Abstract
Glial cell line-derived neurotrophic factor (GDNF) is a potent growth factor essential to the development, survival, and function of dopaminergic neurons (Airaksinen and Saarma, 2002). The molecular mechanisms underlying GDNF expression remain elusive, thus, we set out to identify a signaling pathway that governs GDNF levels. We found that treatment of both differentiated dopaminergic-like SH-SY5Y cells and rat midbrain slices with the dopamine D2 receptor (D2R) agonist, quinpirole, triggered an increase in the expression of GDNF that was temporally preceded by an increase in the levels of Zif268, a DNA-binding transcription factor encoded by an immediate-early gene. Moreover, the D2R inhibitor raclopride blocked the increase of both GDNF and Zif268 expression following potassium-evoked dopamine release in SH-SY5Y cells. We used adenoviral delivery of small hairpin RNA (shRNA) targeting Zif268 to downregulate its expression and found that Zif268 is specifically required for the D2R-mediated upregulation of GDNF. Furthermore, the D2R-mediated induction of GDNF and Zif268 expression was dependent on Gβγ-mediated signaling and activation of extracellular signal-regulated kinase 1/2 (Erk1/2). Importantly, using chromatin immunoprecipitation (ChIP) assay, we identified a direct association of Zif268 with the GDNF promoter. These results suggest that D2R activation induces a Gβγ- and Erk1/2-dependent increase in the level of Zif268, which functions to directly upregulate the expression of GDNF.
Keywords: Dopamine D2 receptor, GDNF, Zif268, Gβγ, Erk1/2
Introduction
GDNF, a secreted growth factor belonging to the transforming growth factor-β (TGF-β) superfamily, was first isolated from a rat glioma cell line (Lin et al., 1993). The expression of GDNF is widespread throughout the central nervous system (CNS) during development (Choi-Lundberg and Bohn, 1995; Schaar et al., 1993; Stromberg et al., 1993) but becomes largely relegated to discrete regions in the adult brain, such as the striatum, hippocampus, cortex, and thalamus (Barroso-Chinea et al., 2005; Golden et al., 1998; Golden et al., 1999; Trupp et al., 1997). In addition, neurons, as opposed to glia, appear to be the main source of GDNF expression in the adult brain (Pochon et al., 1997). GDNF plays an important role in the function and survival of dopaminergic neurons in the midbrain (Airaksinen and Saarma, 2002), where its receptor, the Ret receptor tyrosine kinase, and its co-receptor, GDNF family receptor-α 1 (GFRα1), are expressed (Glazner et al., 1998; Trupp et al., 1997). GDNF’s interaction with GFRα1, promotes the recruitment, dimerization, and autophosphorylation of the Ret receptor (Airaksinen and Saarma, 2002). This leads to the subsequent activation of the Erk1/2, phosphoinositide 3-kinase (PI3K), phospholipase C-γ (PLCγ), and other signaling pathways (Airaksinen and Saarma, 2002). The potent neuroprotective and neuroregenerative effects of GDNF on midbrain dopaminergic neurons present great therapeutic potential for the treatment of Parkinson’s disease, a condition characterized by the specific loss of dopaminergic neurons in the substantia nigra (SN) region of the midbrain (Rangasamy et al., 2010). GDNF plays an important role in the regulation of dopaminergic neuron firing rates (Wang et al., 2010; Yang et al., 2001), dopamine release (Barak et al., 2011b; Wang et al., 2010), and to the maintenance of learning processes throughout senescence (Miyazaki et al., 2003). Interestingly, low levels of GDNF are associated with alcohol addiction in humans (Heberlein et al., 2010), and infusion of GDNF reduces the intake of alcohol in rats (Barak et al., 2011a; Carnicella et al., 2009b; Carnicella et al., 2008; Carnicella and Ron, 2009). Finally, GDNF-mediated signaling in the CNS was shown to prevent depression- and anxiety-like behaviors in mice subjected to chronic stress (Uchida et al., 2011).
Constitutive, non-activity-dependent (e.g., passive) release comprises a major mode of GDNF secretion (Lonka-Nevalaita et al., 2010; Oh-hashi et al., 2009), indicating that the transcription and translation of GDNF is an important regulatory step for the function of this growth factor. However, little is known about the control of GDNF expression, thus we set out to identify a possible signaling mechanism that regulates the expression of this growth factor. Previously, in vitro treatment of midbrain neuronal (Guo et al., 2002) or astrocytic (Ohta et al., 2000) cultures with apomorphine, which activates both dopamine D1 and D2 receptors, results in the upregulation of GDNF mRNA. In addition, we previously demonstrated that the dopamine D1/D2 receptor agonist, cabergoline, increases the expression of GDNF in the human dopaminergic-like SH-SY5Y cell line, as well as in the midbrain of rats and mice following in vivo systemic administration of this compound (Carnicella et al., 2009a). Interestingly, endogenous GDNF expression levels are partially reduced in both the midbrain and striatum of mice lacking the dopamine D2 Gαi protein-coupled receptor (D2R) (Bozzi and Borrelli, 1999; Saavedra et al., 2008). Together, these studies suggest that D2R-mediated signaling may contribute the expression of GDNF. Therefore, we tested whether, and if so, how, specific activation of D2Rs results in the induction of GDNF expression.
Methods
Materials
TRIzol reagent, and pre-cast SDS-PAGE gels were purchased from Invitrogen (Carlsbad, CA). Phosphatase inhibitor cocktails II and III, deoxyribonuclease (DNase) and ethidium bromide were purchased from Sigma (St. Louis, MO). The protease inhibitor mini-tablets were purchased from Roche (Indianapolis, IN). The reverse transcription system and 2X PCR master mix were purchased from Promega (Madison, WI). Quinpirole, Raclopride, U0126, PD98059, and gallein were purchased from Tocris Bioscience (Minneapolis, MN). The BCA Protein Assay Kit was purchased from Pierce Biotechnology (Rockford, IL). The enhanced chemiluminescence (ECL) detection reagents were purchased from Fisher Scientific (Pittsburgh, PA). The pRNAT-H1.1/Shuttle vector was obtained from the GenScript Corporation (Piscataway, NJ). The Adeno-X vector, Expression System, and Purification and Rapid Titer kits were purchased from Clontech (Mountain View, CA). The Chromatin Immunoprecipitation (ChIP) Assay Kit was obtained from Millipore (Billerica, MA).
Antibodies
Anti-GDNF antibody (AF-212-NA) was obtained from R&D systems (Minneapolis, MN). Anti-Zif268 antibody (#4153) was purchased from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase (HRP)-conjugated secondary antibodies, anti-Erk2 (sc-1647), anti-phosphoErk1/2 (pErk1/2; sc-7976), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sc-25778) polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Dilutions of the antibodies were as follows: anti-GDNF, 1:250; anti-GAPDH, 1:5000; anti-Erk2, 1:2000; anti-pErk1/2, 1:2000; and anti-Zif268, 1:500.
Cell culture
The human dopaminergic-like SH-SY5Y cells were plated at a density of 2 × 105 cells/ml in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with fetal bovine serum (FBS, 10%), penicillin/streptomycin, and non-essential amino acids. Cells were differentiated into a neuronal-like phenotype by supplementing a 1% FBS DMEM solution containing 10 µM retinoic acid (RA) for 3 days, at which point the medium was changed again, to 1% FBS DMEM without RA for an additional 18–24 hours. This was done to washout the RA, which can itself activate intracellular signaling pathways, such as Erk1/2 (Miloso et al., 2004). Cells were then treated with quinpirole (50 µM) in saline vehicle. For indicated pretreatments, U0126, PD98059, or gallein were added to the cell medium 10 minutes before the addition of quinpirole. All inhibitors were prepared in DMSO, and remained in the cellular medium throughout the quinpirole treatment. Controls were treated with DMSO at a final concentration of 0.1%. For KCl-mediated evoked dopamine release, the cellular medium was supplemented with 100 mM KCl. At the end of treatments, cells were briefly washed in phospho-buffered saline (PBS), then lysed and collected in either TRIzol reagent, for RT-PCR analysis, or radio-immunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 7.4, 5mM EDTA, 120 mM NaCl, 1% NP-40, 0.1% deoxycholate, and 0.5% SDS), for western blot analysis. Alternatively, for ChIP experiments, cells were treated with 1% paraformaldehyde (PFA) for 10 minutes at 37°C to cross-link DNA and proteins.
