Abstract
Bipolar disorder (BD) is a pervasive neuropsychiatric disorder characterized by episodes of mania and depression. The switch between mania and depression may reflect seasonal changes and certainly can be affected by alterations in sleep and circadian control. The circadian locomotor output cycles kaput (CLOCK) protein is a key component of the cellular circadian clock. Mutation of the Clock gene encoding this protein in Clock△19 mutant mice leads to behavioral abnormalities reminiscent of BD mania. To date, however, these mice have not been assessed in behavioral paradigms that have cross-species translational validity. In the present studies of Clock△19 and wildtype (WT) littermate mice, we quantified exploratory behavior and sensorimotor gating, which are abnormal in BD manic patients. We also examined the saccharin preference of these mice and their circadian control in different photoperiods. Clock△19 mice exhibited behavioral alterations that are consistent with BD manic patients tested in comparable tasks, including hyperactivity, increased specific exploration, and reduced sensorimotor gating. Moreover, compared to WT mice, Clock△19 mice exhibited a greater preference for sweetened solutions and greater sensitivity to altered photoperiod. In contrast with BD manic patients however, Clock△19 mice exhibited more circumscribed movements during exploration. Future studies will extend the characterization of these mice in measures with cross-species translational relevance to human testing.
Keywords: Clock, bipolar disorder, mania, cycling, PPI, hedonia, circadian
1. INTRODUCTION
Bipolar disorder (BD) in its various forms affects approximately 3% of the population and is a debilitating illness that impacts every aspect of the lives of sufferers and their loved ones [1]. Current treatments for BD have been found serendipitously as no treatments have been developed specifically to target the mechanism(s) underlying this disorder [2]. This lack of treatment development could reflect the simplicity of behavioral models used to date [2, 3] that neither recreate the mechanism underlying BD [4] nor reflect the complexity of BD symptoms. BD is a unique disorder in that it is characterized by sufferers cycling through periods of mania and depression, the symptoms of which differing markedly in these phases [4, 5]. Mania is associated with hyperactivity, hypersexuality, risk-taking, less need for sleep, aggression, and hedonic behavior [6]. Depression is largely the opposite however, with symptoms including low sex-drive, increased sleep, lethargy, and anhedonia [6]. Surprisingly, sufferers of BD can cycle between these two states, often linked to the seasons of the year [7, 8]. Such cycling may be explained by the evolutionary origin theory of BD, postulating that BD may have first arisen in people from the northern hemisphere where lengthening and shortening photoperiods (daylight length) in the summer and winter respectively induced mania- and depressive-like behaviors [9]. This theory provides an avenue by which BD may be modeled since this theory suggests that alterations in the photoperiod length underlie BD. Hence, by examining mechanisms regulating circadian rhythms, it may be possible to model aspects of BD.
The basic molecular loop that regulates circadian rhythms consists of transcription factors regulating their own expression over 24 hours [10]. The circadian locomotor output cycles kaput (CLOCK) protein binds to brain and muscle ARNT-like protein 1 (BMAL1). The heterodimer then regulates the expression of the period (Per) and cryptochrome genes, which bind together as proteins, enter the nucleus, and inhibit CLOCK and BMAL1 activity [11]. These systems are entrained by light via the suprachiasmatic nucleus [12], which may explain why light therapy works for sufferers of seasonal affective disorder [13], a depression that occurs during short photoperiod seasons (i.e., winter). Moreover, it is recognized that there is a disruption in the circadian rhythm in people with BD [14]. Social rhythm therapy – generating rhythms of behavior that are consistent from day to day [15] – or using extended bed rest and darkness [16a] reduced some symptoms of BD. Interestingly, sleep deprivation can alleviate depression symptoms [17], but can also induce a manic episode in people with a predisposition for BD [18]. Thus, altered circadian rhythm can impact the current state of people with BD.
Because behaviorally augmenting the circadian rhythm is beneficial for aspects of BD, it will be useful to investigate whether disrupting these rhythms produces BD-relevant behaviors. Mice with a deletion of exon 19 in the CLOCK gene (Clock△19 mice) exhibit abnormal behaviors that have been interpreted as ‘mania-like’. For example, Clock△19 mice are hyperactive, exhibit an altered circadian rhythm, spend less time immobile in a forced swim test, exhibit a preference for sweet sucrose solution and cocaine, and have lower reward thresholds identified using intra-cranial self-stimulation [19, 20]. Importantly, some of these behaviors are attenuated by lithium treatment [19], a common treatment for BD. The mania-like behavior of these mice may be mediated by increased dopamine firing in the ventral tegmental area, which can also be reversed by lithium treatment [21]. Moreover, genetic associations of a polymorphism in the 3′ flanking region of the CLOCK (3111 T to C) in people with BD was linked with more frequent episodes of mood disturbances and reduced need for sleep [22, 23]. Hence, it has been postulated that Clock△19 mice model aspects of BD.
While evidence continues to be collected that Clock△19 mice may be a viable model for aspects of BD, as yet no studies have directly utilized cross-species tests to examine the validity of this model in terms of behaviors quantified in people with BD. Previously, we utilized measures of behaviors that are available in both humans and rodents, e.g., exploration in the behavioral pattern monitor (BPM) and sensorimotor gating measured by prepulse inhibition (PPI) of the startle reflex, to model aspects of psychiatric disorders [24]. For example, using the BPM we identified that acutely manic patients with BD exhibit hyperactivity [25], increased specific exploration [26], and more direct movements through space [27, 28]. Moreover, this abnormal exploration is consistent over time [29], and can be recreated in mice by selectively reducing the function of the dopamine transporter (DAT) via genetic or pharmacological means [27, 28, 30-33]. Reduced PPI has been observed in people with BD [34], a behavior that can also be modeled in rodents [35, 36]. Similarly, PPI is impaired in mice with a hyperdopaminergic tone due to a lack of DAT, an impairment that can be attenuated with antipsychotic treatment [37, 38]. Assessing Clock△19 mice in tests having cross-species translational validity would test the appropriateness of these mice as a model for BD.
Herein, we utilized the cross-species BPM and PPI paradigms to examine the similarity of profiles of Clock△19 mice to BD mania. Moreover, we examined the behavior of these mice in the saccharin preference test [39], in order to assess hedonia-like behavior, and their circadian rhythm in response to altered photoperiod lengths. In parallel with BD, we hypothesized that Clock△19 mice would exhibit: 1) an abnormal exploratory profile of increased activity and specific exploration; more straight-line movements through space; 2) impaired sensorimotor gating; 3) hedonia-like preference for rewarding stimuli; and 4) less control over their circadian rhythm in response to altered photoperiod lengths.
2. METHODS
2.1. Animals
Clock△19 mutant mice with a dominant-negative CLOCK protein defective in transcriptional activation activity were created through N-ethyl-N-nitrosourea mutagenesis as described [40]. Male (n=20) and female (n=13) Clock△19 mutant mice and male (n=17) and female (n=22) wildtype (WT) littermate controls on a mixed BALBc:C57BL/6 background were used throughout the different studies. Clock△19 heterozygous breeders were sent to our laboratory from David Welsh, (University of California San Diego; UCSD). All Clock△19 WT and mutant mice used in the present studies resulted from a heterozygous breeding colony in the vivarium at UCSD. Mice were group housed (maximum 4/cage, 2/cage for the saccharin and circadian rhythm tests), maintained in a temperature controlled vivarium (21±1 °C) on a reversed day-night cycle (lights on at 19:00, off at 07:00 hrs), and tested during the dark phase between 8:00 and 13:00hrs. Mice were 3 - 5.5 months old at the time of all tests except the for the saccharin and circadian rhythm tests, at which time mice were 11 months old. Mice had ad libitum access to water and food (Harlan, Madison, WI, USA) except during testing. All procedures were approved by the UCSD Institutional Animal Care and Use Committee. The UCSD animal facility meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care.
