Abstract
Background
Randomized controlled trials focusing on the effects of antidepressant treatment in cardiac patients have found modest effects on depressive symptoms but not on cardiac outcomes. A secondary analysis was conducted on data from the Enhancing Recovery in Coronary Heart Disease trial to assess whether changes in somatic or cognitive depressive symptoms following acute MI predicted event-free survival and whether the results differed per treatment arm (cognitive behavior therapy or care as usual).
Methods
Patients who met depression criteria and completed the 6th month depression assessment (n=1254) were included in this study. Measurements included demographic and clinical data and the Beck Depression Inventory at baseline and 6 months. The primary endpoint was a composite of recurrent MI and mortality over 2.4 years (standard deviation=0.9 years).
Results
Positive changes (per 1 point increase) in somatic depressive symptoms (HR: 0.95; 95% CI: 0.92–0.98; p=0.001) but not in cognitive depressive symptoms (HR: 0.98; 95% CI: 0.96–1.01; p=0.19) were related to a reduced risk of recurrent MI and mortality after adjustment for baseline depression scores. There was a trend for an interaction effect between changes in somatic depressive symptoms and the intervention (p=0.08). After controlling for demographic and clinical variables, the association between changes in somatic depressive symptoms and event-free survival remained significant in the intervention arm (HR: 0.93; 95% CI: 0.88–0.98; p=0.01) only.
Limitations
Secondary analyses.
Conclusions
Changes in somatic depressive symptoms, and not cognitive symptoms, were related to improved outcomes in the intervention arm, independent of demographic and clinical variables.
Keywords: Depression, Dimensions, Myocardial infarction, Mortality, Cognitive behavior therapy
1. Introduction
Depression is associated with morbidity and mortality in patients with coronary heart disease (CHD) and this association appears to be independent from medical variables, including measures of cardiac disease severity (Barth et al., 2004; Meijer et al., 2011). Randomized controlled trials focusing on the effects of antidepressant treatment in cardiac patients have found modest effects on depressive symptoms but not on cardiac outcomes (Glassman et al., 2002; Berkman et al., 2003; van Melle et al., 2007). Further analyses of these studies revealed that patients who did not respond to antidepressant treatment were at increased risk of adverse outcomes (Glassman et al., 2009; Carney et al., 2004; de Jonge et al., 2007). Several recent studies suggest that somatic symptoms of depression (e.g. fatigue, sleep problems), but not cognitive symptoms (e.g. shame, guilt) are related to adverse cardiac prognosis in patients with myocardial infarction (MI) (de Jonge et al., 2006; Martens et al., 2010; Smolderen et al., 2009). Although the association between somatic symptoms of depression and adverse prognosis was partly confounded by somatic health status, somatic symptoms of depression remained predictive of cardiac outcomes after adjustment for measures of disease severity (de Jonge et al., 2006; Martens et al., 2010). No previous studies have focused on the changes in cognitive and somatic depressive symptoms after depression treatment and their potentially differential associations with event-free survival.
This is a secondary analysis of data from the Enhancing Recovery in Coronary Heart Disease (ENRICHD) trial. We assessed whether somatic and cognitive depressive symptoms improved after cognitive behavior therapy (CBT) and whether changes in somatic or cognitive depressive symptoms following acute MI were related to event-free survival. We also assessed whether these associations differed by treatment arm since an earlier study based on the ENRICHD trial showed that intervention patients whose depression did not improve were at higher risk for late mortality than were patients who responded to treatment (Carney et al., 2004). We hypothesized that positive changes in somatic symptoms of depression are associated with a reduced rate of recurrent MI and all-cause mortality.
2. Methods
2.1. Subjects
Participants were patients recruited within 28 days following an acute MI who met ENRICHD-modified Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association, 1994) criteria for major depressive disorder, minor depressive disorder with a history of major depressive disorder, or dysthymia using the Depression Interview and Structured Hamilton (DISH) (Freedland et al., 2002). Under these criteria, patients were eligible if depressive symptoms had been present for at least 7 days, provided patients had a history of major depression (The ENRICHD Investigators, 2000; Berkman et al., 2003). Patients admitted between October 1996 and November 1999 to coronary care units at eight ENRICHD clinical trial sites for an MI were screened for eligibility. MI was documented by cardiac enzymes and by chest pain compatible with acute MI, characteristic evolutionary ST–T changes, or new Q waves. Details of the methods and design of the ENRICHD clinical trial are available elsewhere (Berkman et al., 2003; The ENRICHD Investigators, 2000). Briefly, patients were excluded if they:
had other life-threatening medical illnesses, cognitive impairment, other major psychiatric disorders, or were at imminent risk of suicide;
were too ill or logistically unable to participate;
had been taking a non-study antidepressant for less than 21 days; or
were exempted by their cardiologist from participating in the study.
For the present analyses, patients included in ENRICHD with low perceived social support but no depression were excluded, as well as patients who had a recurrent MI or died before the assessment of depression outcomes (6 months).