Acute slice treatments
Male Sprague Dawley rats (P23-26) were purchased from Harlan. All experimental protocols involving rats were approved by the Ernest Gallo Research Center Institutional Animal Care and Use Committee (IACUC), and were conducted in agreement with the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. Rats were deeply anesthetized with isoflurane, then rapidly decapitated. The brains were quickly removed and immediately placed in ice-cold artificial cerebral spinal fluid (aCSF; 126 mM NaCl, 1.2 mM KCl, 1.2 mM NaH2PO4, 1 mM MgCl2, 2.4 mM CaCl2, 18 mM NaHCO3, 11 mM glucose) saturated with oxygen (95% O2 and 5% CO2 mixture). Horizontal slices (150 µm) containing the midbrain were prepared with a Leica vibratome in ice-cold aCSF. The ventral midbrain, containing the ventral tegmental area (VTA) and the substantia nigra (SN), were dissected from the slices, and allowed to rest in oxygen-saturated aCSF at room temperature for 45 minutes prior to treatment. Slices were treated with 50 μM QP for an additional 30 or 240 minutes in oxygen-saturated, room temperature aCSF. At the end of the treatment, the slices were mechanically homogenized in TRIzol in preparation for RT-PCR analysis.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from cells lysed in TRIzol reagent according to the manufacturer’s recommended protocol. Following DNase treatment, messenger RNA (mRNA) was selectively reverse transcribed using the Reverse Transcription System and oligo(dT) primers. The resulting cDNA was used in PCR reactions for GDNF, Zif268, and the housekeeping gene GAPDH using the following primers: human GDNF upstream: 5’- TGC CAG AGG ATT ATC CTG ATC AGT TCG ATG -3’; human GDNF downstream: 5’- GAT ACA TCC ACA CCT TTT AGC GGA ATG CTT -3’; human Zif268 upstream: 5’- TGA CCG CAG AGT CTT TTC CT -3’; human Zif268 downstream: 5’- TGG GTT GGT CAT GCT CAC TA -3’; human GAPDH upstream: 5’- TGA AGG TCG GTG TCA ACG GAT TTG GC -3’; human GAPDH downstream: 5’- CAT GTA GGC CAT GAG GTC CAC CAC -3’. For GDNF, 33–35 amplification cycles were used, while 27 cycles were used for Zif268 and GAPDH. Equal volumes of the PCR products were resolved on 1.8% agarose gels containing 0.05% ethidium bromide for visualization under UV light. Images were captured using the Eagle Eye 2 software. Relative band intensities were quantified using NIH ImageJ software, and normalized to GAPDH.
Western blot
Cells were lysed and collected in RIPA buffer containing protease and phosphatase inhibitors. Lysates were briefly sonicated, and then placed on ice for 30 minutes. Protein concentrations were determined using the BCA Protein Assay kit. Equal amounts of protein (15–25 µg) were resolved on NuPAGE 10% (for Zif268 and Erks 1/2) or 4–12% gradient (for GDNF) Bis-Tris gels, and transferred onto nitrocellulose membranes overnight. For pErk1/2 and Erk2 detection, membranes were first probed for pErk1/2, then incubated in stripping buffer (25 mM glycine-HCl, 1% SDS, pH 3, for 30 minutes at room temperature) and reprobed for Erk2. Membranes were incubated with primary antibody for 4 hours at RT, followed by a 2-hour RT incubation with the appropriate HRP-conjugated secondary antibodies to detect immunoreactivity via an ECL reaction. Images were developed on Kodak film, and digitally scanned for relative densitometric quantification using NIH ImageJ software.
Chromatin immunoprecipitation (ChIP)
Following the PFA-mediated cross-linking of DNA and proteins, cells were washed twice, briefly, with PBS and collected. ChIP was conducted as described in the manufacturer’s protocol. Shearing of DNA was accomplished by using 3 rounds of 30-pulse, low-power sonication. Zif268-containing complex was IP-ed overnight at 4°C with 10 µg of anti-Zif268 (rabbit polyclonal IgG). Control IP with normal rabbit IgG was conducted in parallel. Putative Zif268-binding sites were identified by manually scanning the 1000 base pair region immediately upstream of the transcription start site of the human GDNF gene (NCBI Ref Seq: NG_011675.2) for the Zif268 consensus binding sequence. Primer sequences for the PCR reactions were as follows: putative Zif268 binding site #1 upstream: 5’- CTC GGA CCT CGG CTT CTG -3’; putative Zif268 binding site #1 downstream: 5’- AAC AGG TCA GGG GCA CGC GT -3’; putative Zif268 binding site #2 upstream: 5’- AGC TCC TTT TCT GCC ACT -3’; putative Zif268 binding site #2 downstream: 5’- GGG TGG ATC AAA AAT CGA GA -3’; negative control upstream: 5’- CTC GCT GCT CTC CTC TCC T -3’; negative control downstream: 5’- AGT CCC GTG AAG ACA TGA GG -3’. PCR products were amplified using 42 amplification cycles, and were resolved on a 1.8% agarose gel. Bands were visualized as described above for RT-PCR.
Adenoviral-mediated shRNA down-regulation of Zif268
A double-stranded oligonucleotide encoding a 21-base pair short hairpin RNA (shRNA) sequence against base pairs 1,236–1,257 of the coding region of Zif268 mRNA was subcloned into the BamHI and HindIII sites of the pRNAT-H1.1/Shuttle vector containing a green fluorescent protein (GFP) marker. This sequence was previously shown to silence the expression of human Zif268 (Ma et al., 2009). The resulting shZif268 expression cassette was then subcloned into the Adeno-X viral genome. Adenoviral particles were packaged and amplified in HEK293 cells as described in the Adeno-X Maxi Purification kit protocol. The adenoviral titer was determined using the Adeno-X Rapid Titer kit. Preparation of a control virus expressing a non-related control sequence was performed as previously described (Jeanblanc et al., 2009). For adenoviral delivery to SH-SY5Y cells, 2 × 106 infectious units (ifu)/ml of either virus was added to the cell medium after differentiation. All experiments were conducted 48 hours later.
Statistical analysis
Data are expressed as the mean ± SEM. One- or two-way analysis of variance (ANOVA) were used to determine the statistical significance in experiments comparing more than two groups. Significant main effects or interactions of the ANOVAs were further investigated with post-hoc Bonferroni or Student-Newman-Keuls tests, as indicated.
Results
Activation of D2Rs upregulates GDNF expression
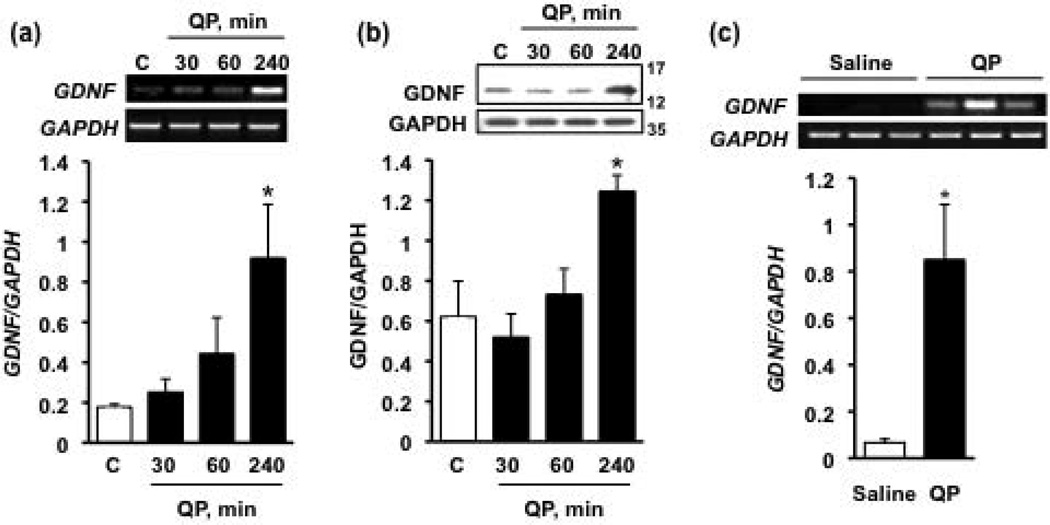
Differentiated human SH-SY5Y cells exhibit a number of features characteristic of dopaminergic neurons. For example, once differentiated, these cells cease dividing and assume neuron-like morphology (Pahlman et al., 1984) and express tyrosine hydroxylase, dopamine transporter, and dopamine β-hydroxylase, proteins necessary for the biosynthesis, uptake, and metabolism of dopamine (Ault and Werling, 2000). In addition, these cells form synaptic vesicles and synapses (Sarkanen et al., 2007), propagate action potentials (Tosetti et al., 1998), and are capable of releasing neurotransmitter following potassium-mediated depolarization (Ault and Werling, 2000; Gibb et al., 2011; Murphy et al., 1991). Thus, we first tested whether activation of D2Rs results in increased GDNF expression in this widely-used dopaminergic cell culture model. To do so, cells were treated with the D2R agonist quinpirole (50 µM), and the mRNA and protein levels of the growth factor were assessed. As shown in Fig. 1, quinpirole treatment caused a significant upregulation of GDNF mRNA (Fig. 1a), which corresponded to an increase of the level of GDNF protein (Fig. 1b). Next, we tested whether D2R activation would enhance GDNF expression in the midbrain, where approximately 75% of neurons are dopaminergic (Fields et al., 2007) and endogenously express the D2R (Chen et al., 1991). To do so, we treated slices prepared from the rat ventral midbrain for 240 minutes with 50 µM quinpirole and found that activation of D2Rs caused an upregulation of GDNF expression in midbrain neurons (Fig. 1c). Together these results suggest that the expression of this growth factor in dopaminergic neurons is increased following D2R activation.
