Abstract
Conditioned pain modulation (CPM) refers to the diminution of perceived pain intensity for a test stimulus following application of a conditioning stimulus to a remote area of the body, and is thought to reflect the descending inhibition of nociceptive signals. Studying CPM in children may inform interventions to enhance central pain inhibition within a developmental framework. We assessed CPM in 133 healthy children (mean age = 13 years; 52.6% girls) and tested the effects of sex and age. Participants were exposed to four trials of a pressure test stimulus before, during, and after the application of a cold water conditioning stimulus. CPM was documented by a reduction in pressure pain ratings during cold water administration. Older children (12–17 years) exhibited greater CPM than younger (8–11 years) children. No sex differences in CPM were found. Lower heart rate variability (HRV) at baseline and after pain induction was associated with less CPM controlling for child age. The findings of greater CPM in the older age cohort suggest a developmental improvement in central pain inhibitory mechanisms. The results highlight the need to examine developmental and contributory factors in central pain inhibitory mechanisms in children to guide effective, age appropriate, pain interventions.
Keywords: diffuse noxious inhibitory controls, experimental pain, descending modulation, endogenous inhibition, pediatric pain
Introduction
The dynamic test paradigm known as conditioned pain modulation (CPM), also known as diffuse noxious inhibitory control (DNIC), has been used in adults to examine central endogenous pain inhibition. This “pain inhibits pain” paradigm assesses perceived pain intensity to a test stimulus prior to, during, and after the application of a conditioning stimulus to a remote (heterotopic) area of the body. CPM refers to the reduction in perceived pain intensity for the test stimulus following application of the conditioning stimulus. Deficits in CPM may reflect impairments in central descending inhibitory systems that have been posited as an underlying mechanism in chronic pain.48 Supporting this hypothesis, studies have frequently found less efficient CPM responses in adults with chronic pain conditions relative to controls (for review, see 48,54).
The only existing pediatric study of CPM by Goffaux and colleagues found that full-term children and pre-term children without early pain exposure exhibited CPM and increased heart rate in response to pain induction, whereas pre-term children with early pain exposure showed an absence of CPM and lacked the normal physiological arousal associated with acute pain induction.13 Sex and age effects were not tested because of small sample size (n = 26) and limited age range (7–11 years). Testing the effects of sex and age on CPM may yield information that can be used to develop interventions for children that take into account developmental changes in pain modulation as well as individual differences in development of these central processes.
Among adults, sex differences in CPM have been studied to determine if differences in endogenous pain modulation underlie the female predominance in clinical pain syndromes. A recent review suggested that men exhibit more CPM than women.35 Epidemiological data indicates that at least during adolescence, girls appear to have a higher prevalence of chronic pain than boys (for review, see 20). Yet, static tests of experimental pain in healthy children have shown inconsistent results, with some studies showing higher pain reactivity in girls 25,28,55 and others finding no sex differences.4,9,47 Unlike the equivocal findings of sex in acute pain responses during childhood, studies of children through adolescence have demonstrated less acute pain reactivity in adolescents than in younger children.4,14
The present study examined CPM in healthy children (aged 8–17 years) to test the following primary hypotheses: 1) CPM will be evident in the total sample across age; 2) girls will exhibit less CPM than boys; and, 3) there will be developmental differences in CPM with younger children exhibiting less CPM than older adolescents. As suggested in a recent review of CPM methods and mechanisms,48 additional data are needed to understand the extent to which CPM is influenced by psychological (cognitive) and emotional (arousal) states. To address this question, we conducted exploratory analyses examining the relationships between CPM and pain catastrophizing as well as anxiety. We expected that as in adult samples, 15,32,53 higher catastrophizing and anxiety would be associated with lower CPM. Additionally, we explored the relationship of CPM to arousal as indexed by autonomic nervous system (ANS) activity; ANS processes have been linked to mechanisms of pain modulation.48 Heart-rate variability (HRV) is a noninvasive indicator of ANS balance3 with low HRV and high HRV indicative of greater sympathetic or parasympathetic dominance respectively. Low HRV has been associated with parasympathetic withdrawal (low cardiac vagal tone) and has been linked to chronic pain conditions in children.41 Our exploratory hypothesis was that greater CPM would be correlated with higher HRV.
Materials and Methods
Participants
Data for the current analyses were derived from a study of laboratory pain responses in children ages 8–17 years. The present group consisted of 133 healthy children (70 girls, 52.6%), with a mean age of 13.0 years (SD = 2.9, range = 8–17 years) (see Table 1 for additional demographic information). Participants were recruited through advertisements, community events, and referrals from previous participants. Study advertisements were posted on online forums (e.g., Craigslist) and at physical locations (e.g., libraries, pediatricians’ offices, etc.). Participants were also recruited at community events (festivals/fairs, etc.). Previous participants were offered the opportunity to refer their friends/neighbors and earn an additional $25 for each referred family that completed the study.
Table 1.
Demographic information for boys, girls, and the total sample.
| Boys (n = 60) | Girls (n = 64) | Younger (n = 44) | Older (n = 80) | Total Sample (N = 124) | |
|---|---|---|---|---|---|
| Mean age in years (SD) | 13.0 (3.1) | 13.4 (2.8) | 9.9 (1.2) | 15.0 (1.7) | 13.2 (2.9) |
| Ethnicity [n (%)] | |||||
| Hispanic/Latino | 20 (33.3%) | 19 (29.7%) | 15 (34.1%) | 24 (30.0%) | 39 (31.5%) |
| Non-Hispanic/Non-Latino | 40 (66.7%) | 45 (70.3%) | 29 (65.9%) | 56 (70.0%) | 85 (68.5%) |
| Race [n (%)] | |||||
| White | 27 (45.8%) | 28 (45.2%) | 21 (48.8%) | 34 (43.6%) | 55 (45.5%) |
| African-American | 15 (25.4%) | 15 (24.2%) | 11 (25.6%) | 19 (24.4%) | 30 (24.8%) |
| Asian | 1 (1.7%) | 1 (1.6%) | 0 (0%) | 2 (2.6%) | 2 (1.7%) |
| American Indian/Alaska Native | 1 (1.7%) | 0 (0%) | 1 (2.3%) | 0 (0%) | 1 (0.8%) |
| Multi-Racial | 15 (25.4%) | 18 (29.0%) | 10 (23.3%) | 23 (29.5%) | 33 (27.3%) |
Note: Racial data was unavailable for 3 participants (1 boy and 2 girls).