2.2. Mouse Behavioral Pattern Monitor
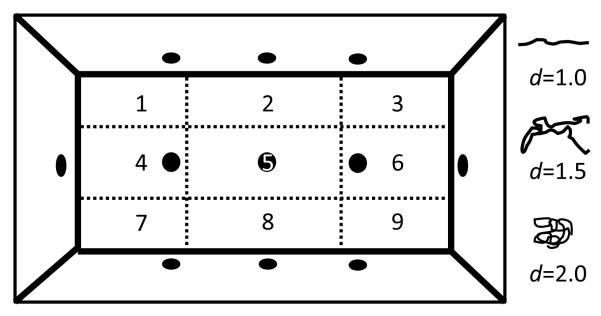
Locomotor behavior and exploration was examined in eight mouse BPM chambers (BPM; San Diego Instruments, San Diego, CA) as described previously [41-43]. In brief, each Plexiglas chamber consists of a 30.5 × 61 × 38-cm area, equipped with three floor holes and eight wall holes (three along each side of the long walls and one in each of the short walls; 1.25 cm in diameter, 1.9 cm from the floor; see Figure 1), containing infrared photobeams to detect holepoking behavior. Each chamber is enclosed in an outer box to minimize external light and noise, with an internal white light above the arena (producing 350 lux in the center and 92 lux in the four corners). Subject activity was obtained from a grid of infrared photobeams 1 cm above the floor (2.5 cm apart along the length and the width of the chamber; 24 × 12 X-Y array), recording the location of the mouse every 0.1 s. Rearing behavior was detected by another set of 16 photobeams, located on the Y-axis only and placed 2.5 cm above the floor. The subject’s position was defined across nine unequal regions (four corners, four walls and center [44]). At the start of the session, the mouse was placed in the bottom left-hand corner of the arena and the test session started immediately for a period of 60 min. Primary measures obtained were transitions across the defined regions and center entries (locomotor activity), holepoking, rearing, and center duration (exploratory behavior), and entropy (h) and scaling measures (locomotor patterns). Lower values of h suggest predictable, ordered sequences of activity, while higher values of h indicate greater variety or disorder of movement. Spatial d quantifies the geometric structure of the locomotor path (see Figure 1), where a value of 1 represents a path in a straight distance-covering line, and 2 highly circumscribed small-scale movements [45]. The spatial coefficient of variation (CV) is a measure of the X-Y pattern representing the variation of transitions across the nine regions. Spatial CV increases when the mouse repeats certain transitions across the chamber regions. The temporal CV measures the amount of time spent in each region, where a high temporal CV indicates a substantial preference for some region(s) over others [44].
Figure 1. Schematic of the mouse Behavioral Pattern Monitor.
The arena was divided into nine unequal regions (1 – 9) on which transitions, center time, center duration, and the coefficient of variation calculations are based. The quantifiable measure spatial d was used to describe the subject’s pattern of movement with values represented in the schematic. The location of the mouse was obtained from a grid of infrared photobeams (24 × 12 X-Y array) located 1 cm above the floor. Another set of 16 photobeams located 2.5 cm above the floor on the Y-axis only was used to detect rearing behavior. The chamber is equipped with three floor holes and eight wall holes (1.2 cm diameter), each containing an infrared photobeam to detect holepoking behavior.
2.2.2. BPM – Initial Assessment
Male (n=20) and female (n=13) Clock△19 mutant mice and male (n=17) and female (n=22) WT littermate controls were tested in the BPM to examine the exploratory profiles of these mice.
2.2.3. BPM – Repeated test to examine reproducibility of effect
A subgroup of mice from experiment 1 (Clock△19 mutant male, n=6; female, n=4; WT male, n=7; female, n=7) were retested in the BPM one week after their initial testing. This test was conducted 7to determine whether any abnormal exploratory behavior exhibited by mutant mice would be reproducible.
2.3. Sensorimotor gating of the acoustic startle response
Sensorimotor gating of the acoustic startle response of a behaviorally naïve cohort of Clock△19 mice (WT male, n=7; female, n=7; mutant male, n=6; female, n=4) was examined in eight startle chambers (SR-LAB, San Diego Instruments, San Diego, CA), each consisting of a Plexiglas cylinder, 5 cm in diameter, resting on a platform in a ventilated sound-attenuating chamber as described previously [37, 46]. Speakers mounted 33 cm above the cylinders produced all acoustic stimuli and an interface and computer assembly stored and digitized movements of the animal transduced by piezoelectric accelerometers mounted under the cylinders. A 65 dB background sound and light delivered by an incandescent bulb located on the ceiling of the chamber were presented continuously throughout the session. Mice were placed into the startle chambers and testing was initiated after a 5 min acclimation period. Startle pulses were 40 ms and prepulses were 20 ms in duration. The inter-trial interval between stimulus presentations ranged between 3- 12 s (7 s average) for both experiments. The acoustic startle sessions included five blocks. The first block included only five 120 dB pulses. The second block consisted of three different prepulse trials: 69, 73, and 81 dB prepulses preceding a 120 dB pulse. The third block included acoustic startle responding only and included stimulus intensities of 80, 90, 100, 110, and 120 dB. The fourth block varied the inter-stimulus interval (ISI), consisting of 73 dB prepulses preceding pulses at 120 dB by 25, 50, 100, 200, and 500 ms. The fifth and final block delivered five 120 dB pulses and together with the first block served to assess habituation. This test session has been used and described previously [46]. The amount of PPI was calculated as a percentage score for each prepulse intensity based on the 120 dB pulse within that block: %PPI = 100 – [(startle magnitude for prepulse + pulse / startle magnitude for pulse alone) × 100].
2.4. Sweet solution preference
The design of this test was based on previous mania-modeling studies of [39]. A subset of male Clock△19 mutant (n=8) and WT (n=8) mice from experiment 2.2.2. were supplied with a bottle of 1.0% saccharin sodium dihydrate solution (Sigma, St, Lewis, MI), dissolved in tap water, on top of the regular supply of water and food. Both the regular water bottle and the saccharin solution bottle were available to the mice throughout the entire sweet solution preference test. Both bottles were weighed at the beginning of the study and 24 h thereafter, for four days. Sweet solution preference was calculated daily as a percentage of saccharin solution out of total liquid consumption.
2.5. Measuring running wheel activity to assess circadian rhythm
After experiment 2.4., male Clock△19 mice (WT n=8, mutant=8) were housed by genotype with 2 mice per cage. Mice had access to a running wheel (Silent Spinner; Forest city, Iowa). The level of running wheel activity was measured using a cyclcomputer (Easton-Bell Sports; Van Nuys, CA) to determine the distance traveled by mice over time. Measurements were taken at 07:00 and 19:00 hrs daily for 10 days, coinciding with the time room lights were turned off and on respectively as a surrogate measure of circadian rhythm. Mice were initially exposed to a 12 hr light/dark (LD) cycle. After stable running wheel activity was established (Day 7, see 3.5.), the lighting of the room was altered to increase the inactive light period. Thus, while lights continued to be turned off at 07:00 hrs, lights were turned back on at 08:00 hrs (LD 23:1). The running wheel activity of mice continued to be measured at 07:00 and 19:00 hrs.