Figure 1. D2R activation increases GDNF mRNA and protein levels.
Cells were treated with the D2R agonist quinpirole (QP, 50 µM) for 30, 60 or 240 minutes (black bars) or saline (white bars). RT-PCR was used to assess the mRNA levels of GDNF (a) and western blot analysis was used to measure protein levels of GDNF (b). Images are representative of 4 independent experiments. Bar graphs depict the mean GDNF mRNA or protein levels (upper panels of images) normalized to the housekeeping gene GAPDH (lower panels of images) ± SEM. *p < 0.05, as compared with saline (one-way ANOVA with post hoc Student-Newman-Keuls).(c) Rat ventral midbrain slices were treated with 50 µM QP (black bar) or saline (white bar) for 240 minutes, and GDNF mRNA levels were determined via RT-PCR. *p < 0.05, as compared with saline (Student’s t-test); n = 3 samples per treatment (2 animals per sample).
Activation of D2Rs upregulates Zif268 expression
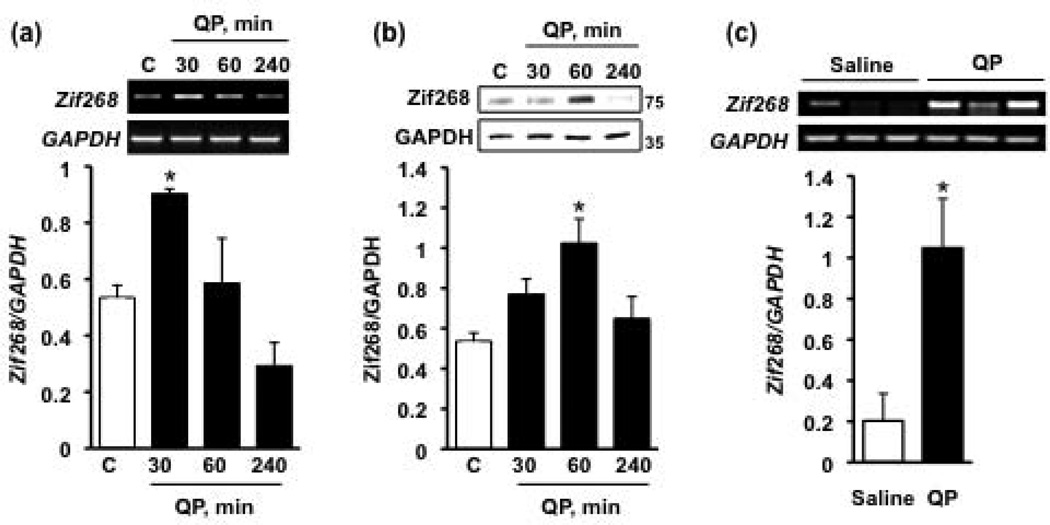
Next, we set out to determine the molecular mechanism of the D2R-mediated upregulation of GDNF expression. Zinc-finger protein 268 (Zif268; also known as early growth response protein-1 [Egr-1], Krox24, and nerve growth factor-induced gene A [NGF-IA]) belongs to the immediate-early gene family of transcription factors (Knapska and Kaczmarek, 2004). Interestingly, the upregulation of Zif268 expression in response to fibroblast growth factor-2 (FGF2) and fibroblast growth factor-1 (FGF1) treatment enhanced the expression of GDNF in cultured rat astrocytes and the rat PC12 cell line, respectively (Lin et al., 2009; Shin et al., 2009). If Zif268 mediates the increase in GDNF expression in response to D2R activation, then the transcription factor expression should be induced first in response to D2R agonist treatment. Cells were therefore treated with quinpirole and the mRNA and protein levels of the transcription factor were assessed. We found a significant, and transient, increase in the levels of Zif268 mRNA (Fig. 2a) and protein (Fig. 2b) 30 and 60 minutes following quinpirole treatment, respectively. Thus, D2R activation leads to a time-dependent increase in Zif268 mRNA, which consequently translates to an increase in Zif268 protein levels. Importantly, a 30-minute quinpirole treatment of rat ventral midbrain slices likewise resulted in an increase in the mRNA levels of the transcription factor (Fig. 2c). Together, these findings suggest that activation of D2Rs results in an increase in the levels of this transcription factor.
Figure 2. D2R activation induces a rapid upregulation of Zif268.
Cells were treated with the D2R agonist quinpirole (QP, 50 µM) for 30, 60 or 240 minutes (black bars) or saline (white bars). RT-PCR was used to assess the mRNA levels of Zif268 (a), while western blot analysis was used to measure protein levels of the transcription factor (b). Images are representative of 4 independent experiments. Bar graphs depict the mean Zif268 mRNA or protein levels (upper panels of images) normalized to GAPDH (lower panels of images) ± SEM. *p < 0.05, as compared with saline-treated cells (one-way ANOVA with post hoc Student-Newman-Keuls). (c) Rat ventral midbrain slices were treated with 50 µM QP (black bar) or saline (white bar) for 30 minutes, and Zif268 mRNA levels were determined via RT-PCR. *p < 0.05, as compared with saline (Student’s t-test); n = 3 samples per treatment (2 animals per sample).
Evoked dopamine release results in the sequential upregulation of Zif268 and GDNF in a D2R-dependent mechanism
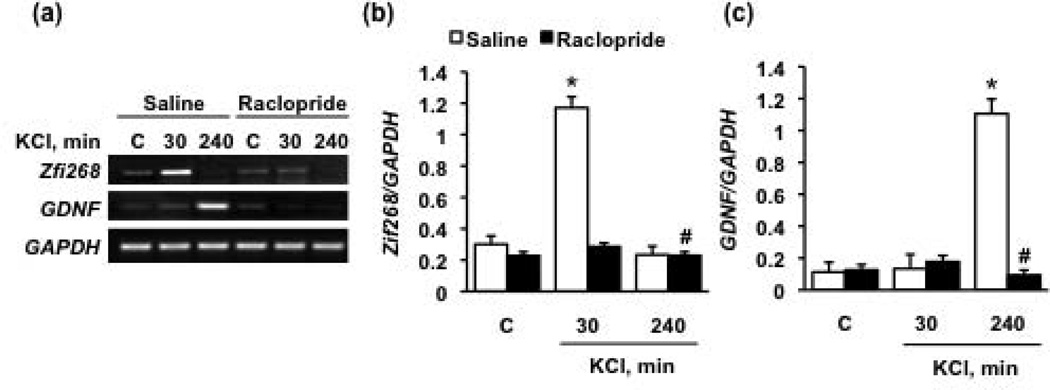
As previously mentioned, the dopaminergic SH-SY5Y cells rapidly release the neurotransmitter dopamine following potassium-induced depolarization (Ault and Werling, 2000; Gibb et al., 2011). We therefore tested whether evoked dopamine release, and the subsequent activation of D2Rs causes an upregulation of Zif268 and/or GDNF. To do so, cells were treated with 100 mM KCl in the presence and absence of the D2R inhibitor, raclopride. We observed an upregulation of Zif268 30 minutes following KCl treatment (Fig. 3a and b), which was followed by an increase in the level of GDNF mRNA at 240 minutes (Fig. 3a and c). Importantly, raclopride attenuated both of these effects (Fig. 3a–c). These findings suggest that the KCl-induced dopamine release leads to the upregulation of these transcription and growth factors is via D2R-mediated signaling.
Figure 3. Dopamine-mediated upregulation of Zif268 and GDNF is D2R-dependent.