Eligibility was confirmed by telephone. Parents were interviewed by a trained research assistant to ascertain whether they or their child met any of the following exclusionary criteria: having a chronic pain problem; acute illness or injury that may potentially affect participation in the laboratory session (e.g., fever, flu symptoms), or that affected sensitivity of the extremities (e.g., Reynaud’s disease, hand injuries); daily use of opioids at the time of study participation; or developmental delay, autism, or significant other impairment that may preclude comprehension of study procedures, or participation in pain induction procedures. If the family had more than one child who met inclusion criteria, only one child per family was enrolled in the study. Of the 187 families screened for eligibility, 5 children (2.7% of those screened) were excluded due to acute or chronic illness, use of medications that could affect study outcomes, or developmental delay. Of the 182 families invited to participate, 47 (25.8%) declined participation due to lack of interest or scheduling difficulties. Two children were removed from the sample after participation because ineligibilities were discovered after study completion (child younger than minimum required age and child developmental delay).
The study was approved by the UCLA Institutional Review Board. Each participant received $50 cash for participation.
Procedure
Parents completed written consent and children separately provided written assent. Children then completed questionnaires using an online system. Children were interviewed about their recent pain history and, for girls, their menstrual history. Children’s height and weight were recorded, as well as any medication use that day.
The laboratory session began with attachment of leads for physiological recording and participants were instructed on the use of the 0-10 Numerical Rating Scale (NRS), with 0 indicating no pain and 10 worst pain. In order to assess comprehension of the NRS, six baseline items were administered (see Measures below). Instructions for using the NRS and baseline items were repeated with practice items until participants fully understood the scale.
A 5 minute habituation period for recording of baseline physiology followed during which participants were instructed to sit quietly and to watch a neutral nature video with no sound. Participants were then administered a series of laboratory pain tasks. These tasks are described in detail elsewhere.31 In brief, the tasks included the evoked pressure and cold pressor tasks (described below), and a single trial of focal pressure through a dull lucite point to the second dorsal phalanx of the middle finger of the right hand. There was a 3 minute interval between each task. After these three tasks were completed, and following a 3 minute interval during which participants sat quietly, the CPM task was administered. After completion of the CPM task, there was another 5-minute period during which participants sat quietly for recording of post-task period physiology. Two female experimenters conducted the laboratory sessions and were present during the entire testing period.
CPM Task
Test Stimulus: Evoked Pressure
Pressure pain sensitivity was assessed by applying discrete 5-second pressure stimuli to the fixated thumbnail of the left hand with a 1 × 1 cm hard rubber probe.12 The rubber probe was attached to a hydraulic piston that was controlled by a computer-activated pump to provide repeatable pressure-pain stimuli of rectangular waveform. First, a series of stimuli were presented in a predictable, “ascending” manner, beginning at .066 kg/cm2 and increasing in .132 kg/cm2 intervals up to the participant’s report of moderate pain (a rating of 6 on the 0-10 NRS) or to a maximum of 1.12 kg/cm2. Then, pressure stimuli were delivered at 15-second intervals in random order, using the multiple random-staircase (MRS) pressure-pain sensitivity method.16 The MRS method is a validated approach to reliably and efficiently determine the stimulus levels required to elicit several specific sensory thresholds. For this study two thresholds were used: ‘mild pain’ (3 on the 0-10 NRS scale), and ‘moderate pain’ (6 on the 0-10 NRS scale). The stimuli for the mild and moderate pain determinations were given in a ‘staircase’ fashion with each stimulus adjusted up or down based on the response to the last stimulus in that sequence. The two staircases were administered simultaneously and stimuli were presented in random order between the two staircases. The final four pressure steps on the high staircase were averaged to produce a value representing the amount of pressure that would reliably induce a rating of moderate pain in that participant. This value was rounded up to one of 24 pressure levels (0.05–1.20 kg/cm2) and this pressure level was then used for the test stimulus (TS) in the CPM task.
Conditioning Stimulus: Cold Pressor Task
Participants placed their right hand in a cold pressor unit (Techne TE-10D Thermoregulator, B-8 Bath, and RU-200 Dip Cooler; Techne, Burlington, NJ) with water maintained at a temperature of 5 degrees Celsius that was circulated to prevent localized warming around the hand. The participant’s hand was submerged up to approximately 2″ above the wrist. Participants were instructed to leave their hand in the water for 30 seconds and told that they would be informed by the research assistant when this time period had elapsed; participants were also told that they could remove their hand before the end of the 30 seconds if it became too uncomfortable/painful.
CPM procedure
Figure 1 shows the timing of the test stimulus and the conditioning stimulus administration. The test stimulus (TS; phasic pressure delivered to the left thumbnail) was administered first without the cold water conditioning stimulus to obtain a baseline TS pain rating (TS1). Then, participants were administered the cold pressor task and, while the right hand was immersed in the cold water, the TS was administered a second time (TS2). Fifteen seconds after removal of the hand from the cold water, the TS was administered a third time (TS3). Finally, the TS was administered a fourth time (TS4) 50 seconds later. TS pain ratings using the 0-10 NRS were made immediately following each 5 sec TS administration.
Figure 1.
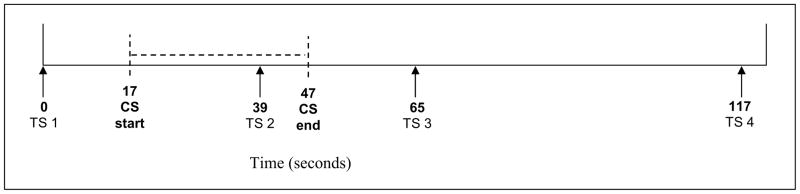
Timing of CPM stimuli administration.