2.2. Procedure
2.2.1. Assessment
Depressive symptoms were measured with the Beck Depression Inventory (BDI), a 21-item self-report measure developed to assess the presence and severity of depressive symptoms (Beck et al., 1961). Each item is rated on a 0–3 scale with higher scores reflecting greater severity. The BDI is a valid and reliable measure of depressive symptoms in cardiac patients (Davidson et al., 2006). For the present study, we used the BDI at the time of randomization and at 6 months post-randomization to calculate difference scores comparable to the time frame of the active treatment arm. Depressive symptoms were defined as cognitive or somatic using the original division proposed by Beck and Steer (1987). Therefore, the items concerning sadness, pessimism, sense of failure, dissatisfaction, guilt, punishment, self-dislike, self-accusations, suicidal ideas, crying, irritability, social withdrawal, and indecisiveness were summed to obtain scores for the cognitive depressive dimension and the items concerning body image change, work difficulty, insomnia, fatigue, loss of appetite, weight loss, somatic preoccupation, and loss of libido were summed to obtain scores for the somatic depressive dimension. This division was chosen because in recent studies in patients with CHD, items divergently loaded on the somatic and cognitive dimension (de Jonge et al., 2006; Martens et al., 2010; Roest et al., 2011). However, a 2 factor solution resembling the original division is found in most cases. Further, significant change in somatic depressive symptoms was defined as a standardized effect size (Cohen’s d) ≥0.5, which is widely accepted as representing a clinically meaningful improvement in depression (Denollet and Brutsaert, 2001). We assessed whether patients who significantly improved were at decreased risk of adverse outcomes and we compared baseline characteristics of patients who significantly improved with characteristics of patients who did not improve.
2.3. Design and treatment
After written informed consent was obtained, the participants were randomly assigned by an automated system located at the ENRICHD coordinating center within 28 days of their index MI, to receive either the intervention or usual care. Both groups received the American Heart Association’s Active Partnership ™ health education booklet. The usual care patients received no further contact from study personnel except for follow-up.
The intervention consisted of CBT and was guided by two standard CBT manuals (Beck et al., 1979; Beck, 1995). The Beck Institute for Cognitive Therapy and Research provided the training and quality control for the intervention. The therapists were supervised by clinical investigators at each site and by Beck Institute Personnel. The intervention, training and quality control procedures are described in detail elsewhere (The ENRICHD Investigators, 2001). Participants randomized to the intervention arm who were severely depressed (Hamilton Rating Scale for Depression score >24) at enrollment or who did not show at least a 50% reduction in BDI scores after five weeks of CBT were referred to a study psychiatrist for concurrent pharmacotherapy. For these patients, unless contraindicated, sertraline was initiated at 50 mg per day. Patients who were unable to tolerate or who were unresponsive to sertraline were switched to an alternative antidepressant. The maximum duration of treatment was 6 months of CBT and 12 months of sertraline.
2.4. Endpoints
The primary endpoint for the present analysis was a composite of all-cause mortality and recurrent MI, consistent with the primary endpoint of the ENRICHD randomized trial (Berkman et al., 2003). All-cause mortality was a secondary endpoint. These endpoints were ascertained by follow-up assessments (including a medical history, physical examination and a resting electrocardiograph) and phone calls, routine hospital surveillance, and contacts with the patient’s physician. Since this study focused on the effects of changes in depressive symptoms on event-free survival, only deaths and MIs that occurred after the assessment of depression outcomes (6 months) were taken into account, which is consistent with previous studies (Carney et al., 2004; de Jonge et al., 2007). The mean follow-up period was 2.4 years (standard deviation [SD]=0.9 years).
2.5. Statistical analysis
Discrete and continuous variables were compared with the chi-square test and the independent-samples t-test. Changes in somatic and cognitive depressive symptoms were calculated by subtracting the 6 month sum scores from the baseline sum scores. Cox regression analyses were used to model the covariate-adjusted effect of changes in cognitive and somatic depressive symptoms between enrollment and the 6-month follow-up on event-free survival beyond 6 months. The Hazard Ratios (HR) represent the change in risk associated with a 1-point increase in the respective depressive symptom dimension. Further, we assessed whether there was an interaction effect between treatment and changes in somatic or cognitive depressive symptoms concerning the endpoints. To control for the possible adverse effects of baseline depressive symptoms, the hazard estimates of changes in depressive symptoms were adjusted for these baseline scores as is consistent with other studies (Carney et al., 2004).
Multivariable analyses were adjusted for demographic and medical factors that predicted mortality and recurrent MI in the ENRICHD trial (Jaffe et al., 2006), including age, diabetes, renal insufficiency, Left Ventricular Ejection Fraction (LVEF), creatinine, history of pulmonary disease, history of Congestive Heart Failure (CHF), prior Transient Ischemic Attack (TIA) or stroke, Coronary Artery Bypass Graft Surgery (CABG) after index MI, and use of non-angiotensin converting enzyme vasodilators. Missing scores on the covariates were imputed using a multiple imputation procedure with 50 iterations. SAS software 9.1.3 was used to perform the multiple imputation and SPSS version 17.0 and STATA version 8 were used for the statistical analyses. All statistical tests were 2-tailed, with p<0.05 denoting significance.
3. Results
Eleven patients had a missing baseline measurement on the BDI and 341 patients did not complete the 6th month depression assessment, leaving 1254 participants, 654 in the intervention and 600 in the control group, for further analyses. Forty-six patients met criteria for dysthymia, 555 for minor depression and 653 for major depression. Patients who did not complete the 6th month depression assessment were more likely to be in the control group (p=0.01) but there were no differences concerning baseline depression severity (p=0.77), age (p=0.87), and gender (p=0.07). During the follow-up period, which started after the 6th month depression assessment, 179 patients died or had a recurrent MI. Table 1 shows the demographic and clinical characteristics of the sample.
Table 1.