Dopamine release from cells was evoked with KCl (100 mM, final concentration) in the presence of either saline (white bars) or 10 µM Raclopride (black bars). (a) Levels of Zif268 and GDNF mRNA were determined via RT-PCR 30 and 240 minutes after the addition of KCl. Image is representative of 3 independent experiments. Bar graphs depict the mean Zif268 (b) or GDNF (c) mRNA (upper and middle panels in (a), respectively) normalized to the housekeeping gene, GAPDH (lower panel in (a)) ± SEM. *p < 0.05, as compared with no KCl, and **#p < 0.05, as compared with Saline (two-way ANOVA with post hoc Student-Newman-Keuls pair-wise comparisons).
D2R activation upregulates GDNF via Zif268
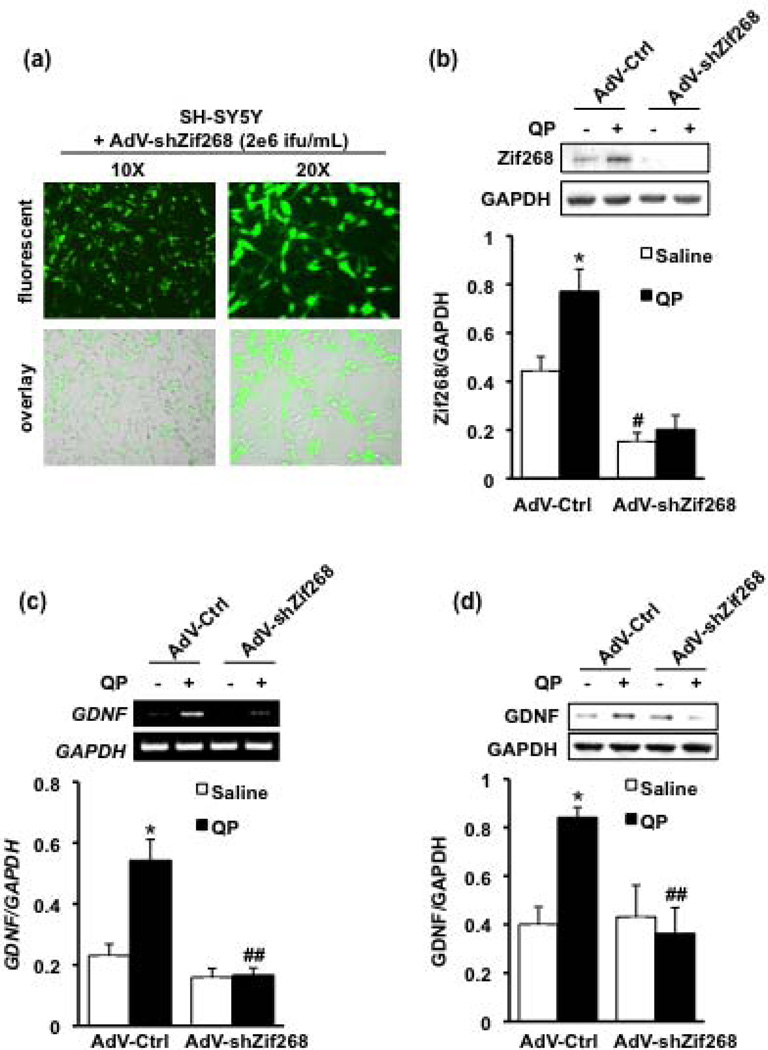
Since the rise in Zif268 levels in response to D2R activation is followed at a later time point by the upregulation of GDNF, we hypothesized that D2R-mediated GDNF expression involves the Zif268 transciption factor. To address this possibility, we constructed an adenovirus expressing green fluorescence protein (GFP) and shRNA targeting the coding region of Zif268 (AdV-shZif268). Cells were first infected with either the AdV-shZif268, or adenovirus expressing a non-related control sequence (AdV-Ctrl) (Jeanblanc et al., 2009), and the level of Zif268 protein was assessed 48 hours later in the presence or absence of quinpirole. Visualization of GFP confirmed a highly level of expression of the shZif268 construct (Fig. 4a), and AdV-shZif268 significantly reduced basal Zif268 protein levels compared with AdV-Ctrl (Fig. 4b). Importantly, treatment of cells expressing the shZif268 construct with quinpirole (50 µM for 60 minutes) blocked the D2R-mediated induction of Zif268, as compared with the quinpirole-treated AdV-Ctrl cells (Fig. 4b).
Figure 4. Adenoviral-mediated knockdown of Zif268 attenuates the D2R-induced upregulation of GDNF.
(a & b) Cells were infected with adenovirus expressing shRNA targeting Zif268 (AdV-shZif268), or a non-related control sequence (AdV-Ctrl), at a concentration of 2 × 106 infectious units (ifu)/ml. (a) Viral infection of the SH-SY5Y cells was confirmed by GFP fluorescence 48 hours after the addition of the virus to the cell medium. (b) Cells were then treated with quinpirole (QP, 50 µM) for 60 minutes (black bars), or saline (white bars), and western blot analysis was conducted to assess Zif268 protein levels. Image is representative of 4 independent experiments. Bar graph depicts the mean Zif268 protein (upper panel) normalized to the housekeeping gene GAPDH (lower panel) ± SEM. *p < 0.05, as compared with saline, and #p < 0.05, as compared with AdV-Ctrl (two-way ANOVA with post hoc Student-Newman-Keuls pair-wise comparisons). (c & d) Cells were infected with AdV-shZif268 or AdV-Ctrl, and treated 48 hours later with 50 uM quinpirole (QP) for 240 minutes (black bars) or saline (white bars). Control groups were treated with saline. (c) RT-PCR analysis was used to assess GDNF expression. Image is representative of 4 independent experiments. (d) GDNF protein levels were evaluated by western blot. Image is a representative of 4 independent experiments. Bar graphs depict the mean GDNF mRNA (c) or protein (d) (upper panels of images) normalized to GAPDH (lower panels of images) ± SEM. *p < 0.05 as compared with the saline-treated, AdV-Ctrl group; ##p < 0.01 as compared with the quinpirole-treated, AdV-Ctrl group (two-way ANOVA with post hoc Student-Newman-Keuls pair-wise comparisons).
As shown in Figures 4c and 4d, quinpirole treatment caused an increase in the mRNA and protein levels of GDNF in the AdV-Ctrl-infected cells. However, infection of cells with AdV-shZif268 blocked the D2R-mediated upregulation of GDNF (Figs. 4c–d). Together, these results indicate that Zif268 is required for the increase in GDNF expression resulting from D2R activation.
