Abstract
Objective
To quantify the natural decline in fecundability by age and assess the effect of selected volitional factors.
Design
Prospective cohort study of women attempting conception.
Setting
General population cohort of Danish women aged 20–40 years.
Participants
2,820 women without infertility, trying to conceive for less than 3 cycles at study entry.
Intervention
none.
Outcome measure
fecundability.
Results
Age had little effect on fecundability except for women 35–40 yrs, for whom it was 0.77 relative to women aged 20–24. Male age showed a similar but smaller drop, declining to 0.95 for men aged 35–39. The effect of age differed for parous and nulliparous women, with the latter experiencing much stronger age-related declines relative to fecundability at age 20. Frequency of intercourse, use of nonhormonal birth control as the last method, and timing of intercourse each had small effects on fecundability. Women who were in the high-fecundability categories for all three of these volitional factors had an estimated probability of conceiving of 88% (95% CI: 83%–93%). Unlike age, these factors represent individual choices that together can offset some of the age-related decline in fecundability.
Conclusion
Fecundability peaks around age 30, slightly earlier for nulliparous than for parous women, and then declines. The decline with age is more modest for males. Couples will experience a compounded effect of their separate age-related declines. At age 40, a couple’s fecundability would be approximately half of what it is at age 30, but some of this decline can be counteracted by volitional factors affecting conception.
MeSH words: fecundability, fertility, age, behavioral factors, cohort study
Introduction
Age is a strong determinant of fecundability (the probability of conception during a single menstrual cycle with unprotected intercourse) (1–5), but it is not modifiable, whereas other strong determinants of fecundability, such as frequency and timing of intercourse, are volitional and may be modified to offset declines in fecundability with age. To the extent that this compensatory behavior occurs, the natural decline in fecundability with age may be partially masked.
The age-related decline in fecundability has been studied mostly in women receiving donor insemination or from retrospective data collected from women who have already conceived. Schwartz and colleagues (1) studied fecundability among women with azoospermic partners receiving donor insemination with frozen semen. They reported a probability of conception of 73% within 12 cycles for women under age 30, declining to 61% for women 30–34 years of age and to 54% for women older than 34. van Noord-Zaadstra et al. (2) found similar results in a Dutch population of women receiving donor insemination. Studies in historical populations that did not use contraception show accelerating decreases in fecundability as women age from their mid-20s into their 40s (4, 5). Studies based on women who succeed in getting pregnant are subject to selection biases that may distort the age relation (6).
Couples also appear to experience declining fecundability with increasing age of the male partner. Homan et al. reported a decline in fecundability for couples in which the male is older than 40 years (7); Baird et al. mention a decline in fertility success in attempts at assisted reproduction when the sperm comes from males over 50 years (8). Hassan and Killick found increased time to pregnancy with increasing age of the male partner (9). These studies are reinforced by findings of declining semen volume, sperm motility, and sperm morphology (but not sperm concentration) with increasing age of the male partner (10).
No study has examined the age-related decline in fecundability in a population-based prospective cohort study of women trying to conceive, while controlling for volitional factors. We therefore examined the pattern of age-related fecundability after controlling for several volitional factors, together with other factors that might confound the fecundability estimates. We also examined the decline in fecundability in relation to age of the male partner.
Methods
Study population and data collection
The Snart Gravid prospective cohort study enrolled Danish women from the general population who stopped using contraception because they wished to become pregnant (11,12). The cohort was recruited and followed for up to one year via the internet, beginning in June of 2007. Potential participants learned about the study from an advertisement on a Danish health-related website (www.netdoktor.dk) or through other publicity, such as magazine articles. The study website contained an on-line consent form and a screening questionnaire. Eligible women were residents of Denmark, age 18–40, in a stable relationship with a male partner of any age, not using any form of contraception or fertility treatment. Participants provided proof of identity and record linkage through their Civil Registration Number (CRN). They also gave an email address and completed an extensive baseline questionnaire. Shorter follow-up questionnaires were completed at intervals of eight weeks to ascertain pregnancies and to update exposures. Follow-up continued for up to 12 months (6 follow-up questionnaires), but ceased if the woman reported being pregnant, stopped trying to become pregnant, or began fertility treatment. 82% of women were followed for all 12 months, or until a study event occurred (13).
On the baseline and follow-up questionnaires, women were asked “Are you or your partner doing anything to help you time intercourse (e.g., keeping a record of basal body temperature, monitoring changes in cervical mucus, using LH or ovulation testing kits)?” On the baseline questionnaire women were also asked to specify which methods they were using (indicating all that applied): basal body temperature, monitoring of cervical mucus, LH/ovulation testing kit, or other. The most common response for the “other” category was “counting days of menstrual cycle”. At baseline and at each follow-up, women were asked to report on their frequency of sexual intercourse (in the past month) in categories of “<1 per month, once per month, 2–3 times per month, once per week, 2–3 times per week, 4–6 times per week, and daily.”