Statistical analyses
Data from the BPM were analyzed using two- or three-way analyses of variance (ANOVA), with sex and genotype as between-subject factors and trial period (three 20 min periods) as a within-subject factor. PPI data were analyzed using a two- or three-way ANOVA, with prepulse intensity as a within-subject factor and genotype and sex as a between subject-factor. Further assessments used startle-matched subgroup comparisons and loglinear regression analyses with weight to assess PPI. Sweet solution preference was analyzed using a repeated measure ANOVA with day as a within-subject factor and genotype as a between-subject factor. Running wheel activity was assessed using a three-way ANOVA with genotype as a between subjects factor while time of measurement and day were within-subjects factors. Tukey post hoc analyses were performed where applicable. When no effect of sex or interaction with sex was observed, data were pooled and reanalyzed. Pearson r correlation coefficients measured the relationship between BPM measures from the first to the second test. All BPM and PPI data were analyzed using Biomedical Data Programs statistical software (Statistical Solutions Inc., USA), while sweet solution preference and running wheel activity levels were analyzed using SPSS (19.0, Chicago, IL, USA). The α level was set to 0.05.
3. RESULTS
3.1. BPM Exploration: Initial characterization
To assess the exploratory profile of Clock△19 mice, mutant (n=33) and WT littermate (n=39) mice were tested in the BPM for 60 min. There were no interactions with sex for any of the measures. Male and female data were therefore pooled and analyzed together. Data are presented with variables grouped into domains of locomotor activity, specific exploration (holepoking) and diversive expoloration (rearing), and locomotor patterns based on the primary variables affected in people with BD mania [28], as well as factor analyses of rat and mouse BPM behavior [43, 47].
3.1.1. Locomotor activity
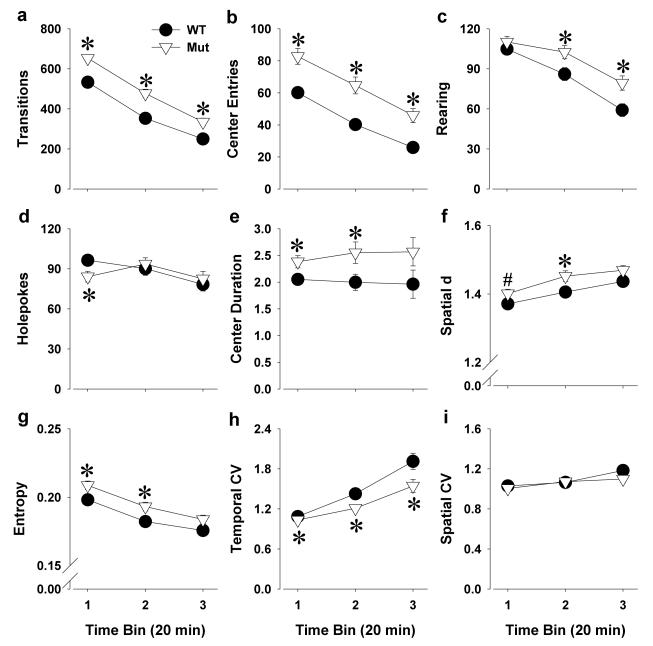
Clock△19 mutant mice were hyperactive with representative X-Y patterns and average activity level heat maps, presented in Figure 2. The hyperactivity of mutant mice was quantified by increased transitions (F(1,70)=19.8, p<0.0001; Figure 3a) and increased center entries (F(1,70)=18.6, p<0.0001; Figure 3b) compared to WT mice. A trend towards a time by genotype interaction was observed for transitions (F(2,140)=2.5, p<0.1). Post hoc analyses revealed that mutant mice exhibited more transitions compared to WT mice in each time period however (p<0.05).
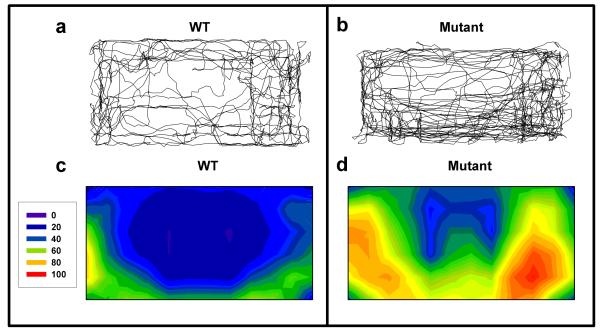
Figure 2. X-Y plots and heat maps of Clock△19 WT and mutant mice.
Representative X-Y plots of wildtype (WT) and mutant mice (a-b) as well as heat maps representing the average group data based on 72 evenly distributed sector entries (c-d) are displayed. Clock△19 mutant mice (b and d) exhibited increased activity and center entries compared to WT mice (a and c). Moreover, more disordered patterns of movement were noticeable in the mutant mice compared to WT mice.
Figure 3. The exploratory profile of Clock△19 WT and mutant mice in the BPM.
Clock△19 mutant (Mut) mice were hyperactive compared to wildtype (WT) littermate mice as measured by increased transitions (a) and center entries (b). Mutant mice exhibited more specific exploration compared to WT mice as measured by increased rearing (c), but not holepoking (d). Mutant mice also spent significantly more time in the center of the arena (e). Mutant mice also exhibited more circumscribed or disordered patterns of movement compared to WT mice as reflected by a higher spatial d (f) and entropy h (g). Compared to WT mice, mutant mice exhibited less preference for specific regions in the arena as reflected by lower temporal CV (h), without an effect on spatial CV (i). Data are presented as mean +S.E.M. *p<0.05 and #p<0.1 when compared to WT mice.
3.1.2. Exploratory behavior
Over 60 min, Clock△19 mutant mice exhibited greater exploration as reflected by increased rearing (F(1,70)=5.9, p<0.05; Figure 3c), but not holepoking (F<1, ns; Figure 3d) compared to WT mice. Analyzed within time however, interactions with genotype interactions were observed for both rearing (F(2,140)=4.0, p<0.05) and holepoking (F(2,140)=5.4, p<0.01). Post hoc analyses revealed that mutant mice made fewer holepokes than WT mice only in time period 1 (p<0.05), while exhibiting increased rearing compared with WT mice in the latter 2 time periods (p<0.05). Mutant mice also spent significantly more time in the center compared to WT mice (center duration; F(1,70)=6.6, p<0.05; Figure 3e), indicative of higher specific exploration [43].
3.1.3. Locomotor patterns
Clock△19 mutant mice moved in more circumscribed patterns compared to WT mice as reflected by increased spatial d (F(1,70)=4.6, p<0.05; Figure 3f). Mutant mice also exhibited a higher entropy (F(1,70)=5.0, p<0.05; Figure 3g) and lower temporal CV (F(1,70)=7.0, p<0.05; Figure 3h). Although there was a time by genotype interaction for temporal CV (F(2,140)=3.5, p<0.05) post hoc analyses revealed that mutant mice exhibited a lower temporal CV in each time period however (p<0.05). No differences between genotypes were observed for spatial CV (F=1.1, ns; Figure 3i).