D2R activation increases Zif268 and GDNF expression via Gβγ and Erk1/2
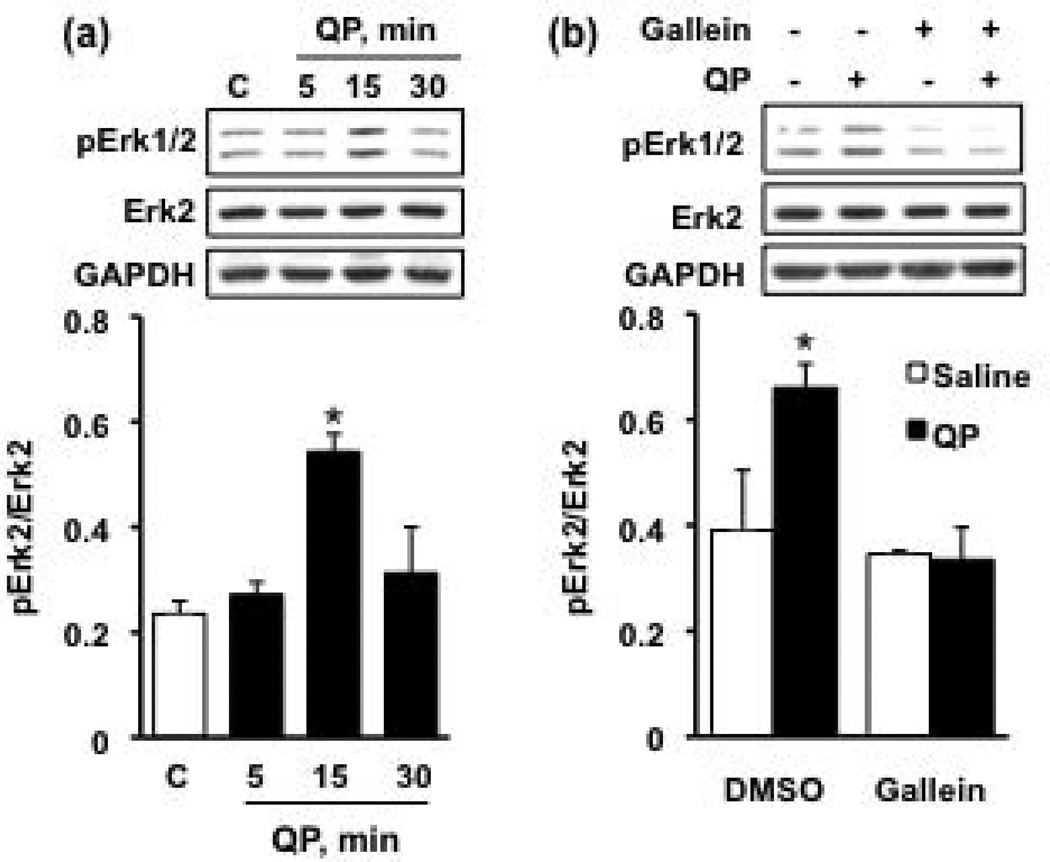
Next, we set out to determine which signaling pathway underlies the D2R-mediated increase of Zif268 expression. Activation of D2R, a Gαi protein-coupled receptor, has been shown to stimulate the Erk1/2 signaling pathway through a mechanism involving the Gβγ subunit complex (Beaulieu and Gainetdinov, 2011; Choi et al., 1999; Faure et al., 1994; Ghahremani et al., 2000). Additionally, increases in the level of Zif268 in vitro in response to cholinergic agonists (Greenwood and Dragunow, 2002), calcium influx (Rusanescu et al., 1995), or growth factor treatments (Kumahara et al., 1999; Shin et al., 2009) are dependent upon the Erk1/2 signaling pathway (Knapska and Kaczmarek, 2004). We therefore tested whether the D2R-mediated upregulation of Zif268 is a consequence of a Gβγ-mediated activation of Erk1/2. First, we established that Erk1/2 is activated in response to quinpirole treatment in our model system. To do so, we measured levels of phosphorylated, and thus activated, Erk1/2 (pErk1/2) in the presence and absence of quinpirole. As shown in Fig. 5a, quinpirole treatment caused a significant increase in the levels of pErk1/2 within 15 minutes. To test whether the D2R-mediated activation of Erk1/2 requires Gβγ, we pretreated the cells with gallein, a small molecule that binds to a domain on Gβγ that is essential for its association with downstream targets (Bonacci et al., 2006). As shown in Fig. 5b, we found that pretreatment of cells with gallein (20 µM) blocked the increase in Erk1/2 phosphorylation in response to quinpirole. Together, these results show that D2R activation leads to a Gβγ-dependent activation of the Erk1/2 signaling pathway. To determine whether the D2R-mediated upregulation of Zif268 expression and the subsequent increase in GDNF are the consequence of the Gβγ-mediated activation of the Erk1/2 signaling pathway, cells were pretreated for 10 minutes with either gallein (20 µM), or the mitogen-activated protein kinase kinase (MEK, the kinase immediately upstream of Erk1/2) inhibitors U0126 (10 μM) or PD98059 (10 µM) prior to a 30- or 240-minute treatment with 50 µM quinpirole, and the levels of Zif268 and GDNF mRNA, respectively, were assessed. As shown in Fig. 6a, the Gβγ inhibitor attenuated the D2R-mediated increase of Zif268, as compared with the vehicle-pretreated cells. In addition, both MEK inhibitors lowered the basal level of Zif268 expression and blocked the D2R-mediated upregulation of Zif268 mRNA, as compared with the vehicle-pretreated controls (Fig. 6a). Moreover, the expression levels of GDNF after D2R activation were also attenuated in the presence of either the MEK or the Gβγ inhibitors (Fig. 6b). Taken together, our findings suggest that quinpirole-mediated activation of the D2R leads to a Gβγ-dependent activation of the Erk1/2 signaling pathway, which is necessary for both Zif268 and GDNF expression.
Figure 5. Activation of the D2R in SH-SY5Y cells results in a Gβγ-dependent increase in Erk1/2 phosphorylation.
(a) Cells were treated with quinpirole (QP, 50µM) for 5, 15, and 30 minutes (black bars) or saline (white bar). Western blot was used to measure levels of phosphorylated Erk1/2 (pErk1/2). Image is a representative of 4 independent experiments. Levels of pErk2 (lower band in the upper panel) were normalized to the total Erk2 protein levels. GAPDH was used as a loading control. Bar graph represents the mean immunoreactivity signal of pErk2/Erk2 ± SEM. *p < 0.05, as compared with saline (one-way ANOVA with post-hoc Student-Newman-Keuls). (b) Cells pretreated for 10 minutes with the Gβγ inhibitor gallein (20µM, in DMSO vehicle) or an equal volume of DMSO at a final concentration of 0.1%. Cells were then treated with 50 µM quinpirole (black bars) or saline (white bars) for 15 minutes, the timepoint of maximal Erk1/2 pathway activation determined in (a). Western blot analysis was used to assess the level of pErk1/2, as in (a). Image is a representative of 4 independent experiments. Bar graph represents the mean immunoreactivity signal of pErk2/Erk2 ± SEM. *p < 0.05, as compared with the saline-treated control (two-way ANOVA with post-hoc Bonferroni comparisons within each pretreatment).
Figure 6. D2R-mediated upregulation of Zif268 and GDNF is Gβγ- and Erk1/2-dependent.
Cells were pretreated for 10 minutes with the Gβγ inhibitor gallein (20 µM, in DMSO vehicle), or the MEK inhibitors U0126 or PD98059 (both at a concentration of 10 µM, in DMSO vehicle), or an equal volume of DMSO at a final concentration of 0.1%. Cells were then incubated for 30 minutes (a) or 240 minutes (b) with quinpirole (QP; 50 µM; black bars) or saline (white bars). The level of Zif268 (a) and GDNF (b) expression was determined by RT-PCR. Images are representative of 4 independent experiments. Zif268 and GDNF levels (upper panels) were normalized to those of GAPDH (lower panels). Bar graphs depict the mean Zif268 or GDNF/GAPDH ± SEM (white bars: saline-treated groups; black bars: QP-treated groups). ***p < 0.001, as compared with the saline-treated control (two-way ANOVA with post-hoc Bonferroni comparisons within each pretreatment).
D2R activation induces a direct interaction of Zif268 with the human GDNF promoter region
Finally, we set out to determine whether Zif268 directly associates with the GDNF promoter after D2R activation. Zif268 binds DNA at its consensus sequence (5’-GCG[G/T]GGGCG-3’), thus directly influencing gene expression (Christy and Nathans, 1989). By searching for this consensus sequence in the 1000-bp region upstream of the GDNF transcription start site, we identified two putative Zif268 binding sites (Fig. 7a). The ChIP technique was then used to determine whether Zif268 directly binds the GDNF promoter at either of these sites in the presence of quinpirole. Following a 60-minute quinpirole treatment, Zif268-containing DNA-protein complexes were IP-ed from cross-linked cellular lysates, and PCR was conducted to assess whether Zif268 was associated with either putative Zif268 binding site #1 and/or #2 (PCRs 1 and 2; Fig. 7a) in the presence or absence of quinpirole. As a negative control, we conducted a PCR to amplify an intermediate region of the GDNF promoter that was not expected to associate with Zif268 (PCR 3; Fig. 7a). We found that activation of the D2R caused an association of Zif268 with putative binding site #2, but not with putative binding site #1 (Fig. 7b). As expected, Zif268 did not associate with the intermediate region, either in the presence or absence of quinpirole (Fig. 7b). These findings indicate that D2R-mediated signaling promotes the association of Zif268 with a novel binding site at the GDNF promoter.
Figure 7. D2R activation results in direct association of Zif268 at a novel Zif268 binding sequence within the GDNF promoter.
(a) Schematic representation of the human GDNF promoter region 1000 base pairs upstream of the transcription start site (designated as +1). ChIP PCR products for putative Zif268 binding sites #1 (PCR1) and #2 (PCR2), as well as an intermediate region not expected to associate with Zif268 (PCR3) are depicted with solid lines. Dashed arrows represent the primers used for each PCR reaction. (b) Cells were treated with 50 µM quinpirole (QP) or saline for 60 minutes, followed by ChIP using rabbit anti-Zif268 antibodies, or normal rabbit IgG, as a control. PCRs were conducted with the resulting precipitated DNA. As a positive control (input), total DNA was used. Image is representative of 3 independent ChIP experiments.
Discussion
Here, we show that dopamine, via the activation of the D2R, causes a Gβγ-dependent phosphorylation, and thus activation, of Erk1/2 that leads to an increase in the level of the transcription factor, Zif268. Zif268 then directly interacts with a specific site at the GDNF promoter and upregulates the levels of the growth factor (model, Fig. 8). Together, our findings illustrate a novel mechanism by which the release of dopamine and the consequent activation of the D2R increases the expression of GDNF.