Baseline demographic and clinical characteristics.
| Characteristic |
Event-
free N=1075 |
Death/
MI N=179 |
p | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Intervention | 570 | 53.0 | 84 | 46.9 | 0.13 |
| Demographic characteristics | |||||
| Mean | SD | Mean | SD | ||
| Age | 59.0 | 12.1 | 64.1 | 11.7 | <0.001 |
| N | % | N | % | ||
| Male gender | 575 | 53.5 | 88 | 49.2 | 0.28 |
| Caucasian | 727 | 67.6 | 118 | 65.9 | 0.65 |
| Education≥12 years | 781 | 74.1 | 117 | 68.0 | 0.10 |
| History of depression | 817 | 77.4 | 128 | 71.9 | 0.11 |
| Medical characteristics | |||||
| N | % | N | % | ||
| Hypertension | 622 | 58.6 | 118 | 68.2 | 0.02 |
| High cholesterol | 623 | 62.9 | 118 | 69.8 | 0.08 |
| Diabetes | 320 | 30.0 | 87 | 48.9 | <0.001 |
| Renal insufficiency | 69 | 6.5 | 38 | 22.0 | <0.001 |
| Smoking history | 698 | 65.6 | 119 | 67.6 | 0.60 |
| LVEF≤40 | 191 | 21.7 | 60 | 42.0 | <0.001 |
| Killip II-III-IV | 199 | 20.2 | 59 | 37.3 | <0.001 |
| Mean | SD | Mean | SD | ||
| BMI | 29.2 | 6.0 | 28.1 | 5.7 | 0.02 |
| Creatinine (mg/dl) | 1.07 | 0.6 | 1.43 | 1.1 | <0.001 |
| N | % | N | % | ||
| history of pulmonary disease | 180 | 16.9 | 50 | 28.4 | <0.001 |
| Prior MI | 237 | 22.4 | 72 | 41.9 | <0.001 |
| History of CHF | 99 | 9.7 | 44 | 27.0 | <0.001 |
| prior TIA or stroke | 75 | 7.1 | 31 | 17.9 | <0.001 |
| Prior CABG | 105 | 9.8 | 39 | 22.2 | <0.001 |
| Prior PTCA | 144 | 13.6 | 35 | 20.2 | 0.02 |
| Prior peripheral vascular disease | 115 | 10.9 | 40 | 23.1 | <0.001 |
| Treatment of index MI | |||||
| N | % | N | % | ||
| Thrombolytic therapy | 398 | 37.6 | 58 | 32.8 | 0.22 |
| CABG | 191 | 18.2 | 28 | 16.3 | 0.53 |
| PTCA | 547 | 51.5 | 66 | 38.2 | 0.001 |
| Medications | |||||
| ACE inhibitors | 455 | 44.3 | 94 | 55.6 | 0.01 |
| Vasodilators (non-ACE) | 405 | 39.7 | 83 | 49.7 | 0.02 |
| anticoagulants | 191 | 18.9 | 46 | 27.4 | 0.01 |
| aspirin | 925 | 88.4 | 141 | 82.5 | 0.03 |
| Beta-blockers | 793 | 76.2 | 116 | 69.0 | 0.05 |
| Lipid-lowering drugs | 469 | 45.7 | 79 | 47.6 | 0.65 |
| Antidepressant medication use at baseline |
89 | 8.3 | 9 | 5.0 | 0.13 |
| Antidepressant medication use during follow-up |
289 | 26.9 | 47 | 26.3 | 0.86 |
ACE=angiotensin-converting enzyme; BMI= body mass index; CABG= coronary artery bypass graft; CHF= congestive heart failure; LVEF= left ventricular ejection fraction; MI= myocardial infarction; PTCA= percutaneous transluminal coronary angioplasty; SD= standard deviation; TIA= transient ischemic attack.
3.1. Changes in depressive symptoms
At baseline mean BDI score was 17.7 (SD=7.8) for the total group and 17.5 (SD=8.0) for the intervention and 17.9 (SD=7.6) for the care as usual group. At 6 months follow-up the mean score was 10.3 (SD=8.9) for the total group and 8.8 (SD=8.4) for the intervention and 12.0 (SD=9.2) for the care as usual group. Patients had a mean positive change of 4.0 (SD=6.0) in cognitive depressive symptoms and a positive change of 3.3 (SD=4.5) in somatic depressive symptoms from baseline to the 6 month follow-up. The mean change was significantly larger for the intervention group for both the cognitive symptoms (4.7 [SD=6.1] versus 3.2 [SD=5.9]; p<0.001) and the somatic symptoms, (mean=4.0 [SD=4.4] versus 2.7 [SD=4.5]; p<0.001) compared to the care as usual group.
3.2. Changes in depressive symptoms as predictors of event-free survival
Baseline cognitive depressive symptoms (HR: 1.00; 95% confidence interval (CI): 0.97–1.03; p=0.99) were not related to mortality and recurrent MI at follow-up, while baseline somatic depressive symptoms were (HR: 1.05; 95% CI: 1.01–1.09; p=0.01). After adjustment for baseline cognitive depressive symptom scores, changes in cognitive depressive symptoms were not associated with improved outcome (HR: 0.98; 95% CI: 0.96–1.01; p=0.19). However, positive changes in somatic depressive symptom scores were significantly related to event-free survival after controlling for baseline somatic depressive scores (HR: 0.95; 95% CI: 0.92–0.98; p=0.001). There was no interaction effect between changes in cognitive symptoms and treatment arm (p=0.25) but there was a trend for an interaction effect between changes in somatic depressive symptoms and the intervention (p=0.08), indicating that the effect of changes in somatic depressive symptoms on prognosis tended to differ by treatment group. We further assessed the association between changes in somatic depressive symptoms and adverse outcomes in the two treatment groups. After adjustment for demographic and medical covariates, change in somatic depressive symptoms was not related to prognosis in the control group (HR: 1.00; 95% CI: 0.95–1.05; p=0.96), but was an independent predictor of event-free survival in the intervention group (HR: 0.93; 95% CI: 0.88–0.98; p=0.01) (Table 2). Because antidepressant use differed between the intervention and control group, we additionally adjusted for use of selective serotonin reuptake inhibitors, but this did not change the association between changes in somatic depressive symptoms and event-free survival in the intervention group (HR: 0.92; 95% CI: 0.87–0.97; p=0.004).
