454–461. doi: 10.1002/pds.1731. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Stopper H, Dekant W. Biotransformation of the anthraquinones emodin and chrysophanol by cytochrome P450 enzymes. Bioactivation to genotoxic metabolites. Drug Metab Dispos. 1998;26:540–546. [PubMed] [Google Scholar]
- Mukhopadhyay MJ, Saha A, Dutta A, De B, Mukherjee A. Genotoxicity of sennosides on the bone marrow cells of mice. Food Chem Toxicol. 1998;36:937–940. doi: 10.1016/s0278-6915(98)00049-0. [DOI] [PubMed] [Google Scholar]
- Nesslany F, Simar-Meintieres S, Ficheux H, Marzin D. Aloe-emodin-induced DNA fragmentation in the mouse in vivo comet assay. Mutat Res. 2009;678:13–19. doi: 10.1016/j.mrgentox.2009.06.004. [DOI] [PubMed] [Google Scholar]
- NIH Public Health Service Policy on Humane Care and Use of Laboratory Animals. 2002.
- Novartis Product informatoin for ex-lax maximum strength. 2010 http://ex-lax.com/product-max.shtml.
- NTP [Retrieved January 11, 2012];2011 http://ntp-apps.niehs.nih.gov/ntp_tox/index.cfm?searchterm=senna&fuseaction=ntpsearch.searchresults.
- Occhipinti KE, Di Palma JA. How to choose the best preparation for colonoscopy. Nat Rev Gastroenterol Hepatol. 2009;6:279–286. doi: 10.1038/nrgastro.2009.42. [DOI] [PubMed] [Google Scholar]
- Pritchard JB, French JE, Davis BJ, Haseman JK. The role of transgenic mouse models in carcinogen identification. Environ Health Perspect. 2003;111:444–454. doi: 10.1289/ehp.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandnes D, Johansen T, Teien G, Ulsaker G. Mutagenicity of crude senna and senna glycosides in Salmonella typhimurium. Pharmacol Toxicol. 1992;71:165–172. doi: 10.1111/j.1600-0773.1992.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Satia JA, Littman A, Slatore CG, Galanko JA, White E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiol Biomarkers Prev. 2009;18:1419–1428. doi: 10.1158/1055-9965.EPI-09-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold U, Landauer N, Hillebrand S, Goebel FD. Senna-induced hepatitis in a poor metabolizer. Ann Intern Med. 2004;141:650–651. doi: 10.7326/0003-4819-141-8-200410190-00024. [DOI] [PubMed] [Google Scholar]
- Shah ND, Chitkara DK, Locke GR, Meek PD, Talley NJ. Ambulatory care for constipation in the United States, 1993-2004. Am J Gastroenterol. 2008;103:1746–1753. doi: 10.1111/j.1572-0241.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- Siegers CP, Siemers J, Baretton G. Sennosides and aloin do not promote dimethylhydrazine-induced colorectal tumors in mice. Pharmacology. 1993a;47(Suppl 1):205–208. doi: 10.1159/000139859. [DOI] [PubMed] [Google Scholar]
- Siegers CP, von Hertzberg-Lottin E, Otte M, Schneider B. Anthranoid laxative abuse--a risk for colorectal cancer? Gut. 1993b;34:1099–1101. doi: 10.1136/gut.34.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitherman LC, Janisse J, Mathur A. The use of folk remedies among children in an urban black community: remedies for fever, colic, and teething. Pediatrics. 2005;115:e297–304. doi: 10.1542/peds.2004-1443. [DOI] [PubMed] [Google Scholar]
- Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233–240. doi: 10.1038/35106009. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Winter ML, Weber JA, Krenzelok EP, Anderson DL, Ryan ML. Skin breakdown and blisters from senna-containing laxatives in young children. Ann Pharmacother. 2003;37:636–639. doi: 10.1345/aph.1C439. [DOI] [PubMed] [Google Scholar]
- Tennant RW, French JE, Spalding JW. Identifying chemical carcinogens and assessing potential risk in short-term bioassays using transgenic mouse models. Environ Health Perspect. 1995;103:942–950. doi: 10.1289/ehp.95103942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda K, Nishikawa A, Furukawa F, Kawanishi T, Hayashi Y, Takahashi M. Cell proliferation induced by laxatives and related compounds in the rat intestine. Cancer Lett. 1994;83:43–49. doi: 10.1016/0304-3835(94)90297-6. [DOI] [PubMed] [Google Scholar]
- Tozzi F, Thornton LM, Mitchell J, Fichter MM, Klump KL, Lilenfeld LR, Reba L, Strober M, Kaye WH, Bulik CM. Features associated with laxative abuse in individuals with eating disorders. Psychosom Med. 2006;68:470–477. doi: 10.1097/01.psy.0000221359.35034.e7. [DOI] [PubMed] [Google Scholar]
- U. S. Food and Drug Administration Laxative Drug Products for Over-the Counter Human Use. Federal Register. 1999;64:4535–4540. [PubMed] [Google Scholar]
- van Gorkom BA, Karrenbeld A, van Der Sluis T, Koudstaal J, de Vries EG, Kleibeuker JH. Influence of a highly purified senna extract on colonic epithelium. Digestion. 2000;61:113–120. doi: 10.1159/000007743. [DOI] [PubMed] [Google Scholar]
- Vanderperren B, Rizzo M, Angenot L, Haufroid V, Jadoul M, Hantson P. Acute liver failure with renal impairment related to the abuse of senna anthraquinone glycosides. Ann Pharmacother. 2005;39:1353–1357. doi: 10.1345/aph.1E670. [DOI] [PubMed] [Google Scholar]
- Westendorf J, Marquardt H, Poginsky B, Dominiak M, Schmidt J. Genotoxicity of naturally occurring hydroxyanthraquinones. Mutat Res. 1990;240:1–12. doi: 10.1016/0165-1218(90)90002-j. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Yagi T, Kuwano S. Suppression of the purgative action of rhein anthrone, the active metabolite of sennosides A and B, by calcium channel blockers, calmodulin antagonists and indomethacin. Pharmacology. 1993;47(Suppl 1):22–31. doi: 10.1159/000139839. [DOI] [PubMed] [Google Scholar]
- Zuckerman V, Wolyniec K, Sionov RV, Haupt S, Haupt Y. Tumour suppression by p53: the importance of apoptosis and cellular senescence. J Pathol. 2009;219:3–15. doi: 10.1002/path.2584. [DOI] [PubMed] [Google Scholar]