3.2. BPM exploration; examining the consistency of the exploratory profile
To assess the consistency of the altered exploratory profile of Clock△19 mice, mutant (n=10) and WT littermate (n=14) mice were tested in the BPM for 60 min a second time one week later. Again, because there were no interactions with sex for any of the measures, data from male and female were pooled and analyzed together. Intra-subject comparisons between the two tests revealed significant correlations for all primary measures (Table 1).
Table 1.
Test-retest reliability of all primary measures in the BPM between testing days 1 and 2 as determined by correlation coefficients
| Measure | R-value | P-value |
|---|---|---|
| Transitions | 0.76 | <0.001 |
| Holepoking | 0.63 | <0.005 |
| Rearing | 0.70 | <0.001 |
| Spatial d | 0.84 | <0.001 |
| Entropy (h) | 0.71 | <0.001 |
| Spatial CV | 0.59 | <0.005 |
| Temporal CV | 0.45 | <0.05 |
| Center duration | 0.52 | <0.05 |
| Center entries | 0.81 | >0.001 |
CV = coefficient of variation
3.2.3. Locomotor activity
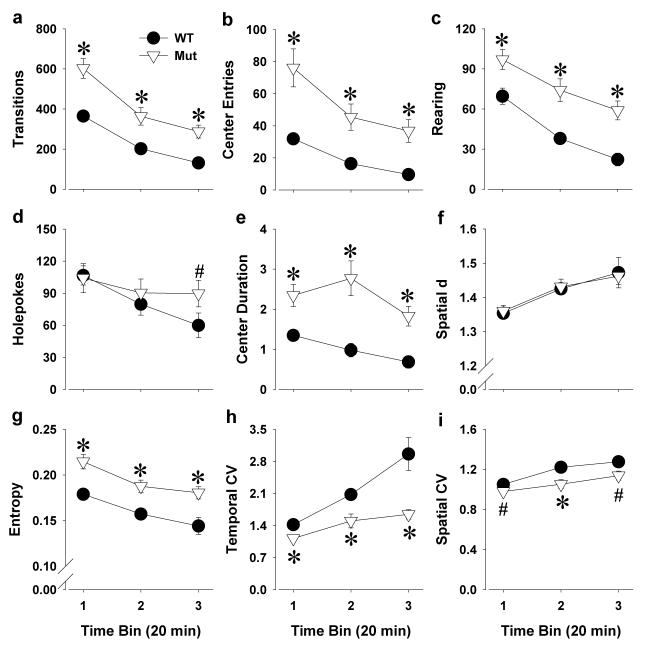
Clock△19 mutant mice were hyperactive as reflected by increased transitions (F(1,22)=26.7, p<0.0001; Figure 4a) and increased center entries (F(1,22)=19.7, p<0.0005; Figure 4b) compared to WT mice. Time by genotype interactions were observed for transitions (F(2,44)=3.2, p<0.05) and center entries (F(2,44)=4.1, p<0.05). Post hoc analyses revealed that mutant mice exhibited increased transitions and center entries compared with WT mice in each time point (p<0.05).
Figure 4. The exploratory profile of Clock△19 WT and mutant mice tested in the BPM a second time, one week after their initial testing.
Clock△19 mutant (Mut) mice remained hyperactive compared to wildtype (WT) littermate mice even upon retesting as measured by increased transitions (a) and center entries (b). More specific exploration was observed in mutant mice compared to WT mice as again reflected by increased rearing (c), but not so much holepoking (d). Mutant mice spent more time in the center of the arena compared to WT mice (e). Spatial d did not differ by genotype (f) in this second test, while mutant mice still exhibited disordered patterns of movement compared to WT mice as reflected by higher entropy h (g). Compared to WT mice, mutant mice exhibited a lower temporal and spatial CV, reflecting less preference for and reduced repetitive transitions between specific regions. Data are presented as mean +S.E.M. *p<0.05 and #p<0.1 when compared to WT mice.
3.2.2. Exploratory behavior
Clock△19 mutant mice exhibited higher exploration as reflected by increased rearing (F(1,22)=22.1, p<0.0001; Figure 4c), but again not for holepoking (F<1, ns; Figure 4d) compared to WT mice. Genotype interacted with time period to affect holepoking (F(2,44)=3.3, p<0.05), and post hoc analyses revealed that mutant mice exhibited a trend toward increased holepoking compared with WT mice in the last time period (p<0.1). Support for increased exploration in mutant mice was also observed with these mice spending more time in the center compared to WT mice (center duration; F(1,22)=25.1, p<0.0001; Figure 4e). A trend toward a time by genotype interaction was observed for center duration (F(2,44)=2.9, p<0.1), with post hoc analyses revealing that mutant mice spent more time in the center than WT mice at each time point (p<0.05)
3.2.3 Locomotor patterns
Spatial d did not differ between genotypes (F<1, ns; Figure 2f), but Clock△19 mutant mice exhibited a higher entropy (F(1,22)=16.3, p<0.0005; Figure 4g) and lower temporal (F(1,22)=15.6, p<0.001; Figure 4h) and spatial CV (F(1,22)=8.8, p<0.01; Figure 4i). A time by genotype interaction was observed for temporal CV (F(2,44)=4.1, p<0.05). Post hoc analyses revealed that mutant mice exhibited a lower temporal CV in each time period however (p<0.05).
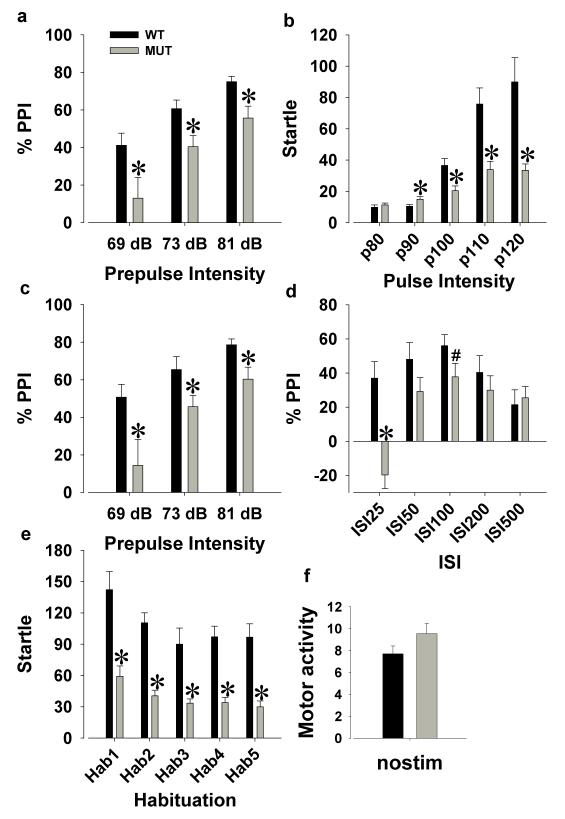
3.3. Sensorimotor Gating
To assess the sensorimotor gating of Clock△19 mice, mutant (n=10) and WT littermate (n=14) mice were tested on prepulse inhibition (PPI) in the acoustic startle test. There were no interactions with sex for any of the acoustic startle measures. Male and female data were therefore pooled and analyzed together.