Note: TS = test stimulus (pressure pain); CS = conditioning stimulus (cold water). TS1 = baseline pressure pain rating (before onset of the CS); TS2 = pressure pain rating during the CS; TS3 = pressure pain rating approximately 15 sec after termination of CS; TS4 = pressure pain rating approximately 50 sec after termination of CS.
Measures
Numeric Rating Scale (NRS) was used to assess anticipatory anxiety and pain intensity. For anticipatory anxiety, participants were asked to rate, “how nervous, afraid, or worried,” they felt immediately before the start of the CPM protocol. For pain intensity, participants were asked, “at its worst, how much pain did you feel?” Prior to the start of the pain tasks, the following 6 practice items were administered to ensure comprehension of the scale: (1) “How much pain do you feel right now?”; (2) “How painful would it be to walk up 2 steps?”; (3) “How painful would it be to touch a hot stove?”; (4) “What might cause a 5 on this scale?”; (5) “How nervous, afraid, or worried do you feel right now?”; (6) “How nervous, afraid, or worried would you be before taking a test?” The NRS has been shown to be valid and reliable for children as young as 8 years.52 Participants’ comprehension of the scale was determined by their responses to practice items #2 and #3. If participants’ ratings of item #3 (“touch a hot stove”) were higher than item #2 (“walking up two steps”), it was determined they understood the scale. The mean difference in ratings between the two items was 7.0 (SD = 2.5). Only 1 participant (13 year old girl) rated both items #2 and #3 the same (both were rated as“0”). However, this participant’s responses to item #4 (“what might cause a 5 on the scale,” i.e., “ankle twisting in a pothole”), and the difference between her ratings for item #5 (“how nervous…are you right now;” “5”) and item #6 (“how nervous…would you be before taking a test;” “8”), indicated that she understood the scale.
Pain Catastrophizing was assessed using the Pain Catastrophizing Scale for Children (PCS-C),10 a revised version of the original PCS43 measuring the extent to which participants worry, amplify and feel helpless about the experience of pain. Thirteen items are rated from 0 (not at all) to 4 (extremely); total scores range from 0 to 52. The PCS-C has demonstrated reliability and validity in children aged 8–16 years.10 In the current sample, reliability was high with Cronbach’s alpha = .86.
Pain Anxiety was assessed using the Child Pain Anxiety Symptoms Scale (CPASS),30 a revised version of the adult PASS-2027 measuring the cognitive, emotional, behavioral, and physiological reactions to the anticipation and/or experience of pain. Twenty items are rated from 0 (Never) to 5 (Always); total scores range from 0 to 100. The CPASS has demonstrated reliability and validity in children aged 8–18 years.30 Reliability in the present sample was high (Cronbach’s alpha = .89).
Bodily Pain was assessed using the bodily pain (BP) subscale of the Child Health Questionnaire (CHQ).22 The CHQ CF-87 is a child-self report questionnaire designed to assess the physical and psychosocial well-being of children with and without chronic conditions. The CHQ is among the most widely used measures for children; reliability and validity tests have been extensive.22 Subscale items are averaged to attain raw scores which are then transformed into a 0–100 continuum. Higher scores on the BP subscale indicate less pain.
Heart Rate Variability (HRV) was recorded using a two lead electrocardiogram (ECG) attached to the upper chest. The signal was sampled at 1000 Hz and timing of each R-wave performed using a BIOPAC recording system and AcqKnowledge software (BIOPAC Systems Inc., Santa Barbara, CA, USA). The data were also visually checked for quality control. Periods with excessive noise, usually due to movement, were eliminated from the analysis. HRV was recorded for a 5-minute pre-task baseline period at the beginning of the laboratory session prior to the start of the pain tasks, and again after the completion of the CPM task (post-task period). Heart period data were imported into the Kubios HRV software44 and HRV measures were determined for 2 two-minute epochs from each period. For each period, the HRV measures for the two epochs were averaged. If, due to artifact only one epoch was available, this was used (10.9% of pre-task baseline files; 18.9% of post-task period files). Heart rate data for 5 subjects’ pre-task baseline periods and 6 post-task periods did not contain sufficient clean data to score HRV (because of movement artifacts). The extent of HRV was assessed by calculation of the root mean square of successive differences (rMSSD) using the procedures described in the Kubios HRV User’s Guide.44 Specifically, rMSSD is a measure of beat-to-beat variance in heart period. Increasing rMSSD is related to respiratory sinus arrhythmia and is used as a marker of cardiac vagal tone or parasympathetic predominance.
Statistical Analysis
To examine differences in CPM based on age, the sample was divided into children (age 8–11 years; n = 44) and adolescents (aged 12–17 years; n = 80). This age cut-off was based on data related to neurohormonal development. Significant neural development appears to occur as children mature from younger (9–11 years) to older ages (12 years and above), particularly in brain regions associated with cognitive-affective processing.21 These findings support our age categorization for the study of CPM in which neurodevelopment would be expected to play a salient role.
Preliminary analyses tested for differences in demographic characteristics [age, race (Caucasian vs. non-Caucasian), ethnicity (Latino vs. non-Latino)] between boys and girls using independent t-tests for continuous data and chi-square tests for categorical data. The same approach was used to test for differences in race, ethnicity, and sex between younger and older children. Additional independent t-tests compared boys and girls as well as older and younger children on the psychosocial measures (PCS-C, C-PASS, BP), CPM anticipatory anxiety, and HRV.
Confirmatory analyses to test our a priori hypotheses employed a 2 (Time; TS1, TS2) × 2 (Sex; male vs. female) × 2 (Age; younger vs. older) repeated-measures ANOVA. Given that the amount of pressure administered as the test stimulus varied across participants (i.e., the amount of test stimulus pressure was determined by participant’s rating of moderate pain during the evoked pressure task), an additional 2 (Time) × 2 (Sex) × 2 (Age) repeated measures ANCOVA with the test stimulus pressure as a covariate was also conducted. These analyses were used to examine whether the results of the confirmatory analyses differed after accounting for the amount of pressure administered.