After 54 months of recruitment, 5,920 women were enrolled in the cohort. Many of these women had been trying to conceive for several months before they entered. For this analysis, we excluded the 1,948 women who had been trying to conceive for >3 cycles at study entry, to focus on women who were just recently attempting to conceive. We also excluded 38 women and 2 male partners aged <20 at study entry, 236 women with a history of infertility, 296 women with insufficient or implausible information about their last menstrual period date or length of time trying to conceive, and 580 women who completed only the baseline questionnaire. Thus, our cohort for this analysis comprised 2,820 women. Of these, 69% had been trying to become pregnant for 1 month or less at enrollment.
Data analysis
We examined the relation between female age at the time of study enrollment and conception during the following 12 menstrual cycles by applying life-table methods to estimate the cumulative probability of conception within four age categories of women, 20–24, 25–29, 30–34, and 35–40 years. Within each of these age categories, we examined cumulative probabilities of conception for three subsets of women: 1) women with higher frequency of intercourse (≥2 times/week); 2) women who were timing intercourse to become pregnant; and 3) women who used non-hormonal methods as their last method of contraception, because women using hormonal methods have a short delay in return to fertility (14). We also examined fecundabilty for the 319 women who met all three criteria. We then fit a proportional probabilities regression model to compute fecundability ratios (FRs) and 95% confidence intervals (CIs) by age category, adjusting for cycle at risk using left-truncation and controlling for potential confounders including vigorous physical activity, female body mass index (BMI), education, alcohol consumption, current smoking, pack-years of smoking (ever smokers), male BMI, male smoking, cycle length, cycle regularity, intercourse frequency, last method of contraception, parity, and methods used to time intercourse (15). The FR represents the average per cycle probability of conception for women (or men) of a given age relative to those at a referent age. A FR below one indicates reduced fecundability of exposed persons relative to unexposed persons.
We used menstrual cycles rather than calendar time as the time metric in the regression model. We asked women whether they had regular menstrual cycles (defined as “usually being able to predict from one menstrual period to the next about when the next menstrual period would start”). Women with regular cycles were asked to report their usual cycle length. For women with irregular cycles or missing data, we estimated cycle length from the date of last menstrual period (LMP) at the baseline questionnaire, and from the actual LMP dates recorded during each follow-up questionnaire. We estimated time to pregnancy in cycles based on the following formula:
We added one cycle to account for the average woman being at midcycle when she filled out the baseline questionnaire. Observed cycles at risk were defined as those occurring after study entry.
We censored follow-up if and when participants (1) reported use of fertility treatments or change in intention to become pregnant, (2) became lost to follow-up or actively resigned from the study, or (3) reached the end of the observation period (no conception after 12 menstrual cycles). Using the same methods, we also examined the relation between male age and fecundability in five age categories, 20–24, 25–29, 30–34, 35–39, and ≥40 years. Finally we fitted smoothed curves to depict the trend in fecundability ratio with age, using restricted cubic splines (16, 17). A spline curve is a concatenated series of polynomial functions with segments joined at points called “knots,” intended to depict the underlying trend. The slopes of the polynomial segments are constrained to fit smoothly at the knots, and the end segments (in a restricted spline model) are kept linear to avoid the undue influence of outliers.
We used multiple imputation methods to impute missing data (18). The proportion of missing data at baseline ranged from as low as 0.13% (age of the male partner) to as high as 4% (pack-years of smoking); proportions were 0.39% for last method of contraception, 0.42% for frequency of intercourse, and 0.58% for actions to improve chances of fertility success (e.g., timing intercourse). There were no missing data for age of female (by design). In SAS statistical software (version 9.2), we used PROC MI to create five imputed datasets and PROC MIANALYZE to combine results across the five datasets (19). All potential confounders were included in the imputation procedure.
Ethical Review
The Snart Gravid study was approved by the Danish Data Protection Board and the Institutional Review Board at Boston University, and consent was obtained from all participants via the internet.
Results
Characteristics of the study population according to female and male age at baseline are shown in Table 1. Female age was positively associated with male partner’s age, regular menstrual cycles, short cycle length, higher education, parity, pack-years of smoking, alcohol intake, use of barrier methods as last method of contraception, and attempting to time intercourse. An intercourse frequency of 4+ times per week was highest for women under age 25 and diminished progressively with increasing age. Female age was not appreciably associated with BMI. Similar patterns were observed for male age.
Table 1.