A main effect of prepulse (F(2,44)=51.9, p<0.0001) and no interaction with genotype (F<1, ns) revealed that the sensorimotor gating of mice improved with higher prepulse intensities. Importantly, mutant mice exhibited a significant PPI deficit compared with WT mice (F(1,22)=8.4, p<0.01; Figure 5a) at every prepulse intensity (p<0.05). Mutant mice exhibited a lower startle amplitude than WT mice (F(1,22)=13.5, p<0.005; Figure 5b), with a pulse by genotype interaction (F(4,88)=7.4, p<0.005). Post hoc analyses revealed that mutant mice exhibited lower startle than WT mice at pulse intensities 90-120 (p<0.05). An increased startle amplitude with higher pulse intensities was observed for both genotypes (F(4,88)=22.9, p<0.0001). Consistent with previous studies when startle differences were observed [48], PPI was re-examined in WT and mutant mice matched for startle reactivity. Following baseline matching (WT, n=7; mutant, n=8), Clock△19 mutant mice still exhibited a significantly lower PPI compared to WT mice (F(1,13)=5.8, p<0.05; Figure 5c). We also addressed the potential influence of weight on startle measures and observed no difference in weight between WT (M=24.1 g) and mutant (M=27.1 g) mice (T=-1.7, ns), including the subgroup matched for startle reactivity (WT; M=24.1 g, mutant; M=27.3 g, T=-1.4, ns). Furthermore, weight did not influence PPI or startle reactivity as measured by linear regression (overall, F(1,22)<1, ns; in WT only, F(1,12)<1, ns; or in mutant only, F(1,8)<2, ns). . There was a trend effect of mutant mice exhibiting lower PPI than WT mice when split by ISI (F(1,22)=3.6, p<0.1; Figure 5d), with an ISI by genotype interaction (F(4,88)=8.9, p<0.0001). Post hoc analyses revealed that mutant mice exhibited a PPI deficit at ISI 25 (p<0.05) and a trend towards a deficit at ISI 100 (p<0.1). Although both WT and mutant mice habituated over time (F(4,88)=5.5, p<0.001), mutant mice again exhibited significantly lower startle levels (F(1,22)=34.6, p<0.0001; Figure 5e), with post hoc analyses revealing the presence of lower startle at each habituation phase. No difference between genotypes was observed for movements when no stimulus was presented (F=2.5, ns; Figure 5f).
Figure 5. Evaluation of the sensorimotor gating of the acoustic startle response of Clock△19 WT and mutant mice.
Clock△19 mutant (Mut) mice exhibited significantly lower prepulse inhibition (PPI) compared to wildtype (WT) littermate mice (a), but also exhibited reduced overall amplitude of the startle response (b). When mice were matched by startle amplitude and compared, mutant mice still exhibited significantly lower PPI compared to WT mice (c). When split by inter-stimulus interval (ISI), mutant mice exhibited a PPI deficit compared to WT mice at ISI 25 and ISI 100 (d). Both WT and mutant mice exhibited habituation over time, although mutant mice had lower startle amplitude compared to WT mice at each habituation phase (e). No difference between genotypes was observed when no stimulus was presented (f). Data are presented as mean ±S.E.M. *p<0.05 and #p<0.1 when compared to WT mice.
3.4. Sweet solution preference
Both WT (±75%) and Clock△19 mutant mice (±89%) exhibited a high sweet solution preference, which decreased over the four testing days (F(3,42)=6.7, p<0.005; Figure 6) independent of genotype. These preference levels are a little higher compared to previously described data of various mouse strains [39]. No main effect of genotype was observed when analyzed over all four testing days (F(1,14)=2.8, p=0.116). Given higher sucrose preference in these mice having been observed before, we examined their preference over individual days. When examined over days, mutant mice exhibited a higher sweet solution preference compared with WT mice on day 1 (p<0.05) and tended to be higher on day 4 (p<0.1).
Figure 6. Preference for 1.0% saccharin solution of Clock△19 WT and mutant mice across a four days test.
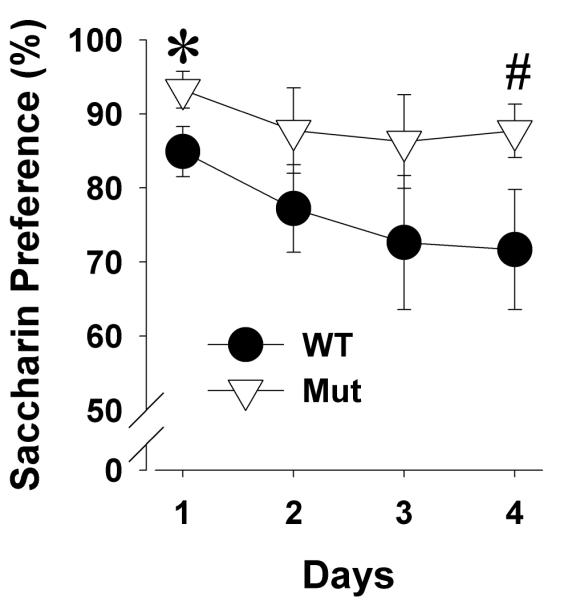
Both WT and mutant (Mut) mice exhibited a preference for the sweet solution that decreased over time. Clock△19 mutant mice exhibited a higher preference compared to WT mice on the first and last day. Data are presented as mean ±S.E.M. *p<0.05 and #p<0.1 when compared to WT mice.
3.5. Running wheel-based assessment of circadian rhythms
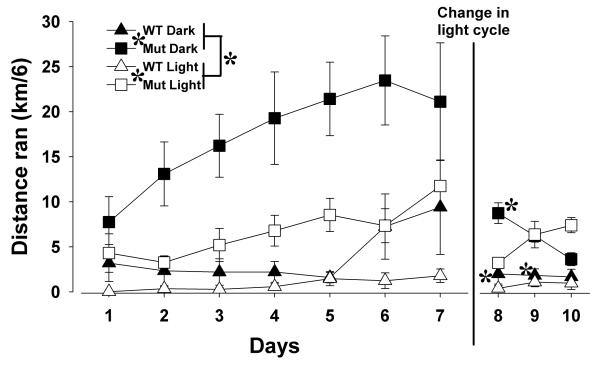
Running wheel activity levels of WT and mutant mice were initially assessed in an LD 12:12 light/dark cycle (12 hrs light, 12 hrs dark; Figure 7). Clock△19 mutant mice exhibited more activity overall (F(1,14)=22.9, p<0.0001), in both the dark (F(1,14)=45.1, p<0.0001) and light (F(1,14)=17.2, p<0.0001) phases. Mice were more active in the dark period (F(1,14)=31.3, p<0.0001), but the size of this effect depended on genotype (F(1,14)=9.5, p<0.01), reflecting a greater increase of dark period activity compared with light period in the mutant mice (large effect size d=1.02) compared with WT mice (medium to large effect size d=0.59). After introduction of the running wheels, all mice increased activity over the days in LD 12:12 (F(6,84)=6.7, p<0.001), independent of genotype (F=2.1, ns), and reached stable levels by day 5, as days 5, 6, and 7 did not differ in the WT or mutant mice (p>0.1).
Figure 7. Home cage running wheel activity of Clock△19 WT and mutant mice across seven days of LD 12:12 and three days of LD 23:1.