Exploratory analyses examined the durability of the CPM effect among children. Existing work in adult samples has generally found that the maximal pain suppressive effects of CPM occur during application of the conditioning stimulus and return to baseline within roughly 5 minutes after conditioning stimulation (for review see 48). Since no previous research has examined the durability of the CPM effect in children, an exploratory 4 (Time; TS1, TS2, TS3, TS4) × 2 (Sex) × 2 (Age) repeated-measures ANOVA was conducted. Additional exploratory analyses examined the relationship between the amount of CPM and the psychosocial measures, CPM anticipatory anxiety, and HRV. These relationships were tested using partial correlations controlling for child age. For all analyses, a standard probability level of p < .05 (two-tailed) was used to evaluate the results.
Results
Preliminary Analyses
Demographic information for the final sample is shown in Table 1. Means for the test stimulus ratings, anticipatory anxiety ratings, and the psychosocial and HRV measures are shown in Table 2. Data for the TS1 and TS2 ratings were missing for 7 participants due to equipment problems. In addition, 2 participants rated TS1 as zero, precluding the possibility of any reduction in TS ratings during administration of the conditioning stimulus. Exclusion of these participants left a final sample of 124 children. Four participants reported taking over-the-counter pain medications on the day of the testing session (ibuprofen; acetaminophen); exclusion of these participants did not change the results. Therefore, the results reported below include all 124 participants in the final sample.
Table 2.
Descriptive data [mean (SD)] for the CPM ratings, psychosocial and HRV measures.
| Boys (n = 60) | Girls (n = 64) | Younger (n = 44) | Older (n = 80) | Total Sample (N = 124) | |
|---|---|---|---|---|---|
| TS1 | 5.32 (2.2) | 5.38 (1.9) | 5.66 (2.1) | 5.18 (2.0) | 5.35 (2.1) |
| TS2 | 3.83 (2.1) | 3.80 (2.0) | 4.68 (2.2) | 3.34 (1.8) | 3.81 (2.0) |
| TS3 | 4.50 (2.4) | 3.98 (1.9) | 4.93 (2.3) | 3.85 (2.0) | 4.23 (2.1) |
| TS4 | 4.97 (2.0) | 5.05 (1.8) | 5.72 (2.0) | 4.62 (1.7) | 5.01 (1.9) |
| Absolute CPM | −1.48 (2.0) | −1.58 (2.2) | −0.98 (2.4) | −1.84 (1.9) | −1.53 (2.1) |
| CPM Percent Change | −21.8% (45.5) | −24.2 (38.3) | −9.2% (44.5) | −30.6% (38.4) | −23.0% (41.8) |
| Anticipatory Anxiety | 4.12 (3.0) | 4.17 (2.8) | 4.70 (3.0) | 3.84 (2.8) | 4.15 (2.9) |
| PCS-C | 15.0 (8.2) | 15.7 (7.9) | 15.2 (7.0) | 15.4 (8.6) | 15.3 (8.0) |
| CPASS | 25.7 (16.8) | 26.4 (15.5) | 28.5 (17.1) | 24.7 (15.5) | 26.1 (16.1) |
| CHQ Bodily Pain Subscale (Transformed Score) | 75.6 (17.7) | 76.8 (20.5) | 81.1 (18.6) | 73.5 (19.1) | 76.2 (19.2) |
| rMSSD | |||||
| Pre-task Baseline | 65.9 (39.1) | 61.2 (39.0) | 65.4 (40.7) | 62.3 (38.2) | 63.4 (38.9) |
| Post-task Period | 61.4 (35.2) | 61.3 (46.7) | 64.5 (42.5) | 59.7 (40.9) | 61.4 (41.4) |
Note: CPM = Conditioned Pain Modulation; TS = Test stimulus; Absolute CPM = TS2 minus TS1; CPM percent change = percent change from TS1 to TS2; PCS-C = Pain Catastrophizing Scale for Children; CPASS = Child Pain Anxiety Symptoms Scale – 20 item version; CHQ = Child Health Questionnaire; rMSSD = Root mean square of successive differences; Psychosocial measures data were unavailable for two participants; TS4 was missing for one participant; Pre-task baseline was missing for five participants; Post-task period data was missing for six participants.
There were no sex differences for the demographic characteristics, psychosocial measures, HRV, or CPM anticipatory anxiety. Younger and older children did not differ in the proportion of girls vs. boys, nor in race or ethnicity. There were no differences for the psychosocial measures, HRV, or CPM anticipatory anxiety based on child age. Congruent with the non-clinical nature of the current sample, means for BP were consistent with normative data (normative transformed BP score for boys and for girls = 80). Scores for the PCS-C were slightly lower than normative values reported in community samples; 10,30 C-PASS scores were consistent with normative values.30
Confirmatory analysis of sex and age differences in CPM
There was a main effect of Time (F(1,120) = 50.91, p < .001), indicating a significant CPM effect for the total sample such that TS2 ratings were significantly lower than TS1 ratings (see Table 2 for means). No main effect of Sex was found nor any interaction effects with Sex. There was however a main effect of Age (F (1,120) = 8.01, p < .01), as well as a Time by Age interaction (F(1,120) = 4.70, p < .04). Younger children reported higher average TS ratings than older children (see Table 2 for descriptive data). The Time by Age interaction is illustrated in Figure 2. Simple effects analysis of the Time by Age interaction indicated that there was a significant effect of Time for both younger and older children [younger - F(1,122) = 9.68, p < .01; older F(1,122) = 62.19, p < .001]. In addition, while TS ratings did not differ between younger and older children at baseline (F(1,122) = 1.58, NS), TS ratings during the conditioning stimulus were significantly lower in older children compared to younger children (F(1,122) = 13.62, p < .01). These results indicate that although both younger and older children demonstrated CPM, the CPM effect was more robust in older than in younger children.
Figure 2.
Age by time interaction for test stimulus ratings.