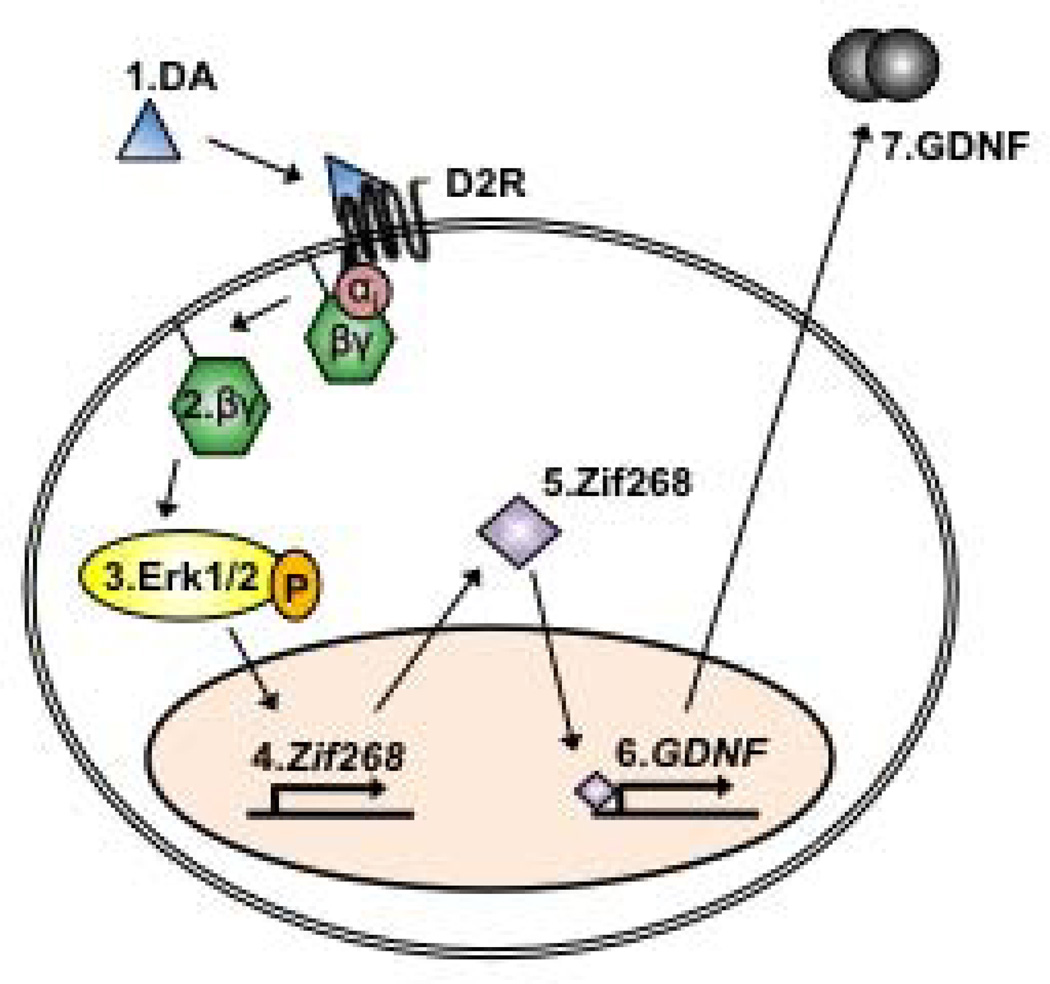
Figure 8. Model: Dopamine-mediated upregulation of GDNF expression occurs via a D2R-Gβγ-Erk1/2-Zif268 pathway.
Dopamine (DA) binds to and activates D2R (1), leading to the dissociation of the of the Gαi subunit from Gβγ (2). Gβγ mediates the activation of MEK and the subsequent activation of Erk1/2 (3). D2R-Gβγ-mediated activation of Erk12 results in an upregulation of Zif268 mRNA (4), which translates to an increase in the protein levels of this transcription factor (5). Zif268 directly binds the GDNF promoter region (6), and specifically promotes the transcription of GDNF, leading to an increase in the levels of GDNF mRNA and protein (7).
D2R signaling induces a Zif268-mediated upregulation of GDNF
We show that potassium-evoked dopamine release in differentiated SH-SY5Y cells and the subsequent activation of D2Rs cause a sequential increase in the expression levels of first the transcription factor, Zif268, and then GDNF. Importantly, we demonstrate that the upregulation of both Zif268 and GDNF via D2Rs occurs in the rat ventral midbrain, a dopaminergic region that endogenously expresses this receptor (Chen et al., 1991). Finally, we found that D2R-mediated upregulation of GDNF expression occurs via the induction of expression of the transcription factor, Zif268. Together, our findings suggest that following physiological activation of D2Rs, the levels of GDNF may be increased in a Zif268-dependent manner. Dopamine, which is released in response to dopaminergic neuron excitation, binds to D2Rs to regulate both dopamine synthesis and release via a negative feedback mechanism, in addition to activating various molecular signaling pathways that contribute to the expression of dopamine-driven functions, such as locomotion and reward-seeking behaviors, including drug addition (Picetti et al., 1997; Usiello et al., 2000). Thus it is highly plausible that neuronal activity that causes the release of dopamine may, under certain circumstances, induce the expression of GDNF via a D2R-mediated increase in Zif268 expression.
Erk1/2-mediated induction of Zif268 expression upregulates GDNF
We show that D2R-mediated activation of the Erk1/2 pathway contributes to an increase in the level of Zif268, and is required for the subsequent expression of GDNF. Erk1/2 activation by several different types of stimuli can result in increased Zif268 levels, consequently altering gene expression (Greenwood and Dragunow, 2002; Kumahara et al., 1999; Rusanescu et al., 1995; Shin et al., 2009). Thus, activation of Erk1/2 signaling through pathways other than that mediated by the D2R, as we describe, may cause an upregulation of GDNF via Zif268. For example, growth factors, such as GDNF, are known to activate Erk1/2 pathway activation (Airaksinen and Saarma, 2002). Interestingly, we previously showed that GDNF-activated Erk1/2 contributes to an upregulation of this growth factor’s own expression, both in SH-SY5Y cells (He and Ron, 2006) and in the VTA of rats in vivo (Barak et al., 2011a). In light of our present findings, it is possible that the positive autoregulation of GDNF mRNA by GDNF-activated Erk1/2 occurs via Zif268, however this possibility is yet to be determined. Interestingly, inhibition of ERK1/2 activation resulted in a decrease in the basal level of Zif268 in SH-SY5Y cells. This is likely due to the reduction of constitutive basal ERK1/2 activity in immortalized cell lines by MEK inhibitors (Kress et al., 2010). This finding puts forward the possibility that a basal level of Zif268 is maintained by baseline ERK1/2 pathway activity. This prospect, as well as whether this is a specific characteristic of dopaminergic cell types remains to be tested.
Direct association of Zif268 with the human GDNF promoter
We found that following D2R activation, Zif268 is directly associated with a binding sequence approximately 700 base pairs upstream of the human GDNF transcription start site, which we describe as putative Zif268 binding site #2. A previous study reported a direct association of Zif268 with the rat GDNF promoter (Shin et al., 2009) following FGF2 treatment of cultured rat astrocytes. We note that the Zif268 binding motif reported by Shin and colleagues bears a close positional homology to what we describe in our study as putative Zif268 binding site #1. However, we found no association of Zif268 with the human putative Zif268 binding site #1 in either the presence or absence of quinpriole. It is possible that the site-specific binding of Zif268 to the GDNF promoter may depend on whether growth factor or neurotransmitter receptors are activated. It is also possible that Zif268 binds to and enhances the activity of the GDNF promoter in a species- or cell type-specific manner. In addition, previous sequence analyses of the human GDNF proximal promoter region have revealed putative binding sites for several transcription factors including specificity protein-1 (Sp1), activating protein-2 (AP2), cAMP response element binding (CREB), nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), and metal regulatory element binding protein (MRE-BP) (Baecker et al., 1999; Woodbury et al., 1998). Thus, whether D2R-induced Zif268 acts alone or in concert with one or more of these transcription factors to upregulate the expression of GDNF remains to be determined.