Both WT and mutant (Mut) mice were more active during the dark (D) than the light (L) phase during the seven days of LD 12:12. For the first two days in LD 23:1, WT mice maintained greater activity during the 12 hrs previously in darkness (active phase). Mutant mice however, rapidly lost maintenance of their circadian rhythm by the second day of LD 23:1, exhibiting equal activity during the 12 hrs previously in darkness (active phase) and the 12 hrs previously in light (rest phase). Finally, mutant mice were more active than the WT mice in both photoperiods. Data are presented as mean home cage running activity ±S.E.M. * denotes p<0.05 when compared with mutant mice, # denotes p<0.05 when compared with activity during what was the 12 hour L cycle.
Subsequently, the light cycle was changed from LD 12:12 to LD 23:1 (23 hrs light, 1 hr dark). Over the next three days, the activity of the mice continued to be measured during the 12 hrs previously in dark (active phase) and the 12 hrs previously in light (rest phase). A significant interaction between day, phase, and genotype was observed (F(2,28)=21.5, p<0.0001), with post hoc analyses revealing that in those three days, WT mice continued to exhibit more activity in active phase compared with rest phase for all three days (F(1,7)=7.2, p<0.05), while Clock△19 mutant mice only exhibited such a distinct difference on day 1 (F(1,7)=6.0, p<0.05), but not on days 2 or 3 (F<1, ns). Despite the change in lighting, mutant mice remained more active than WT mice irrespective of phase (F(1,14)=22.6, p<0.0001).
4. DISCUSSION
Clock△19 mutant mice exhibited abnormal behavior in several cross-species tests that measure aspects of BD mania. Mutant mice were hyperactive and exhibited increased specific exploration in the BPM, consistent with patients with BD in a manic [26, 28] and euthymic phase [29]. The mutant mice also exhibited altered startle responses and modest sensorimotor gating deficits similar to patients with BD [34]. Moreover, we have replicated the preference of mutant mice for sweet solution but using a non-caloric saccharin solution. Finally, we confirmed that these mice are even hyperactive in their home cage and importantly, that mutant mice exert less control of their circadian rhythm of activity in response to altered photoperiods. Thus, here we provide further support that Clock△19 mutant mice share numerous similarities to patients with BD by using cross-species translational tests.
The present studies of increased transitions and center entries support previous reports of hyperactive behavior in Clock△19 mutant mice both in a novel environment and in their home cage [19]. Importantly, these findings are consistent with the increased activity of patients with BD both in a manic and euthymic state [28, 29]. Moreover, because the present studies examined exploratory behaviors of Clock△19 mutant mice in the BPM, we also quantified increased exploration as measured by increased rearing and center duration in these mice, which collectively load onto a diversive exploratory factor [43]. These findings go beyond simple hyperactivity and provide further consistency to increased object interactions of patients with BD [28]. Besides increased exploration, the increased time spent in the center by Clock△19 mutant mice could be related to their reduced anxiety/increased risk-seeking behavior [19]. Future studies on tasks measuring risk-proneness are required however [49, 50]. Furthermore, consistent with patients with BD and in contrast to patients with schizophrenia [28], Clock△19 mice habituated rapidly to their testing environment. In contrast to both patients with BD or schizophrenia however, mutant mice exhibited increased spatial d, reflecting more circumscribed exploratory movement, compared with more linear movement in these patients [28]. We have previously demonstrated that both pharmacological and genetic reduction of DAT functioning, which increases extracellular dopamine [51], resulted in reduced spatial d, consistent with patients with BD and schizophrenia [28, 31-33] that was untreated by chronic valproate [30]. Increased spatial d can occur however, when there is a unilateral increase of dopamine in the brains of mice, such as the chakragati mouse model of schizophrenia (unpublished observations). Clock△19 mice exhibit increased dopamine firing from the VTA [21], but it is unclear whether this is bilateral or unilateral. While BD treatments such as lithium [19, 52-54] and valproate [30] can normalize hyperactivity in animal models of BD mania, normalization of spatial d has yet to be demonstrated. Thus, while Clock△19 mutant mice share many characteristics of the abnormal exploration of patients with BD mania, some differences exist that require investigation.
Psychiatric populations, including patients with BD and schizophrenia exhibit impaired sensorimotor gating, as measured by PPI [34, 55, 56]. Despite the cross-species availability of PPI testing, to date these are the first studies to assess the PPI of Clock△19 mutant mice. We used a paradigm designed to quantify PPI across prepulse intensities and inter-stimulus intervals, startle amplitude in response to varying pulses, and startle habituation over time [57]. This study revealed that Clock△19 mutant mice exhibit reduced PPI, complicated by a reduced startle response in these mice. Importantly however, when mice were matched for baseline startle response [48, 58], the PPI deficit of Clock△19 mutant mice compared with WT mice was still observed. Thus, mutant mice exhibit sensorimotor gating deficits similar to people with BD.
The present data demonstrate that Clock△19 mutant mice share several characteristics with people with BD, but also some with people with schizophrenia as described above. Another characteristic consistently reported in Clock△19 mutant mice is increased reward preference as measured by reduced stimulation threshold and increased preference for sugared solutions [19].
Hedonia, including increased reward seeking, is a defining characteristic of BD mania as described in the DSM IV, and differs from people with schizophrenia whom traditionally are described as anhedonic, reflected by the need of greater stimulation and reduced preference for rewards [59]. The present findings extend the hedonia-like behavior of Clock△19 mutant mice, describing their preference even for non-caloric sweetened solutions (i.e., saccharin solution). Hence, despite some characteristics of the mutant mice that overlap with those of schizophrenia, our findings support these mice as modeling mania, including hedonia-like behaviors.
Previous studies have identified an altered circadian rhythm of Clock△19 mutant mice [60]. Indeed, the present studies support more activity of the mutant compared with WT mice during periods in which the mice should be inactive. Mutant mice were more active than WT mice overall however. Hence, more importantly our findings provide evidence for a direct consequence of the dysregulated circadian rhythm of these mice in response to aberrant photoperiod length. When the Clock△19 mutant and WT mice were challenged with the aberrant LD 23:1 photoperiod, we found increased activity of mutant mice during the rest phase. WT mice continued to exhibit circadian entrainment, suggesting resistance to photoperiod changes. Thus, Clock△19 mutant mice may represent a vulnerability genotype that is more susceptible to changes in photoperiod, which are known to affect mood states in patients with BD [4]. Altering photoperiod or putting nocturnal animals in constant light can induce depressive-like behaviors [61] that can be rescued using antidepressants [62]. Although shRNA-induced knockdown of CLOCK in the VTA of mice induced both mania- and depression-like behaviors in mice [63], to date no one has demonstrated such behaviors in response to environmental manipulations, such as changes in photoperiod. Future studies will determine whether photoperiod challenges will alter the mania-like behavioral phenotype of these Clock△19 mutant mice.