Additional analyses examined the potential impact of test stimulus pressure levels on CPM. Boys and girls did not differ in the amount of pressure administered as the test stimulus (boys – M = 0.60, SD = 0.40; girls – M = 0.58, SD = 0.30). However, the amount of pressure administered to younger children (M = 0.42, SD = 0.27) was significantly lower than the amount administered to older children (M = 0.69, SD = 0.35) (t(122) = −4.46, p < .001). Nevertheless, inclusion of the amount of test stimulus pressure as a covariate in the repeated measures ANCOVA did not change the results of the confirmatory analysis. The amount of pressure was not a significant covariate nor were there any interaction effects between the amount of pressure and any of the other variables.
Exploratory analyses of the durability of CPM
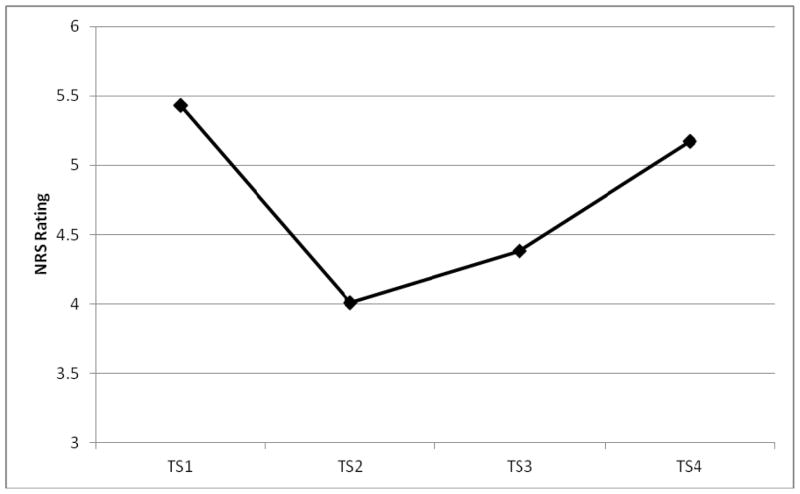
Data on 123 participants were available for this analysis (one child completed the first 3 CPM ratings but did not complete the final CPM rating). Results indicated a main effect of Time (F (3,117) = 18.36, p < .001), and a significant effect of Age (F(1,119) = 11.36, p < .01), but no Time by Age interaction; there was no main effect of Sex nor any interaction effects with Sex. The Age effect indicates that across all TS ratings, younger children reported higher pain intensity than did older children (see Table 2 for descriptive data). The significant Time effect is illustrated in Figure 3. Pairwise comparisons of the TS ratings with Bonferroni adjustments for multiple comparisons indicated that TS1 was significantly different from TS2 (p < .001), and TS3 (p < .001), and that TS4 was also significantly different from TS2 (p < .001), and TS3 (p < .001). As illustrated in Figure 2, TS2 ratings (reported during application of the CS) declined significantly from TS1; the reduction in test stimulus ratings persisted until TS3 ratings which were reported shortly after withdrawal of the CS, but the effect had dissipated by TS4 ratings which were reported roughly 1 minute later.
Figure 3.
Significant time effect across the four test stimulus ratings.
Relationship of CPM to psychosocial and physiological measures
The amount of CPM was calculated as a difference score between TS2 and TS1 (TS2-TS1), as well as percent change [(TS2 minus TS1)/TS1 × 100].54 For both percent change and difference scores, more negative values for the amount of CPM indicate greater CPM; more positive values indicate less CPM. Descriptive data for the difference scores and percent change are shown in Table 2.
The amount of CPM expressed as percent change was inversely correlated with the pre-task baseline rMSSD (r = −.22, p < .02), and there was a trend toward a relationship for CPM absolute values (r = −.18, p = .053). Post-task period rMSSD values were significantly inversely correlated with amount of CPM expressed both as percent change (r = −.22, p < .02), and as absolute values (r = −.21, p < .03). These results indicate that lower pre-task baseline and post-task period rMSSD (i.e., lower HRV) was associated with less CPM. The amount of CPM was not correlated with CPM anticipatory anxiety or with the psychosocial measures. CPM anticipatory anxiety was not correlated with HRV but was correlated with the PCS-C (r = .29, p < .01), and the CPASS (r = .26, p < .01). Thus, higher CPM anticipatory anxiety was associated with higher catastrophizing and higher pain anxiety.
Discussion
Supporting our hypothesis, this sample of healthy children demonstrated CPM, as indexed by a reduction in perceived pain intensity to a phasic pressure test stimulus during application of a heterotopic cold water conditioning stimulus. Also as hypothesized, the amount of CPM differed by age--younger children (aged 8–11 years) demonstrated significantly less CPM than did adolescents (aged 12–17 years). The average reduction in test stimulus pain intensity was, in absolute terms, just under 1 point (on a 0-10 NRS), or about a 9% reduction for younger children, and more than 1.8 points (on a 0-10 NRS), or a greater than 30% reduction for adolescents. Younger children also reported higher average test stimulus pain intensity compared to the pain intensity ratings of adolescents. Contrary to our hypothesis of sex differences, boys and girls did not differ in the magnitude of CPM. Our exploratory analyses of the durability of CPM found that the effect persisted for a brief period (15 sec) after termination of the conditioning stimulus, but was no longer present approximately 1 minute later (see Figure 2), and that this overall pattern did not differ depending on child age or child sex. Finally, lower baseline and post-task rMSSD (HRV) was associated with less CPM, indicating that low HRV was related to less inhibitory pain modulation after controlling for child age.
Our results are clinically important because they document the presence of central pain modulation in healthy children across pubertal development. Our findings are consistent with those of the study by Goffaux et al documenting active pain inhibitory systems in children born at full-term or at pre-term but without neonatal pain exposure. 13 The Goffaux findings suggest that excessive early pain exposure in pre-term children may prevent the normal development of responses triggered by descending inhibitory circuits.13 Thus, early pain exposure may be one factor accounting for individual differences in CPM. However, Goffaux study did not elucidate the normal developmental trajectory of CPM.