Table 2.
Association between positive changes in somatic depressive symptoms and mortality and recurrent MI in the intervention group after multivariable adjustment.
| Variables in model | HR | 95% CI | P |
|---|---|---|---|
| Changes in somatic depressive symptoms | 0.93 | 0.88–0.98 | 0.01 |
| Baseline somatic depressive symptoms | 1.04 | 0.98–1.11 | 0.19 |
| Age | 1.03 | 1.01–1.05 | 0.001 |
| Diabetes | 1.48 | 0.93–2.35 | 0.10 |
| Renal insufficiency | 1.49 | 0.75–2.96 | 0.25 |
| LVEF % | 0.98 | 0.96–1.00 | 0.04 |
| Creatinine | 1.33 | 1.07–1.65 | 0.01 |
| History of pulmonary disease | 1.81 | 1.09–2.99 | 0.02 |
| Prior MI | 1.44 | 0.89–2.32 | 0.13 |
| History of CHF | 1.30 | 0.74–2.31 | 0.36 |
| Prior TIA or stroke | 1.06 | 0.53–2.15 | 0.87 |
| CABG treatment of index MI | 1.00 | 0.53–1.88 | 0.99 |
| Use of vasodilators (non-ACE) | 0.97 | 0.59–1.59 | 0.90 |
ACE=angiotensin-converting enzyme; CABG=coronary artery bypass graft; CHF=congestive heart failure; LVEF=left ventricular ejection fraction; MI=myocardial infarction; TIA=transient ischemic attack.
We found similar results with respect to the secondary endpoint, all-cause mortality. There was a significant interaction effect between changes in somatic depressive symptoms and the intervention concerning the secondary endpoint (p=0.02). Change in somatic depressive symptoms was an independent predictor of all-cause mortality in the intervention group (HR: 0.91; 95% CI: 0.85–0.97; p=0.004) but not in the care as usual group (HR: 1.02; 95% CI: 0.96–1.08; p=0.55) after adjustment for baseline somatic depressive symptoms scores, and demographic and medical covariates.
3.3. Substantial improvement in somatic depressive symptoms
Patients whose somatic depressive symptom scores improved substantially after CBT (standardized effect size ≥0.5) had a mean positive change of 6.0 (SD=3.2) in somatic symptoms compared with a mean negative change of 1.3 (SD=2.7) for patients who did not improve following CBT. Patients whose symptoms improved were at decreased risk of adverse outcomes compared with patients who did not improve. Although this association was not significant for the primary composite endpoint consisting of all-cause mortality or recurrent MI, (HR: 0.70; 95% CI: 0.44–1.09; p=0.11), it was significant for the secondary endpoint, all-cause mortality, (HR: 0.52; 95% CI: 0.29–0.94; p=0.03) (Fig. 1). The association between improvement in somatic depressive symptoms and all-cause mortality in the intervention group remained marginally significant after adjustment for demographic and medical variables (HR: 0.55; 95% CI: 0.30–1.01; p=0.05).
Fig. 1.
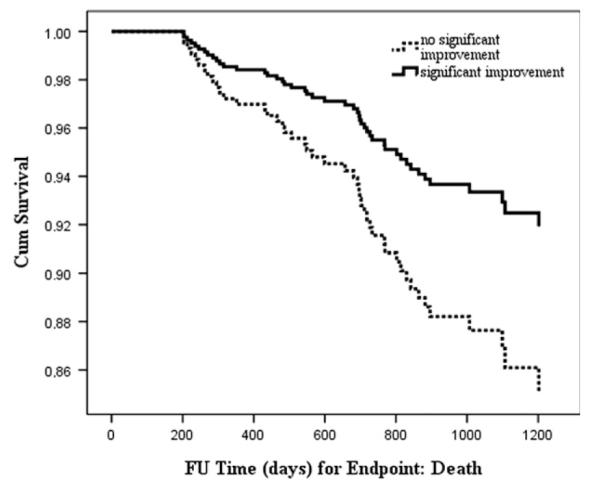
Improvement in somatic depressive symptoms following treatment and association with survival.
3.4. Comparison of demographic and medical characteristics between groups
Demographic and medical characteristics of patients whose somatic depressive symptom scores improved after CBT were compared with patients whose somatic symptoms did not improve during treatment. Patients who did not improve were more likely to be treated with antidepressants during the intervention (p<0.001), which is explained by the design of the intervention, as only patients who were severely depressed at baseline or who did not respond quickly enough to the CBT received antidepressant medication. Further, patients who improved during the intervention were more likely to be female (p=0.01). Finally, patients who did not improve during treatment were more likely to have a history of TIA or stroke (p=0.05) and to have high cholesterol (p=0.05) (Table 3) than those whose somatic depressive symptoms improved.
Table 3.