Clock△19 mutant mice exhibit increased dopaminergic firing in the VTA [21]. This increased firing rate may underlie many of the behavioral abnormalities observed here and elsewhere, given the similar profile of these mice to hyperdopaminergic mice mediated by reduced DAT expression [27, 28, 31-33]. Moreover, additional studies support increased dopamine release and turnover in the striatum of Clock△19 mutants, resulting in increased dopamine D1 and D2 protein expression, with a shift to increased D2 vs. D1 signaling [64]. The dopamine reward hypothesis postulates that striatal dopamine receptors such as D1 and D2 play critical roles in all forms of learning [65, 66]. Thus, altered dopamine D1 and D2 receptor signaling will likely alter learning mechanisms, which can be measured similarly to humans [67]. People with depression and mania exhibit numerous neurocognitive deficits [68, 69]. Such deficits include impaired probabilistic learning and decision-making behavior and are mediated by hypersensitivity to punishment in depression [70] and reward [71] in mania. Hence, tasks such as the Iowa Gambling Task [72] could be used to determine putative changes in ‘mood state’ in these mice resulting from environmental challenges [3]. Hence, future studies will determine the neurocognitive performance of the Clock△19 mutant mice, such as attention in a continuous performance test [73, 74], spatial working memory [75, 76], and decision-making under risk conditions in an Iowa Gambling Task [49, 50]. Such studies will be vital in the future given the correlation between cognition and a subject’s functional capabilities [77, 78].
4.1. Conclusion
In conclusion, we provide further evidence that Clock△19 mutant mice can be used to model aspects of BD mania by using tasks that have been utilized in patients with BD mania. Clock△19 mutant mice are not only hyperactive, but also exhibit increased specific exploration, a key aspect of abnormal exploration in patients with BD. Mutant mice also exhibited impaired sensorimotor gating, which was still evident after normalizing for the reduced baseline startle amplitude observed in these mice. Such characterization provides a platform for putative treatments tested in this model to be validated in equivalent human tests. The increased preference for saccharin solution extends previous findings of hedonia-like behavior observed in Clock△19 mutant mice. Finally, the poor circadian control of the Clock△19 mutant mice in an abnormal photoperiod supports further studies of whether photoperiod challenges can induce depressive-like behaviors in these mice.
Highlights.
Clock△19 mutants may model mania but have yet to be tested in cross-species tasks
Mutant mice are hyperactive and specifically explorative consistent with mania
Mutant mice also exhibit low sensory motor gating consistent with bipolar disorder
Mutant mice are hypersensitive to shortened active photoperiods
5. Acknowledgements
We thank Drs. Mark Geyer, Berend Olivier, David Welsh, William Perry, and Brook Henry, and Ms. Mahalah Buell for their support. These studies were supported by NIMH grant R01 MH071916; as well as by the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center. The authors report no conflict of interest. The experiments were approved by Institutional Animal Care and Use Committees at UCSD and VASDHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–23. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neurosci Biobehav Rev. 2007;31:825–31. doi: 10.1016/j.neubiorev.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Young JW, Henry BL, Geyer MA. Predictive animal models of mania, hits, misses, and future directions. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Malkesman O, Austin DR, Chen G, Manji HK. Reverse translational strategies for developing animal models of bipolar disorder. Dis Model Mech. 2009;2:238–45. doi: 10.1242/dmm.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Belmaker RH, Bersudsky Y. Bipolar disorder: Mania and depression. Discov Med. 2004;4:239–45. [PubMed] [Google Scholar]
- [6].APA . Diagnostic and Statistical Manual of Mental Disorders. 4th edn American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- [7].Cassidy F, Carroll BJ. Seasonal variation of mixed and pure episodes of bipolar disorder. J Affect Disord. 2002;68:25–31. doi: 10.1016/s0165-0327(00)00325-6. [DOI] [PubMed] [Google Scholar]
- [8].Friedman E, Gyulai L, Bhargava M, Landen M, Wisniewski S, Foris J, et al. Seasonal changes in clinical status in bipolar disorder: a prospective study in 1000 STEP-BD patients. Acta Psychiatr Scand. 2006;113:510–7. doi: 10.1111/j.1600-0447.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- [9].Sherman JA. Evolutionary origin of bipolar disorder-revised: EOBD-R. Med Hypotheses. 2012;78:113–22. doi: 10.1016/j.mehy.2011.10.005. [DOI] [PubMed] [Google Scholar]
- [10].Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–7. doi: 10.1093/hmg/ddl207. Spec No 2. [DOI] [PubMed] [Google Scholar]
- [11].McClung CA. Role for the Clock gene in bipolar disorder. Cold Spring Harb Symp Quant Biol. 2007;72:637–44. doi: 10.1101/sqb.2007.72.031. [DOI] [PubMed] [Google Scholar]
- [12].McClung CR. The photomorphogenic protein, DE-ETIOLATED 1, is a critical transcriptional corepressor in the central loop of the Arabidopsis circadian clock. Mol Cell. 2011;43:693–4. doi: 10.1016/j.molcel.2011.08.013. [DOI] [PubMed] [Google Scholar]
- [13].Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–63. doi: 10.1017/s1092852900019611. quiz 72. [DOI] [PubMed] [Google Scholar]
- [14].Sitaram N, Gillin JC, Bunney WE., Jr The switch process in manic-depressive illness. Circadian variation in time of switch and sleep and manic ratings before and after switch. Acta Psychiatr Scand. 1978;58:267–78. doi: 10.1111/j.1600-0447.1978.tb06938.x. [DOI] [PubMed] [Google Scholar]
- [15].Frank E, Swartz HA, Kupfer DJ. Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biol Psychiatry. 2000;48:593–604. doi: 10.1016/s0006-3223(00)00969-0. [DOI] [PubMed] [Google Scholar]
- [16].Wehr TA, Turner EH, Shimada JM, Lowe CH, Barker C, Leibenluft E. Treatment of rapidly cycling bipolar patient by using extended bed rest and darkness to stabilize the timing and duration of sleep. Biol Psychiatry. 1998;43:822–8. doi: 10.1016/s0006-3223(97)00542-8. [DOI] [PubMed] [Google Scholar]
- [17].Bunney BG, Bunney WE. Mechanisms of Rapid Antidepressant Effects of Sleep Deprivation Therapy: Clock Genes and Circadian Rhythms. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.07.020. [DOI] [PubMed] [Google Scholar]
- [18].Salvadore G, Quiroz JA, Machado-Vieira R, Henter ID, Manji HK, Zarate CA., Jr The neurobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010;71:1488–501. doi: 10.4088/JCP.09r05259gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–11. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102:9377–81. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology. 2011;36:1478–88. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, et al. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:23–6. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- [23].Serretti A, Benedetti F, Mandelli L, Lorenzi C, Pirovano A, Colombo C, et al. Genetic dissection of psychopathological symptoms: insomnia in mood disorders and CLOCK gene polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2003;121B:35–8. doi: 10.1002/ajmg.b.20053. [DOI] [PubMed] [Google Scholar]
- [24].Henry BL, Minassian A, Young JW, Paulus MP, Geyer MA, Perry W. Cross-species assessments of motor and exploratory behavior related to bipolar disorder. Neurosci Biobehav Rev. 2010;34:1296–306. doi: 10.1016/j.neubiorev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. J Affect Disord. 2009;120:200–6. doi: 10.1016/j.jad.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA. Quantifying over-activity in bipolar and schizophrenia patients in a human open field paradigm. Psychiatry Res. 2010;178:84–91. doi: 10.1016/j.psychres.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neurosci Biobehav Rev. 2007;31:882–96. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, et al. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–80. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. PLoS One. 2011;6:e24185. doi: 10.1371/journal.pone.0024185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].van Enkhuizen J, Geyer MA, Kooistra K, Young JW. Chronic valproate attenuates some, but not all, facets of mania-like behaviour in mice. Int J Neuropsychopharmacol. 2012:1–11. doi: 10.1017/S1461145712001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010;208:443–54. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacology Biochemistry and Behavior. 2010;96:7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Young JW, Kooistra K, Geyer MA. Dopamine receptor mediation of the exploratory/hyperactivity effects of modafinil. Neuropsychopharmacology. 2011;36:1385–96. doi: 10.1038/npp.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry. 2001;50:418–24. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- [35].Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–53. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- [36].Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0807s03. Chapter 8:Unit 8 7. [DOI] [PubMed] [Google Scholar]