As discussed by van Wijk and colleagues,48 extant work suggests that CPM is mediated by descending endogenous processes involving a region in the caudal medulla, the subnucleus reticularis dorsalis (SRD),5,51 and that this descending mechanism inhibits nociceptive neuronal activity in the spinal cord dorsal horns. Van Wijk et al maintain that the SRD is a key relay station for CPM48 since the SRD is activated by nociceptive information from any part of the body and it contains reciprocal connections with the spinal dorsal horns.2,51 Our finding of an age-related increase in CPM suggests that these endogenous pain inhibitory processes may mature and become more efficient as children develop into adolescents. Emerging evidence on brain development supports a shift with increasing age from the recruitment of “bottom up” processing regions towards “top down” fronto-cortical and fronto-subcortical connections.38 Future work may examine the association between developmental changes in functional as well as structural connectivity and CPM. As Goffaux et al13 maintained, conditions that disrupt the normal neural developmental process of CPM warrant additional research.
Age-related effects in CPM are consistent with prior research testing static acute pain tasks. We previously found that younger children exhibited lower pressure and heat pain tolerance than adolescents.25 Age differences in acute pain sensitivity may relate to cognitive factors such as understanding laboratory pain as temporary and therefore less threatening,25,33 and/or to the ability to employ a larger array of pain coping responses.33 However, less is known about neural development and maturation of cognitive processes in children that influence CPM. By providing normative CPM data on the effects of sex and age in a large cohort of healthy children, our study lays the foundation for others to examine additional psychobiological factors that influence children’s CPM.
Our failure to find sex differences differ from a recent review which found significantly more CPM in men compared to women.35 This divergence might relate to differences in types of pain stimuli used, body locations stimulated, duration, and strength of the pain stimuli, since the magnitude of CPM may depend upon these factors. 36 Alternatively, other factors occurring during and after adolescence may contribute to the sex differences found in adult CPM studies. Investigations of sex differences in acute pain responses among children have yielded mixed results, with some studies reporting more pain responsivity in girls compared to boys (e.g., 25,28,33,39,50,55), but others reporting no sex differences (e.g.,4,9,47). In contrast to prior work which found that older boys showed less acute pain reactivity than younger boys but no age-related differences among girls,28,33,39 we did not find a sex by age interaction in CPM. The present findings should be replicated in other populations of children, especially those with chronic pain to determine if and when sex differences in CPM emerge in that population.
Unlike previous adult investigations,15,32,53 we did not find associations between CPM and measures of pain catastrophizing or pain anxiety. Although some investigators using static pain tasks have found links between catastrophizing and pain reactivity among children,26,33,49 others did not find such relationships.4,39 It may be that the association between CPM and catastrophizing is less robust among children than it is in adults. A related finding of the current study was the lack of a relationship between CPM and anticipatory anxiety. We previously found strong associations between anticipatory anxiety and acute pain responses to the cold pressor and similar tasks in healthy children;45,46 it may be that such relationships may hold primarily for static acute pain paradigms.
Our finding that lower CPM was associated with lower HRV support the Goffaux et al 13 finding that early pain exposure in preterm children altered ANS-driven, cardiovascular responses to pain induction. 13 Both studies underscore the importance of examining developmental neurobiological factors that may influence both CPM and physiological stress reactivity. Lower resting cardiac vagal tone (measured as HRV) has been reported among children with recurrent abdominal pain (RAP) compared to controls,41 although some studies did not find this difference.18,29 Nevertheless, significant links between the autonomic nervous system and pain inhibitory processes have been shown. Parasympathetic function and baroreflex activity have been linked to the efficacy of descending inhibitory circuits.6 Adult studies have also linked parasympathetic withdrawal with development of esophageal hyperalgesia40 and decrements in pain inhibition in fibromyalgia and irritable bowel syndrome.8 Further study is warranted in populations of children with chronic pain as well as with other aspects of autonomic nervous system development (e.g., baroceptor sensitivity).
A limitation of the current study is the lack of data on mechanism(s) of age-related differences in CPM. CPM may result from distraction of attention away from the test stimulus, although adult studies have found only minimal effects of distraction.7,11,19,23,42 Whereas one study found that younger children exhibited higher cold pressor tolerance in response to distraction (compared to focusing on physical sensations), both interventions were equally effective for older children.34 However, another study found that older children demonstrated increased cold pressor tolerance in response to interactive virtual reality distraction than younger children. 24 Thus, the type of distraction and extent of cognitive/attentional processing demands should be considered when investigating distraction effects on CPM among children. The potential role of distraction is underscored by recent findings that, relative to controls, children with RAP displayed an enhanced P3 component of the somatosensory-evoked potential, indicating an attentional bias to painful stimuli which may reflect deficits in activating pain inhibitory processes.17 As discussed by van Wijk et al,48 an alternative explanation for CPM is the “perceptual adaptation level perspective”37 which posits that intense phasic stimuli may be judged as less noxious in the presence of a tonic pain stimulus than when experienced alone, and that this difference is interpreted as “inhibition.” Emerging data suggest that maturity in executive function is achieved by age 12.1 If perceptual adaptation is an explanatory mechanism of CPM, it may be that enhanced cognitive processing abilities in older children are responsible for the observed age-related findings. Finally, we did not assess children’s early pain history and such experiences may have influenced the results.
Our findings indicate that CPM increased with age from younger children to adolescents. However, we did not find sex differences in CPM and no sex by age interaction. Although no correlations were found between CPM and the psychological variables tested, there was a link lower CPM and lower HRV, supporting the hypothesis that parasympathetic withdrawal is connected to reduced central inhibitory control. We view our findings as a first step in understanding the developmental neurobiology of central pain modulation in children. This research is clinically important because it reports normative data on age and sex effects in CPM that can be compared to data from other populations of children. The results also suggest that treatments for pediatric pain may need to consider developmental changes in pain modulation. Our procedures can be used to test interventions aimed at enhancing central pain inhibitory mechanisms, such as hypnotherapy. Research within a developmental framework may help unravel the interconnected neurodevelopmental processes involved in pain inhibition, especially in determining childhood factors for the development and maintenance of chronic pain.
Perspective.
In this healthy sample, younger children exhibited less conditioned pain modulation (CPM) than did older adolescents, suggesting a developmental improvement in CPM. Cardiac vagal tone was associated with CPM across age. The current findings may inform the development of targeted, developmentally appropriate pain interventions for children.