Potential implications
Increased dopamine release in the reward circuitry of the brain is a hallmark of exposure to all drugs of abuse (Nestler, 2001). Intriguingly, the expression of Zif268 may be altered in response to cocaine and alcohol in brain regions that are targeted by drugs of abuse (Bertran-Gonzalez et al., 2008; Koob et al., 1998; Vilpoux et al., 2009). In addition, Zif268 has been found to play a critical role in the reconsolidation of both heroin- and cocaine-associated memories, which ultimately affects drug-seeking behaviors (Hellemans et al., 2006; Lee et al., 2005). Separately, the expression of GDNF, a potent negative regulator of the rewarding properties of drugs of abuse (Carnicella and Ron, 2009; Ghitza et al., 2010), in the CNS is subject to alterations in response to cocaine (Green-Sadan et al., 2003), heroin (Airavaara et al., 2011), phencyclidine (Semba et al., 2004), and alcohol (Ahmadiantehrani et al., 2013). Together with our present findings, it is possible that drug-induced dopamine release can, via a D2R-mediated increase in the levels of Zif268, influence the expression of the growth factor to mediate the physiological effects of drugs of abuse.
The D2R-Gβγ-Zif268-GDNF pathway may also be implicated in hippocampal learning and memory. An age-related decline in the hippocampal expression of endogenous GDNF has been implicated in senescence-related deficits in learning and memory (Miyazaki et al., 2003), while increased levels of GDNF in the hippocampus may function to repair learning and memory impairments caused by chronic stress (Bian et al., 2012). Notably, Zif268 has long been known to play a role in long-term potentiation (LTP) (James et al., 2005; Knapska and Kaczmarek, 2004), as well as in various models of sensory-based learning (Da Costa et al., 1997; Mello, 2002; Mello et al., 1992; Okuno and Miyashita, 1996) and long-term memory (Jones et al., 2001). More recently, long-term potentiation (LTP), synaptic plasticity, and memory formation were found to be dependent upon D2R and Gβγ signaling (Saab et al., 2009). Although GDNF, Zif268, and D2R-activated Gβγ have been separately shown to contribute to the learning and memory functions of the hippocampus, whether they act in concert, or in the sequential pathway that we have identified in this study, remains to be tested.
In summary, we found that dopamine-mediated D2R activation leads to a Gβγ- and Erk1/2-mediated increase in the levels of Zif268. Importantly, this elevation in Zif268 levels is specifically required for the D2R-mediated upregulation of GDNF (model, Fig. 8). Our results are an important contribution to the understanding of the regulation of this critical growth factor. Moreover, these findings illustrate a novel pathway by which dopaminergic signaling, via the D2R, alters GDNF expression, with possible ramifications on learning and memory mechanisms, as well as for the treatment of Parkinson’s disease and drug addiction.
Acknowledgements
This research was supported by funds provided by the National Institutes of Health–National Institute on Alcohol Abuse and Alcoholism (NIH–NIAAA) RO1 AA014366 (D.R.), the state of California for medical research on alcohol and substance abuse through the University of California San Francisco (D.R.), NIH–NIAAA F31 AA017801 (S.A.).
Abbreviations used
- AdV
adenovirus
- ChIP
chromatin immunoprecipitation
- D2R
dopamine D2 receptor
- Erk1/2
extracellular signal-regulated kinase 1/2
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GDNF
glial cell line-derived neurotrophic factor
- GFRα1
GDNF family receptor-α1
- shRNA
small hairpin RNA
- Zif268
zinc-finger protein 268
Footnotes
The authors have no conflict of interest to declare.
References
- Ahmadiantehrani S, Barak S, Ron D. GDNF is a novel ethanol-responsive gene in the VTA: implications for the development and persistence of excessive drinking. Addict Biol. 2013 doi: 10.1111/adb.12028. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen M, Saarma M. The GDNF family: signaling, biological functions and therapeutic value. Nature Reviews Neuroscience. 2002;3:383–393. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Pickens CL, Stern AL, Wihbey KA, Harvey BK, Bossert JM, Liu QR, Hoffer BJ, Shaham Y. Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addict Biol. 2011;16:261–272. doi: 10.1111/j.1369-1600.2010.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault DT, Werling LL. SH-SY5Y cells as a model for sigma receptor regulation of potassium-stimulated dopamine release. Brain Res. 2000;877:354–360. doi: 10.1016/s0006-8993(00)02722-0. [DOI] [PubMed] [Google Scholar]
- Baecker PA, Lee WH, Verity AN, Eglen RM, Johnson RM. Characterization of a promoter for the human glial cell line-derived neurotrophic factor gene. Brain Res Mol Brain Res. 1999;69:209–222. doi: 10.1016/s0169-328x(99)00106-0. [DOI] [PubMed] [Google Scholar]
- Barak S, Ahmadiantehrani S, Kharazia V, Ron D. Positive autoregulation of GDNF levels in the ventral tegmental area mediates long-lasting inhibition of excessive alcohol consumption. Transl Psychiatry. 2011a;2011 doi: 10.1038/tp.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Carnicella S, Yowell QV, Ron D. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J Neurosci. 2011b;31:9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-Chinea P, Cruz-Muros I, Aymerich MS, Rodriguez-Diaz M, Afonso-Oramas D, Lanciego JL, Gonzalez-Hernandez T. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur J Neurosci. 2005;21:1815–1827. doi: 10.1111/j.1460-9568.2005.04024.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Y, Pan Z, Hou Z, Huang C, Li W, Zhao B. Learning, memory, and glial cell changes following recovery from chronic unpredictable stress. Brain Res Bull. 2012;88:471–476. doi: 10.1016/j.brainresbull.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- Bozzi Y, Borrelli E. Absence of the dopamine D2 receptor leads to a decreased expression of GDNF and NT-4 mRNAs in restricted brain areas. Eur J Neurosci. 1999;11:1275–1284. doi: 10.1046/j.1460-9568.1999.00541.x. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, He DY, Nielsen CK, Bartlett SE, Janak PH, Ron D. Cabergoline decreases alcohol drinking and seeking behaviors via glial cell line-derived neurotrophic factor. Biol Psychiatry. 2009a;66:146–153. doi: 10.1016/j.biopsych.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009b;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D. GDNF--a potential target to treat addiction. Pharmacol Ther. 2009;122:9–18. doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Qin ZH, Szele F, Bai G, Weiss B. Neuronal localization and modulation of the D2 dopamine receptor mRNA in brain of normal mice and mice lesioned with 6-hydroxydopamine. Neuropharmacology. 1991;30:927–941. doi: 10.1016/0028-3908(91)90106-l. [DOI] [PubMed] [Google Scholar]
- Choi EY, Jeong D, Park KW, Baik JH. G protein-mediated mitogen-activated protein kinase activation by two dopamine D2 receptors. Biochem Biophys Res Commun. 1999;256:33–40. doi: 10.1006/bbrc.1999.0286. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Bohn MC. Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res Dev Brain Res. 1995;85:80–88. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- Christy B, Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proc Natl Acad Sci U S A. 1989;86:8737–8741. doi: 10.1073/pnas.86.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa AP, Broad KD, Kendrick KM. Olfactory memory and maternal behaviour-induced changes in c-fos and zif/268 mRNA expression in the sheep brain. Brain Res Mol Brain Res. 1997;46:63–76. doi: 10.1016/s0169-328x(96)00272-0. [DOI] [PubMed] [Google Scholar]
- Faure M, Voyno-Yasenetskaya TA, Bourne HR. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Ghahremani MH, Forget C, Albert PR. Distinct roles for Galpha(i)2 and Gbetagamma in signaling to DNA synthesis and Galpha(i)3 in cellular transformation by dopamine D2S receptor activation in BALB/c 3T3 cells. Mol Cell Biol. 2000;20:1497–1506. doi: 10.1128/mcb.20.5.1497-1506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb SL, Jeanblanc J, Barak S, Yowell QV, Yaka R, Ron D. Lyn kinase regulates mesolimbic dopamine release: implication for alcohol reward. J Neurosci. 2011;31:2180–2187. doi: 10.1523/JNEUROSCI.5540-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazner GW, Mu X, Springer JE. Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol. 1998;391:42–49. doi: 10.1002/(sici)1096-9861(19980202)391:1<42::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–150. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Green-Sadan T, Kinor N, Roth-Deri I, Geffen-Aricha R, Schindler CJ, Yadid G. Transplantation of glial cell line-derived neurotrophic factor-expressing cells into the striatum and nucleus accumbens attenuates acquisition of cocaine self-administration in rats. Eur J Neurosci. 2003;18:2093–2098. doi: 10.1046/j.1460-9568.2003.02943.x. [DOI] [PubMed] [Google Scholar]