Characteristics of improved versus unimproved patients in the intervention group.
| Characteristic | Somatic depression unimproved N=182 |
Somatic depression Improved N=472 |
p | ||
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Mean | SD | Mean | SD | ||
| Age (sd) | 59 | 12.1 | 59 | 12.4 | 0.98 |
| N | % | N | % | ||
| Male gender | 115 | 63.2 | 241 | 51.1 | 0.01 |
| Caucasian | 130 | 71.4 | 318 | 67.4 | 0.32 |
| Education≥12 years | 139 | 77.7 | 340 | 73.1 | 0.24 |
| Medical characteristics | |||||
| N | % | N | % | ||
| Hypertension | 112 | 62.2 | 270 | 58.1 | 0.34 |
| High cholesterol | 116 | 69.9 | 268 | 61.3 | 0.05 |
| Diabetes | 56 | 30.9 | 146 | 31.0 | 0.99 |
| Renal insufficiency | 18 | 10.2 | 37 | 8.0 | 0.38 |
| Smoking history | 120 | 66.7 | 302 | 64.7 | 0.63 |
| LVEF <40 | 35 | 23.2 | 95 | 23.9 | 0.87 |
| Killip II-III-IV | 36 | 22.6 | 101 | 23.1 | 0.90 |
| Mean | SD | Mean | SD | ||
| BMI | 29.3 | 5.5 | 28.7 | 5.8 | 0.26 |
| Creatinine (mg/dl) (sd) | 1.2 | 0.8 | 1.1 | 0.7 | 0.23 |
| N | % | N | % | ||
| history of pulmonary disease |
34 | 19.0 | 85 | 18.1 | 0.80 |
| Prior MI | 47 | 26.3 | 103 | 22.3 | 0.29 |
| History of CHF | 24 | 13.8 | 51 | 11.4 | 0.41 |
| prior TIA or stroke | 19 | 10.6 | 28 | 6.0 | 0.05 |
| Prior CABG | 22 | 12.2 | 48 | 10.2 | 0.48 |
| Prior PTCA | 31 | 17.2 | 59 | 12.6 | 0.13 |
| Prior peripheral vascular disease |
28 | 15.9 | 54 | 11.6 | 0.14 |
| Treatment of index MI | |||||
| N | % | N | % | ||
| Thrombolytic therapy | 53 | 29.6 | 173 | 37.0 | 0.08 |
| CABG | 22 | 12.3 | 83 | 18.0 | 0.08 |
| PTCA | 91 | 50.0 | 225 | 48.4 | 0.71 |
| Medications | |||||
| ACE inhibitors | 76 | 42.7 | 190 | 41.9 | 0.86 |
| Vasodilators (non-ACE) | 74 | 42.0 | 181 | 40.6 | 0.74 |
| anticoagulants | 28 | 15.8 | 100 | 22.5 | 0.06 |
| aspirin | 155 | 88.1 | 396 | 86.3 | 0.55 |
| Beta-blockers | 137 | 76.5 | 346 | 76.4 | 0.97 |
| Lipid-lowering drugs | 85 | 48.6 | 194 | 43.0 | 0.21 |
| Antidepressant medication use at baseline |
25 | 13.7 | 41 | 8.7 | 0.06 |
| Antidepressant medication use during follow-up |
74 | 40.7 | 127 | 26.9 | <0.001 |
ACE= angiotensin-converting enzyme; BMI= body mass index; CABG= coronary artery bypass graft; CHF= congestive heart failure; LVEF= left ventricular ejection fraction; MI= myocardial infarction; PTCA= percutaneous transluminal coronary angioplasty; SD= standard deviation; TIA= transient ischemic attack.
4. Discussion
This study is the first to assess changes in somatic and cognitive depressive symptoms after depression treatment and their association with event-free survival in depressed MI patients. Patients who received antidepressant treatment, namely CBT and concurrent antidepressant medication when necessary, had larger changes in cognitive and somatic depressive symptoms compared with patients in the usual care group. Changes in cognitive depressive symptoms were not related to event-free survival. Conversely, changes in somatic depressive symptoms were related to improved outcomes in the intervention group, independent of demographic and clinical variables, including disease severity and use of selective serotonin reuptake inhibitors. This association was not apparent for the control group.
Earlier studies found that somatic depressive symptoms and not cognitive depressive symptoms were independently associated with adverse outcomes in patients following an acute MI (de Jonge et al., 2006; Martens et al., 2010; Smolderen et al., 2009) or acute coronary syndrome (Roest et al., 2011), although not consistently (Frasure-Smith and Lespérance, 2003). Somatic depressive symptoms were also more strongly associated with adverse outcomes in patients with myocardial ischemia (Linke et al., 2009), heart failure (Schiffer et al., 2009), and stable CHD (Hoen et al., 2010), although a study in patients treated with CABG surgery found that cognitive depressive symptoms were predictive of cardiac mortality and somatic depressive symptoms were not (Connerney et al., 2010). The results of the current study add significantly to our understanding of the relationship between treatment for depression and cardiac outcomes in patients with heart disease and suggest that trials aiming to improve cardiac prognosis in depressed MI patients must improve the somatic depressive symptoms in order to improve survival. Although other studies have suggested this possibility (Bekke-Hansen et al., 2012), our study is the first to actually show that improvement in somatic depressive symptoms following CBT in depressed MI patients is related to improved cardiac prognosis.