Acknowledgments
This study was supported by R01DE012754, awarded by the National Institute of Dental and Craniofacial Research (PI: Lonnie K. Zeltzer), by UCLA Clinical & Translational Research Center CTSI Grant UL1RR033176 (PI: Lonnie K. Zeltzer), and by 1K01AT005093, awarded by the National Center for Complementary and Alternative Medicine (PI: Subhadra Evans).
Footnotes
Disclosures
There are no conflicts of interest in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002;8:71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- 2.Bernard JF, Villanueva L, Carroue J, Le Bars D. Efferent projections from the subnucleus reticularis dorsalis (SRD): a Phaseolus vulgaris leucoagglutinin study in the rat. Neurosci Lett. 1990;116:257–262. doi: 10.1016/0304-3940(90)90083-l. [DOI] [PubMed] [Google Scholar]
- 3.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 4.Blankenburg M, Meyer D, Hirschfeld G, Kraemer N, Hechler T, Aksu F, Krumova EK, Magerl W, Maier C, Zernikow B. Developmental and sex differences in somatosensory perception--a systematic comparison of 7- versus 14-year-olds using quantitative sensory testing. Pain. 2011;152:2625–2631. doi: 10.1016/j.pain.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Bouhassira D, Villanueva L, Bing Z, le Bars D. Involvement of the subnucleus reticularis dorsalis in diffuse noxious inhibitory controls in the rat. Brain Res. 1992;595:353–357. doi: 10.1016/0006-8993(92)91071-l. [DOI] [PubMed] [Google Scholar]
- 6.Bruehl S, Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev. 2004;28:395–414. doi: 10.1016/j.neubiorev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in diffuse noxious inhibitory controls. J Pain. 2008;9:759–766. doi: 10.1016/j.jpain.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalaye P, Goffaux P, Bourgault P, Lafrenaye S, Devroede G, Watier A, Marchand S. Comparing pain modulation and autonomic responses in fibromyalgia and irritable bowel syndrome patients. Clin J Pain. 2012;28:519–526. doi: 10.1097/AJP.0b013e31823ae69e. [DOI] [PubMed] [Google Scholar]
- 9.Chambers CT, Craig KD, Bennett SM. The impact of maternal behavior on children’s pain experiences: an experimental analysis. J Pediatr Psychol. 2002;27:293–301. doi: 10.1093/jpepsy/27.3.293. [DOI] [PubMed] [Google Scholar]
- 10.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104:639–646. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 11.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 12.Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9:417–422. doi: 10.1016/j.jpain.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Goffaux P, Lafrenaye S, Morin M, Patural H, Demers G, Marchand S. Preterm births: Can neonatal pain alter the development of endogenous gating systems? Eur J Pain. 2008;12:945–951. doi: 10.1016/j.ejpain.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Goodenough B, Thomas W, Champion GD, Perrott D, Taplin JE, von Baeyer CL, Ziegler JB. Unravelling age effects and sex differences in needle pain: ratings of sensory intensity and unpleasantness of venipuncture pain by children and their parents. Pain. 1999;80:179–190. doi: 10.1016/s0304-3959(98)00201-2. [DOI] [PubMed] [Google Scholar]
- 15.Goodin BR, McGuire L, Allshouse M, Stapleton L, Haythornthwaite JA, Burns N, Mayes LA, Edwards RR. Associations between catastrophizing and endogenous pain-inhibitory processes: sex differences. J Pain. 2009;10:180–190. doi: 10.1016/j.jpain.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Gracely RH, Lota L, Walter DJ, Dubner R. A multiple random staircase method of psychophysical pain assessment. Pain. 1988;32:55–63. doi: 10.1016/0304-3959(88)90023-1. [DOI] [PubMed] [Google Scholar]
- 17.Hermann C, Zohsel K, Hohmeister J, Flor H. Cortical correlates of an attentional bias to painful and innocuous somatic stimuli in children with recurrent abdominal pain. Pain. 2008;136:397–406. doi: 10.1016/j.pain.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Jarrett M, Heitkemper M, Czyzewski D, Zeltzer L, Shulman RJ. Autonomic nervous system function in young children with functional abdominal pain or irritable bowel syndrome. J Pain. 2012;13:477–484. doi: 10.1016/j.jpain.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakigi R. Diffuse noxious inhibitory control - reappraisal by pain-related somatosensory-evoked potentials following co2-laser stimulation. J Neurol Sci. 1994;125:198–205. doi: 10.1016/0022-510x(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 20.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Ladouceur CD. Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Front Integr Neurosci. 2012;6:65. doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landgraf JM, Abetz L, Ware JE. A User’s Manual (2nd printing) The Health Institute; Boston, MA: 1999. The CHQ. [Google Scholar]
- 23.Lautenbacher S, Prager M, Rollman GB. Pain additivity, diffuse noxious inhibitory controls, and attention: A functional measurement analysis. Somatosens Mot Res. 2007;24:189–201. doi: 10.1080/08990220701637638. [DOI] [PubMed] [Google Scholar]
- 24.Law EF, Dahlquist LM, Sil S, Weiss KE, Herbert LJ, Wohlheiter K, Horn SB. Videogame distraction using virtual reality technology for children experiencing cold pressor pain: the role of cognitive processing. J Pediatr Psychol. 2011;36:84–94. doi: 10.1093/jpepsy/jsq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Q, Zeltzer LK, Tsao JCI, Kim SC, Turk N, Naliboff BD. Heart rate mediation of sex differences in pain tolerance in children. Pain. 2005;118:185–193. doi: 10.1016/j.pain.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Lu Q, Tsao JCI, Myers CD, Kim SC, Zeltzer LK. Coping predictors of children’s laboratory-induced pain tolerance, intensity, and unpleasantness. J Pain. 2007;8:708–717. doi: 10.1016/j.jpain.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 27.McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Res Manag. 2002;7:45–50. doi: 10.1155/2002/517163. [DOI] [PubMed] [Google Scholar]