- [37].Powell SB, Young JW, Ong JC, Caron MG, Geyer MA. Atypical antipsychotics clozapine and quetiapine attenuate prepulse inhibition deficits in dopamine transporter knockout mice. Behav Pharmacol. 2008;19:562–5. doi: 10.1097/FBP.0b013e32830dc110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Powell SB, Zhou X, Geyer MA. Prepulse inhibition and genetic mouse models of schizophrenia. Behav Brain Res. 2009;204:282–94. doi: 10.1016/j.bbr.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Flaisher-Grinberg S, Overgaard S, Einat H. Attenuation of high sweet solution preference by mood stabilizers: a possible mouse model for the increased reward-seeking domain of mania. J Neurosci Methods. 2009;177:44–50. doi: 10.1016/j.jneumeth.2008.09.018. [DOI] [PubMed] [Google Scholar]
- [40].King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–53. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, et al. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–67. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D(1), D(2), and D(3) receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–58. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- [43].Tanaka S, Young JW, Halberstadt AL, Masten VL, Geyer MA. Four factors underlying mouse behavior in an open field. Behav Brain Res. 2012;233:55–61. doi: 10.1016/j.bbr.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–88. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- [45].Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- [46].Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA. Delayed procedural learning in alpha7-nicotinic acetylcholine receptor knockout mice. Genes Brain Behav. 2011;10:720–33. doi: 10.1111/j.1601-183X.2011.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Paulus MP, Callaway CW, Geyer MA. Quantitative assessment of the microstructure of rat behavior: II. Distinctive effects of dopamine releasers and uptake inhibitors. Psychopharmacology (Berl) 1993;113:187–98. doi: 10.1007/BF02245696. [DOI] [PubMed] [Google Scholar]
- [48].Young JW, Wallace CK, Geyer MA, Risbrough VB. Age-associated improvements in cross-modal prepulse inhibition in mice. Behav Neurosci. 2010;124:133–40. doi: 10.1037/a0018462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].van Enkhuizen J, Geyer MA, Young JW. Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task : Relevance to mania. Psychopharmacology (Berl) 2013;225:661–74. doi: 10.1007/s00213-012-2854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Young JW, van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. J Psychopharmacol. 2011;25:934–43. doi: 10.1177/0269881111400646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–7. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cappeliez P, Moore E. Effects of lithium on an amphetamine animal model of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:347–58. doi: 10.1016/0278-5846(90)90023-a. [DOI] [PubMed] [Google Scholar]
- [53].Frey BN, Valvassori SS, Reus GZ, Martins MR, Petronilho FC, Bardini K, et al. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci. 2006;31:326–32. [PMC free article] [PubMed] [Google Scholar]
- [54].Gould TJ, Keith RA, Bhat RV. Differential sensitivity to lithium’s reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav Brain Res. 2001;118:95–105. doi: 10.1016/s0166-4328(00)00318-1. [DOI] [PubMed] [Google Scholar]
- [55].Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–43. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- [56].Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–8. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- [57].Young J, Kamenski M, Geyer M. Delayed ‘eureka’ of alpha 7 nicotinic acetylcholine receptor knockout mice in a probabilistic reversal learning paradigm. Schizophrenia Research. 2012;136:S361. [Google Scholar]
- [58].Swerdlow NR, Eastvold A, Uyan KM, Ploum Y, Cadenhead K. Matching strategies for drug studies of prepulse inhibition in humans. Behav Pharmacol. 2001;12:45–52. doi: 10.1097/00008877-200102000-00005. [DOI] [PubMed] [Google Scholar]
- [59].Schurhoff F, Szoke A, Bellivier F, Turcas C, Villemur M, Tignol J, et al. Anhedonia in schizophrenia: a distinct familial subtype? Schizophr Res. 2003;61:59–66. doi: 10.1016/s0920-9964(02)00237-2. [DOI] [PubMed] [Google Scholar]
- [60].Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, et al. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci U S A. 2006;103:9327–32. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, et al. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205:349–54. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- [62].LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–8. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–11. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Spencer S, Torres-Altoro MI, Falcon E, Arey R, Marvin M, Goldberg M, et al. A mutation in CLOCK leads to altered dopamine receptor function. J Neurochem. 2012;123:124–34. doi: 10.1111/j.1471-4159.2012.07857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Glimcher PW. Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15647–54. doi: 10.1073/pnas.1014269108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- [67].Ragland JD, Cools R, Frank M, Pizzagalli DA, Preston A, Ranganath C, et al. CNTRICS final task selection: long-term memory. Schizophr Bull. 2009;35:197–212. doi: 10.1093/schbul/sbn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Goodwin GM, Martinez-Aran A, Glahn DC, Vieta E. Cognitive impairment in bipolar disorder: neurodevelopment or neurodegeneration? An ECNP expert meeting report. Eur Neuropsychopharmacol. 2008;18:787–93. doi: 10.1016/j.euroneuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- [69].Burdick KE, Braga RJ, Goldberg JF, Malhotra AK. Cognitive dysfunction in bipolar disorder: future place of pharmacotherapy. CNS Drugs. 2007;21:971–81. doi: 10.2165/00023210-200721120-00002. [DOI] [PubMed] [Google Scholar]
- [70].Han G, Klimes-Dougan B, Jepsen S, Ballard K, Nelson M, Houri A, et al. Selective neurocognitive impairments in adolescents with major depressive disorder. J Adolesc. 2012;35:11–20. doi: 10.1016/j.adolescence.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Linke J, Sonnekes C, Wessa M. Sensitivity to positive and negative feedback in euthymic patients with bipolar I disorder: the last episode makes the difference. Bipolar Disord. 2011;13:638–50. doi: 10.1111/j.1399-5618.2011.00956.x. [DOI] [PubMed] [Google Scholar]
- [72].Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- [73].Harms LR, Turner KM, Eyles DW, Young JW, McGrath JJ, Burne TH. Attentional Processing in C57BL/6J Mice Exposed to Developmental Vitamin D Deficiency. PLoS One. 2012;7:e35896. doi: 10.1371/journal.pone.0035896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav Brain Res. 2011;222:183–92. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, et al. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196:207–13. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tarantino IS, Sharp RF, Geyer MA, Meves JM, Young JW. Working memory span capacity improved by a D2 but not D1 receptor family agonist. Behav Brain Res. 2011;219:181–8. doi: 10.1016/j.bbr.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67 Suppl 9:3–8. discussion 36-42. [PubMed] [Google Scholar]
- [78].Torres IJ, Defreitas CM, Defreitas VG, Bond DJ, Kunz M, Honer WG, et al. Relationship between cognitive functioning and 6-month clinical and functional outcome in patients with first manic episode bipolar I disorder. Psychol Med. 2010:1–12. doi: 10.1017/S0033291710001613. [DOI] [PubMed] [Google Scholar]