Changes in somatic depressive symptoms were associated with improved outcomes in the intervention group only. Although the changes were larger for the intervention group, the control group also showed positive changes in somatic depressive symptoms suggesting that size of the symptom reduction does not fully explain the differential relationship between improvement in somatic depressive symptoms and event-free survival for the two groups. There may be several other reasons why changes in somatic symptoms were associated with event-free survival only in the intervention group. First, the association between changes in somatic depressive symptoms with event-free survival might be caused by specific effects of the therapy, for example if behavioral activation (The ENRICHD Investigators, 2001) improved feelings of fatigue and sleeping problems. Since the patients who failed to improve in the intervention arm did so despite intensive treatment, a second explanation is that the association between changes in somatic depressive symptoms with event-free survival is the result of the presence of a treatment-resistant form of depression (Carney et al., 2004; de Jonge et al.,. 2007; Carney and Freedland, 2009). This subtype of depression might be characterized by persistent somatic depressive symptoms associated with a high risk for mortality. A recent secondary analysis of the ENRICHD clinical trial data showed that somatic depressive symptoms at 12 months post-MI predicted mortality, while baseline somatic depressive symptoms did not (Bekke-Hansen et al., 2012). Also, residual symptoms after depression treatment appeared to be more often somatic, namely lack of energy and sleeping problems (Conradi et al., 2010) suggesting that persistent somatic depressive symptoms might be an indication of recurrent or chronic depression. Therefore, in this study, it is possible that the intervention identified patients with a high risk subtype of depression unresponsive to standard antidepressant treatment (Carney and Freedland, 2009). How this subgroup can be further specified remains unclear, although there are several propositions. First, patients who do not respond to antidepressant medication are more likely to have elevated levels of inflammation (Lanquillon et al., 2000). Since inflammatory processes have been associated with recurrent cardiac events (Pearson et al., 2003) inflammation is a potential mechanism linking poor treatment response to standard antidepressant treatment with adverse cardiac outcomes (Carney and Freedland, 2009). Another factor related to non-response to antidepressant treatment is obstructive sleep apnea. A study with patients with CHD found that patients with comorbid sleep apnea were less likely to respond to antidepressant medication as compared to patients without sleep apnea (Roest et al., 2012). Treatment resistant depression may be a marker of underlying sleep apnea (Roest et al., 2012) which is a risk factor for MI and sudden cardiac death in patients with CHD (Somers et al., 2008).
It has been shown repeatedly that different treatment forms (e.g. antidepressants, psychotherapy) improve all types of depressive symptoms (Bhar et al., 2008; Stewart and Harkness, 2012). However, substantial heterogeneity exists with respect to symptom patterns of depression in patients with CHD (Ormel and de Jonge 2011; Poole et al., 2011). This study indicates that besides treating depression as a homogeneous disorder, researchers and clinicians should pay special attention to somatic depressive symptoms. Close monitoring of changes in somatic depressive symptoms might help to improve clinical care for patients with MI. If patients do not respond to antidepressant treatment with significantly improved somatic symptoms, additional treatments (e.g. interventions for sleep disturbances) may be considered, even if overall depression severity improves. Also, specific cardiac risk factors that predict treatment resistance to antidepressant therapy in patients with CHD, e.g. sleep apnea, should be identified and treated, potentially leading to better cardiovascular outcomes. Further, future research should attempt to develop more efficacious treatments for depression in patients with CHD. Depression treatments that also modify cardiac risk markers in patients with CHD might lead to better depression and medical outcomes (Carney and Freedland, 2009) For example, exercise therapy may offer considerable promise in patients with heart disease through a decrease in depressive symptoms and by simultaneously modifying mechanisms by which depression may be associated with increased risk, including autonomic nervous system and hypothalamic pituitaryadrenal axis dysfunction, and increased platelet activation and inflammation (Blumenthal, 2008).
Several studies have shown that somatic depressive symptoms are related to measures of cardiac disease severity such as LVEF and Killip class (de Jonge et al., 2006; Martens, et al., 2010; Roest et al., 2011). Therefore, somatic depressive symptoms might in part be physical manifestations of more severe heart disease and as a result be related to adverse outcomes. However, in these studies the association between somatic depressive symptoms and adverse prognosis remained significant after adjustment for measures of cardiac disease severity (de Jonge et al., 2006; Martens, et al., 2010; Roest et al., 2011). Also in the current study the association between changes in somatic depressive symptoms with event-free survival remained significant after adjustment for established predictors of recurrent MI and mortality following MI, including LVEF, previous MI and comorbid diseases. Also, patients whose somatic depressive symptoms did not improve did not significantly differ on demographic and clinical cardiac risk factors compared with patients whose depressive symptoms improved, other than that patients who did not improve were more likely to be male and have high cholesterol levels. Nevertheless, although affecting only a small group of patients, those whose somatic depressive symptoms did not improve appeared to be more likely to have had a TIA or stroke in the past, supporting the vascular depression hypothesis which suggests that cerebrovascular disease can induce depressive symptoms (Alexopoulos, 2006). Therefore, we cannot exclude the possibility of underlying pathophysiology explaining at least part of the relationship between somatic depressive symptoms and outcomes. Future research should carefully assess the possible confounding role of (changes in) cardiac disease severity in the relationship between (changes in) somatic depressive symptoms and cardiac prognosis.
A limitation of this study is that we did not take into account the possible effects of a worsening of cardiac disease during the six months of depression treatment. However, to minimize this possibility we excluded patients who had a recurrent MI during the time frame of the active treatment arm from our analyses. As a second limitation, there were a considerable number of patients who did not complete the 6th month depression assessment. Further, we defined depressive symptoms as either cognitive or somatic concordant with Beck and Steer (1987). Principal component analyses have shown that, although a 2 factor solution is found in most cases (Martens, et al., 2010; Roest et al., 2011; Bekke-Hansen et al., 2012), the BDI items are often not completely consistent (de Jonge et al., 2006; Martens et al., 2010;Roest et al., 2011; Bekke-Hansen et al., 2012). For example, a recent study on the ENRICHD trial including patients who were depressed or socially isolated at baseline found that body image change loaded on the cognitive dimension instead of the somatic dimension (Bekke-Hansen et al., 2012). However, excluding body image change from the somatic depressive symptom dimension did not change the relationship between changes in somatic depressive symptoms and prognosis in this study (data not shown). Finally, since these are secondary, post hoc analyses of the ENRICHD trial, the results should be interpreted with caution. Although improvement in somatic depressive symptoms was related to event-free survival in the current study, future research should examine whether close monitoring of somatic depressive symptoms and accompanying interventions can effectively improve cardiac prognosis of patients.