- 28.Myers CD, Tsao JCI, Glover DA, Kim SC, Turk N, Zeltzer LK. Sex, gender, and age: contributions to laboratory pain responding in children and adolescents. J Pain. 2006;7:556–564. doi: 10.1016/j.jpain.2006.01.454. [DOI] [PubMed] [Google Scholar]
- 29.Olafsdottir E, Ellertsen B, Berstad A, Fluge G. Personality profiles and heart rate variability (vagal tone) in children with recurrent abdominal pain. Acta Paediatr. 2001;90:632–637. [PubMed] [Google Scholar]
- 30.Page MG, Fuss S, Martin AL, Escobar EM, Katz J. Development and preliminary validation of the Child Pain Anxiety Symptoms Scale in a community sample. J Pediatr Psychol. 2010;35:1071–1082. doi: 10.1093/jpepsy/jsq034. [DOI] [PubMed] [Google Scholar]
- 31.Payne LB, Seidman LC, Lung K, Zeltzer LK, Tsao JCI. Relationship of neuroticism and laboratory pain in healthy children: Does anxiety sensitivity play a role? Pain. 2013;154:103–109. doi: 10.1016/j.pain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piche M, Bouin M, Arsenault M, Poitras P, Rainville P. Decreased pain inhibition in irritable bowel syndrome depends on altered descending modulation and higher-order brain processes. Neuroscience. 2011;195:166–175. doi: 10.1016/j.neuroscience.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 33.Piira T, Taplin JE, Goodenough B, von Baeyer CL. Cognitive-behavioural predictors of children’s tolerance of laboratory-induced pain: implications for clinical assessment and future directions. Behav Res Ther. 2002;40:571–584. doi: 10.1016/s0005-7967(01)00073-0. [DOI] [PubMed] [Google Scholar]
- 34.Piira T, Hayes B, Goodenough B, von Baeyer CL. Effects of attentional direction, age, and coping style on cold-pressor pain in children. Behav Res Ther. 2006;44:835–848. doi: 10.1016/j.brat.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Popescu A, LeResche L, Truelove EL, Drangsholt MT. Gender differences in pain modulation by diffuse noxious inhibitory controls: a systematic review. Pain. 2010;150:309–318. doi: 10.1016/j.pain.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain. 2009;144:16–19. doi: 10.1016/j.pain.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Rollman GB. Signal-detection theory pain measures - empirical validation studies and adaptation-level effects. Pain. 1979;6:9–21. doi: 10.1016/0304-3959(79)90136-2. [DOI] [PubMed] [Google Scholar]
- 38.Rubia K. Functional brain imaging across development. Eur Child Adolesc Psychiatry. 2012 doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz AK, Vierhaus M, Lohaus A. Pain tolerance in children and adolescents: Sex differences and psychosocial influences on pain threshold and endurance. Eur J Pain. 2013;17:124–131. doi: 10.1002/j.1532-2149.2012.00169.x. [DOI] [PubMed] [Google Scholar]
- 40.Sharma A, Paine P, Rhodes S, Warburton F, Chua YC, Aziz Q. The autonomic response to human esophageal acidification and the development of hyperalgesia. Neurogastroenterol Motil. 2012;24:e285–293. doi: 10.1111/j.1365-2982.2012.01929.x. [DOI] [PubMed] [Google Scholar]
- 41.Sowder E, Gevirtz R, Shapiro W, Ebert C. Restoration of vagal tone: a possible mechanism for functional abdominal pain. Appl Psychophysiol Biofeedback. 2010;35:199–206. doi: 10.1007/s10484-010-9128-8. [DOI] [PubMed] [Google Scholar]
- 42.Staud R, Robinson ME, Vierck CJ, Jr, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–174. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 44.Tarvainen MP, Niskanen J. Kubios HRV Version 2.0 User’s Guide. Biosignal Analysis and Medical Imaging Group (BSAMIG), Department of Physics, University of Kuopio; Finland: 2008. [Google Scholar]
- 45.Tsao JCI, Lu Q, Kim SC, Zeltzer LK. Relationships among anxious symptomatology, anxiety sensitivity and laboratory pain responsivity in children. Cogn Behav Ther. 2006;35:207–215. doi: 10.1080/16506070600898272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsao JCI, Myers CD, Craske MG, Bursch B, Kim SC, Zeltzer LK. Role of anticipatory anxiety and anxiety sensitivity in children’s and adolescents’ laboratory pain responses. J Pediatr Psychol. 2004;29:379–388. doi: 10.1093/jpepsy/jsh041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsao JCI, Glover DA, Bursch B, Ifekwunigwe M, Zeltzer LK. Laboratory pain reactivity and gender: relationship to school nurse visits and school absences. J Dev Behav Pediatr. 2002;23:217–224. doi: 10.1097/00004703-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 48.van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain. 2010;11:408–419. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Vervoort T, Goubert L, Vandenbossche H, Van Aken S, Matthys D, Crombez G. Child’s and parents’ catastrophizing about pain is associated with procedural fear in children: a study in children with diabetes and their mothers. Psychol Rep. 2011;109:879–895. doi: 10.2466/07.15.16.21.PR0.109.6.879-895. [DOI] [PubMed] [Google Scholar]
- 50.Vierhaus M, Lohaus A, Schmitz AK. Sex, gender, coping, and self-efficacy: Mediation of sex differences in pain perception in children and adolescents. Eur J Pain. 2011;15 doi: 10.1016/j.ejpain.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Villanueva L, Bouhassira D, Le Bars D. The medullary subnucleus reticularis dorsalis (SRD) as a key link in both the transmission and modulation of pain signals. Pain. 1996;67:231–240. doi: 10.1016/0304-3959(96)03121-1. [DOI] [PubMed] [Google Scholar]
- 52.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143:223–227. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res. 2008;186:79–85. doi: 10.1007/s00221-007-1206-7. [DOI] [PubMed] [Google Scholar]
- 54.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain. 2010;14:339. doi: 10.1016/j.ejpain.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Zeltzer LK, Fanurik D, LeBaron S. The cold pressor pain paradigm in children: feasibility of an intervention model (Part II) Pain. 1989;37:305–313. doi: 10.1016/0304-3959(89)90195-4. [DOI] [PubMed] [Google Scholar]