- Greenwood JM, Dragunow M. Muscarinic receptor-mediated phosphorylation of cyclic AMP response element binding protein in human neuroblastoma cells. J Neurochem. 2002;82:389–397. doi: 10.1046/j.1471-4159.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- Guo H, Tang Z, Yu Y, Xu L, Jin G, Zhou J. Apomorphine induces trophic factors that support fetal rat mesencephalic dopaminergic neurons in cultures. Eur J Neurosci. 2002;16:1861–1870. doi: 10.1046/j.1460-9568.2002.02256.x. [DOI] [PubMed] [Google Scholar]
- He DY, Ron D. Autoregulation of glial cell line-derived neurotrophic factor expression: implications for the long-lasting actions of the anti-addiction drug, Ibogaine. Faseb J. 2006;20:2420–2422. doi: 10.1096/fj.06-6394fje. [DOI] [PubMed] [Google Scholar]
- Heberlein A, Muschler M, Wilhelm J, Frieling H, Lenz B, Groschl M, Kornhuber J, Bleich S, Hillemacher T. BDNF and GDNF serum levels in alcohol-dependent patients during withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1060–1064. doi: 10.1016/j.pnpbp.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Everitt BJ, Lee JL. Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J Neurosci. 2006;26:12694–12699. doi: 10.1523/JNEUROSCI.3101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AB, Conway AM, Morris BJ. Genomic profiling of the neuronal target genes of the plasticity-related transcription factor -- Zif268. J Neurochem. 2005;95:796–810. doi: 10.1111/j.1471-4159.2005.03400.x. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK. Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Kress TR, Raabe T, Feller SM. High Erk activity suppresses expression of the cell cycle inhibitor p27Kip1 in colorectal cancer cells. Cell Commun Signal. 2010;8:1. doi: 10.1186/1478-811X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumahara E, Ebihara T, Saffen D. Nerve growth factor induces zif268 gene expression via MAPK-dependent and -independent pathways in PC12D cells. J Biochem. 1999;125:541–553. doi: 10.1093/oxfordjournals.jbchem.a022319. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lin WF, Chen CJ, Chang YJ, Chen SL, Chiu IM, Chen L. SH2B1beta enhances fibroblast growth factor 1 (FGF1)-induced neurite outgrowth through MEK-ERK1/2-STAT3-Egr1 pathway. Cell Signal. 2009;21:1060–1072. doi: 10.1016/j.cellsig.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Lonka-Nevalaita L, Lume M, Leppanen S, Jokitalo E, Peranen J, Saarma M. Characterization of the intracellular localization, processing, and secretion of two glial cell line-derived neurotrophic factor splice isoforms. J Neurosci. 2010;30:11403–11413. doi: 10.1523/JNEUROSCI.5888-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Ren Z, Ma Y, Xu L, Zhao Y, Zheng C, Fang Y, Xue T, Sun B, Xiao W. Targeted knockdown of EGR-1 inhibits IL-8 production and IL-8-mediated invasion of prostate cancer cells through suppressing EGR-1/NF-kappaB synergy. J Biol Chem. 2009;284:34600–34606. doi: 10.1074/jbc.M109.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV. Mapping vocal communication pathways in birds with inducible gene expression. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:943–959. doi: 10.1007/s00359-002-0347-1. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloso M, Villa D, Crimi M, Galbiati S, Donzelli E, Nicolini G, Tredici G. Retinoic acid-induced neuritogenesis of human neuroblastoma SH-SY5Y cells is ERK independent and PKC dependent. J Neurosci Res. 2004;75:241–252. doi: 10.1002/jnr.10848. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Okuma Y, Nomura J, Nagashima K, Nomura Y. Age-related alterations in the expression of glial cell line-derived neurotrophic factor in the senescence-accelerated mouse brain. J Pharmacol Sci. 2003;92:28–34. doi: 10.1254/jphs.92.28. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Ball SG, Vaughan PF. Potassium- and carbachol-evoked release of [3H]noradrenaline from human neuroblastoma cells, SH-SY5Y. J Neurochem. 1991;56:1810–1815. doi: 10.1111/j.1471-4159.1991.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Oh-hashi K, Ito M, Tanaka T, Hirata Y, Kiuchi K. Biosynthesis, processing, and secretion of glial cell line-derived neurotrophic factor in astroglial cells. Mol Cell Biochem. 2009;323:1–7. doi: 10.1007/s11010-008-9958-3. [DOI] [PubMed] [Google Scholar]
- Ohta M, Mizuta I, Ohta K, Nishimura M, Mizuta E, Hayashi K, Kuno S. Apomorphine up-regulates NGF and GDNF synthesis in cultured mouse astrocytes. Biochem Biophys Res Commun. 2000;272:18–22. doi: 10.1006/bbrc.2000.2732. [DOI] [PubMed] [Google Scholar]
- Okuno H, Miyashita Y. Expression of the transcription factor Zif268 in the temporal cortex of monkeys during visual paired associate learning. Eur J Neurosci. 1996;8:2118–2128. doi: 10.1111/j.1460-9568.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Pahlman S, Ruusala AI, Abrahamsson L, Mattsson ME, Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984;14:135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Picetti R, Saiardi A, Abdel Samad T, Bozzi Y, Baik JH, Borrelli E. Dopamine D2 receptors in signal transduction and behavior. Crit Rev Neurobiol. 1997;11:121–142. doi: 10.1615/critrevneurobiol.v11.i2-3.20. [DOI] [PubMed] [Google Scholar]
- Pochon NA, Menoud A, Tseng JL, Zurn AD, Aebischer P. Neuronal GDNF expression in the adult rat nervous system identified by in situ hybridization. Eur J Neurosci. 1997;9:463–471. doi: 10.1111/j.1460-9568.1997.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH. Neurotrophic factor therapy for Parkinson's disease. Prog Brain Res. 2010;184:237–264. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- Rusanescu G, Qi H, Thomas SM, Brugge JS, Halegoua S. Calcium influx induces neurite growth through a Src-Ras signaling cassette. Neuron. 1995;15:1415–1425. doi: 10.1016/0896-6273(95)90019-5. [DOI] [PubMed] [Google Scholar]
- Saab BJ, Georgiou J, Nath A, Lee FJ, Wang M, Michalon A, Liu F, Mansuy IM, Roder JC. NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory. Neuron. 2009;63:643–656. doi: 10.1016/j.neuron.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Saavedra A, Baltazar G, Duarte EP. Driving GDNF expression: the green and the red traffic lights. Prog Neurobiol. 2008;86:186–215. doi: 10.1016/j.pneurobio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Sarkanen JR, Nykky J, Siikanen J, Selinummi J, Ylikomi T, Jalonen TO. Cholesterol supports the retinoic acid-induced synaptic vesicle formation in differentiating human SH-SY5Y neuroblastoma cells. J Neurochem. 2007;102:1941–1952. doi: 10.1111/j.1471-4159.2007.04676.x. [DOI] [PubMed] [Google Scholar]
- Schaar DG, Sieber BA, Dreyfus CF, Black IB. Regional and cell-specific expression of GDNF in rat brain. Exp Neurol. 1993;124:368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- Semba J, Akanuma N, Wakuta M, Tanaka N, Suhara T. Alterations in the expressions of mRNA for GDNF and its receptors in the ventral midbrain of rats exposed to subchronic phencyclidine. Brain Res Mol Brain Res. 2004;124:88–95. doi: 10.1016/j.molbrainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Shin SY, Song H, Kim CG, Choi YK, Lee KS, Lee SJ, Lee HJ, Lim Y, Lee YH. Egr-1 is necessary for fibroblast growth factor-2-induced transcriptional activation of the glial cell line-derived neurotrophic factor in murine astrocytes. J Biol Chem. 2009;284:30583–30593. doi: 10.1074/jbc.M109.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg I, Bjorklund L, Johansson M, Tomac A, Collins F, Olson L, Hoffer B, Humpel C. Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp Neurol. 1993;124:401–412. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Tosetti P, Taglietti V, Toselli M. Functional changes in potassium conductances of the human neuroblastoma cell line SH-SY5Y during in vitro differentiation. J Neurophysiol. 1998;79:648–658. doi: 10.1152/jn.1998.79.2.648. [DOI] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, Suzuki T, Miyata N, Watanabe Y. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 2009;33:945–969. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Ahmadiantehrani S, He DY, Barak S, Kharazia V, Ben Hamida S, Zapata A, Shippenberg TS, Ron D. Nucleus accumbens-derived glial cell line-derived neurotrophic factor is a retrograde enhancer of dopaminergic tone in the mesocorticolimbic system. J Neurosci. 2010;30:14502–14512. doi: 10.1523/JNEUROSCI.3909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury D, Schaar DG, Ramakrishnan L, Black IB. Novel structure of the human GDNF gene. Brain Res. 1998;803:95–104. doi: 10.1016/s0006-8993(98)00627-1. [DOI] [PubMed] [Google Scholar]
- Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, Wu CP, Lu B. GDNF acutely modulates excitability and A-type K(+) channels in midbrain dopaminergic neurons. Nat Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]