In summary, we found that the positive changes in somatic depressive symptoms, and not cognitive depressive symptoms, were related to event-free survival after treatment with CBT in depressed patients with MI. This association was independent of demographic and clinical variables, including disease severity, and was restricted to the intervention arm.
Acknowledgments
We thank Brian Steinmeyer for performing the multiple imputation.
Role of funding source This study was supported by National Institutes of Health contracts NO1-HC-55140, NO1-HC-55141, NO1-HC-55142, NO1-HC-55143, NO1-HC-55144, NO1-HC-55145, NO1-HC-55146, NO1-HC-55147, NO1-HC-55148. Pfizer provided sertraline (Zoloft) for the study.
Dr. de Jonge is supported by a VIDI grant from the Dutch Medical Research Council (Grant 016.086.397).
Dr. Denollet is supported by a VICI grant from the Dutch Organization for Scientific Research (Grant 453.04.004).
Footnotes
Conflict of interest Dr. Carney or a member of his family owns stock in Pfizer, Inc. All other authors report no conflicts of interest.
References
- Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biological Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders IV. Fourth edition. American Psychiatric Association; Washington, DC: 1994. 1994. [Google Scholar]
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosomatic Medicine. 2004;66:802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- Bhar SS, Gelfand LA, Schmid SP, Gallop R, DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Beck AT. Sequence of improvement in depressive symptoms across cognitive therapy and pharmacotherapy. Journal of Affective Disorders. 2008;110:161–166. doi: 10.1016/j.jad.2007.12.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JS. Cognitive Therapy: Basics and Beyond. Guilford Press; New York: 1995. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. Guilford Press; New York: 1979. [Google Scholar]
- Beck AT, Steer RA. Manual for the Revised Beck Depression Inventory. Psychological Corp.; San Antonio, Tex: 1987. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bekke-Hansen S, Trockel M, Burg MM, Taylor CB. Depressive symptom dimensions and cardiac prognosis following myocardial infarction: results from the ENRICHD clinical trial. Psychological Medicine. 2012;42:51–60. doi: 10.1017/S0033291711001000. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Enhancing Recovery in Coronary Heart DiseasePlease check the journal title in Ref. Berkman et al., 2003. Patients Investigators (ENRICHD), 2003. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. Journal of the American Medical Association. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA. Depression and coronary heart disease: association and implications for treatment. Cleveland Clinic Journal of Medicine. 2008;75(2):S48–S53. doi: 10.3949/ccjm.75.suppl_2.s48. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Freedland KE, Youngblood M, Veith RC, Burg MM, Cornell C, Saab PG, Kaufmann PG, Czajkowski SM, Jaffe AS, ENRICHD Investigators Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosomatic Medicine. 2004;66:466–474. doi: 10.1097/01.psy.0000133362.75075.a6. 2004. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE. Treatment-resistant depression and mortality after acute coronary syndrome. American Journal of Psychiatry. 2009;166:410–417. doi: 10.1176/appi.ajp.2008.08081239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connerney I, Sloan RP, Shapiro PA, Bagiella E, Seckman C. Depression is associated with increased mortality 10 years after coronary artery bypass surgery. Psychosomatic Medicine. 2010;72:874–881. doi: 10.1097/PSY.0b013e3181f65fc1. [DOI] [PubMed] [Google Scholar]
- Conradi HJ, Ormel J, de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychological Medicine. 2010 Oct 8; doi: 10.1017/S0033291710001911. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- Davidson KW, Kupfer DJ, Bigger JT, Califf RM, Carney RM, Coyne JC, Czajkowski SM, Frank E, Frasure-Smith N, Freedland KE, Froelicher ES, Glassman AH, Katon WJ, Kaufmann PG, Kessler RC, Kraemer HC, Krishnan KR, Lespérance F, Rieckmann N, Sheps DS, Suls JM, National Heart, Lung, and Blood Institute Working Group Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosomatic Medicine. 2006;68:645–650. doi: 10.1097/01.psy.0000233233.48738.22. 2006. [DOI] [PubMed] [Google Scholar]
- de Jonge P, Honig A, van Melle JP, Schene AH, Kuyper AM, Tulner D, Schins A, Ormel J, MIND-IT Investigators Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. American Journal of Psychiatry. 2007;164:1371–1378. doi: 10.1176/appi.ajp.2007.06091492. 2007. [DOI] [PubMed] [Google Scholar]
- de Jonge P, Ormel J, van den Brink RH, van Melle JP, Spijkerman TA, Kuijper A, van Veldhuisen DJ, van den Berg MP, Honig A, Crijns HJ, Schene AH. Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. American Journal of Psychiatry. 2006;163:138–144. doi: 10.1176/appi.ajp.163.1.138. [DOI] [PubMed] [Google Scholar]
- Denollet J, Brutsaert DL. Reducing emotional distress improves prognosis in coronary heart disease: 9-year mortality in a clinical trial of rehabilitation. Circulation. 2001;104:2018–2023. doi: 10.1161/hc4201.097940. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F. Depression and other psychological risks following myocardial infarction. Archives of General Psychiatry. 2003;60:627–636. doi: 10.1001/archpsyc.60.6.627. [DOI] [PubMed] [Google Scholar]
- Freedland KE, Skala JA, Carney RM, Raczynski JM, Taylor CB, Mendes de Leon CF, Ironson G, Youngblood ME, Krishnan KR, Veith RC. The Depression Interview and Structured Hamilton (DISH): rationale, development, characteristics, and clinical validity. Psychosomatic Medicine. 2002;64:897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT, Jr, Gaffney M. Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression: seven-year follow-up of SADHART participants. Archives of General Psychiatry. 2009;66:1022–1029. doi: 10.1001/archgenpsychiatry.2009.121. [DOI] [PubMed] [Google Scholar]
- Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, McIvor M, Sertraline Antidepressant Heart Attack Randomized Trial (SADHEART) Group Sertraline treatment of major depression in patients with acute MI or unstable angina. Journal of the American Medical Association. 2002;288:701–709. doi: 10.1001/jama.288.6.701. 2002. [DOI] [PubMed] [Google Scholar]
- Hoen PW, Whooley MA, Martens EJ, Na B, van Melle JP, de Jonge P. Differential associations between specific depressive symptoms and cardiovascular prognosis in patients with stable coronary heart disease. Journal of the American College of Cardiology. 2010;56:838–844. doi: 10.1016/j.jacc.2010.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AS, Krumholz HM, Catellier DJ, Freedland KE, Bittner V, Blumenthal JA, Calvin JE, Norman J, Sequeira R, O’Connor C, Rich MW, Sheps D, Wu C, Enhancing Recovery in Coronary Heart Disease (ENRICHD) Trial Investigators Prediction of medical morbidity and mortality after acute myocardial infarction in patients at increased psychosocial risk in the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study. American Heart Journal. 2006;152:126–135. doi: 10.1016/j.ahj.2005.10.004. 2006. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Linke SE, Rutledge T, Johnson BD, Vaccarino V, Bittner V, Cornell CE, Eteiba W, Sheps DS, Krantz DS, Parashar S, Bairey Merz CN. Depressive symptom dimensions and cardiovascular prognosis among women with suspected myocardial ischemia: a report from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation. Archives of General Psychiatry. 2009;66:499–507. doi: 10.1001/archgenpsychiatry.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EJ, Hoen PW, Mittelhaeuser M, de Jonge P, Denollet J. Symptom dimensions of post-myocardial infarction depression, disease severity and cardiac prognosis. Psychological Medicine. 2010;40:807–814. doi: 10.1017/S0033291709990997. [DOI] [PubMed] [Google Scholar]
- Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. General Hospital Psychiatry. 2011;33:203–216. doi: 10.1016/j.genhosppsych.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Ormel J, de Jonge P. Unipolar depression and the progression of coronary artery disease: Toward an integrative model. Psychotherapy and Psychosomatics. 2011;80:264–274. doi: 10.1159/000323165. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F, Centers for Disease Control and Prevention, American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. 2003. [DOI] [PubMed] [Google Scholar]
- Poole L, Dickens C, Steptoe A. The puzzle of depression and acute coronary syndrome: reviewing the role of acute inflammation. Journal of Psychosomatic Research. 2011;71:61–68. doi: 10.1016/j.jpsychores.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roest AM, Carney RM, Stein PK, Freedland KE, Meyer H, Steinmeyer BC, de Jonge P, Rubin EH. Obstructive sleep apnea and poor response to sertraline in patients with coronary heart disease. Journal of Clinical Psychiatry. 2012;73:31–36. doi: 10.4088/JCP.10m06455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roest AM, Thombs BD, Grace SL, Stewart DE, Abbey SE, de Jonge P. Somatic/affective symptoms, but not cognitive/affective symptoms, of depression after acute coronary syndrome are associated with 12-month all-cause mortality. Journal of Affective Disorders. 2011;131:158–163. doi: 10.1016/j.jad.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Smolderen KG, Spertus JA, Reid KJ, Buchanan DM, Krumholz HM, Denollet J, Vaccarino V, Chan PS. The association of cognitive and somatic depressive symptoms with depression recognition and outcomes after myocardial infarction. Circulation: Cardiovascular Quality Outcomes. 2009;2:328–337. doi: 10.1161/CIRCOUTCOMES.109.868588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer AA, Pelle AJ, Smith ORF, Widdershoven JW, Hendriks EH, Pedersen SS. Somatic versus cognitive symptoms of depression as predictors of all-cause mortality and health status in chronic heart failure. Journal of Clinical Psychiatry. 2009;70:1667–1673. doi: 10.4088/JCP.08m04609. [DOI] [PubMed] [Google Scholar]
- Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Journal of the American College of Cardiology. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Stewart JG, Harkness KL. Symptom specificity in the acute treatment of Major Depressive Disorder: a re-analysis of the treatment of depression collaborative research program. Journal of Affective Disorders. 2012;137:87–97. doi: 10.1016/j.jad.2011.12.015. [DOI] [PubMed] [Google Scholar]
- The ENRICHD Investigators Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD): study design and methods. American Heart Journal. 2000;139:1–9. doi: 10.1016/s0002-8703(00)90301-6. [DOI] [PubMed] [Google Scholar]
- The ENRICHD Investigators Enhancing Recovery in Coronary Heart Disease (ENRICHD) study intervention: rationale and design. Psychosomatic Medicine. 2001;63:747–755. [PubMed] [Google Scholar]
- van Melle JP, de Jonge P, Honig A, Schene AH, Kuyper AM, Crijns HJ, Schins A, Tulner D, van den Berg MP, Ormel J, MIND-IT investigators Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–466. doi: 10.1192/bjp.bp.106.028647. 2007. [DOI] [PubMed] [Google Scholar]