Baseline characteristics of 2,820 Danish women and their male partners participating in a prospective cohort study of pregnancy planners, according to female and male age at baseline
| Female age at baseline | Male age at baseline | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Characteristic | <25 | 25–29 | 30–34 | 35–40 | <25 | 25–29 | 30–34 | 35–39 |
| Number | 466 | 1355 | 791 | 208 | 198 | 1046 | 1048 | 393 |
| Partner’s age, years (mean) | 26.5 | 29.6 | 33.3 | 37.0 | 23.1 | 26.5 | 29.2 | 32.0 |
| Regular cycles (%) | 74.0 | 75.9 | 81.3 | 85.6 | ||||
| Cycle length (mean)a | 28.9 | 28.8 | 28.6 | 28.1 | ||||
| Cycle length <27 days (%)a | 9.6 | 12.9 | 14.0 | 16.9 | ||||
| Cycle length ≥32 days (%)a | 11.0 | 11.6 | 7.8 | 3.4 | ||||
| BMI, kg/m2 (mean) | 24.1 | 23.8 | 23.7 | 24.3 | 25.1 | 25.3 | 25.1 | 25.4 |
| Vigorous physical activity, h/wk (mean) | 1.6 | 1.7 | 1.4 | 1.5 | ||||
| Higher education >4 yrs (%) | 4.7 | 23.5 | 40.5 | 30.3 | ||||
| Parous (%) | 10.1 | 22.3 | 49.3 | 68.3 | ||||
| Current regular smoker, yes (%) | 15.5 | 10.4 | 9.4 | 9.6 | 21.7 | 17.0 | 16.9 | 19.1 |
| Pack-years of ever smoking (mean) | 3.5 | 4.7 | 5.6 | 7.8 | 2.6 | 3.3 | 4.0 | 4.8 |
| Alcohol intake, drinks/wk (mean) | 2.3 | 2.9 | 3.1 | 3.2 | ||||
| Intercourse frequency, ≥4 times/wk (%) | 30.7 | 20.7 | 14.8 | 15.4 | 30.8 | 22.8 | 16.3 | 17.6 |
| Doing something to time intercourse (%) | 40.8 | 40.0 | 42.2 | 49.0 | 43.9 | 41.0 | 39.1 | 43.5 |
| Methods used to time intercourse (%)b | ||||||||
| Basal Body Temperature | 3.0 | 2.0 | 1.9 | 1.4 | 1.5 | 2.7 | 1.6 | 2.0 |
| Cervical mucus | 17.2 | 15.8 | 16.6 | 21.1 | 17.2 | 16.3 | 15.3 | 19.3 |
| LH/ovulation test kit | 13.9 | 12.5 | 14.4 | 14.9 | 15.6 | 14.2 | 11.6 | 14.0 |
| Otherc | 14.6 | 17.5 | 18.1 | 20.7 | 16.7 | 15.8 | 18.1 | 18.8 |
| Last method of contraception (%) | ||||||||
| Barrier methods | 21.9 | 26.3 | 31.7 | 33.7 | 24.7 | 26.8 | 27.9 | 31.3 |
| Hormonal contraceptives | 73.1 | 65.3 | 54.8 | 46.6 | 68.8 | 66.2 | 61.0 | 52.8 |
| Withdrawal, charting, or other | 5.0 | 8.4 | 13.5 | 19.7 | 6.5 | 7.0 | 11.1 | 15.9 |
Among women with regular cycles.
Categories are not mutually exclusive.
Most common response to “other” was “calendar method/counting number of days since LMP.”
Among the 2,820 couples in this analysis, 2,075 became pregnant within 12 menstrual cycles of follow-up, for a crude cumulative probability of conception of 74%. This crude figure does not adjust for the fact that some couples had incomplete follow-up or stopped trying to conceive; when we accounted for these phenomena using life-table methods, the estimated probability of becoming pregnant within 12 months was 83% (95% CI 81%–85%). Women between 25 and 34 years of age had greater fecundability than women in younger or older age categories when other variables were not considered. Table 2 shows how age-specific fecundability changed according to various volitional factors affecting conception. A frequency of intercourse of 2+ times per week, the last method of birth control, and whether intercourse timing was used each had small effects on fecundability, but women who were in the high-fecundability categories for all three of these factors had an estimated probability of conceiving of 88% (95% CI 83%–93%) within 12 cycles, 6% greater in absolute probability than the value of 82% for women who did none of the three. Overall, these results indicate that volitional factors enhance fecundability, although the magnitude does not fully offset the age-related decline. Also, the increase in fecundability attained by timing of intercourse, use of non-hormonal methods of birth control, and greater frequency of intercourse appears to be more modest for older women than for women under age 30.
Table 2.
Number of pregnancies, number of subjects, and cumulative pregnancy proportion within 12 cyclesa by female and male age at baseline, overall and by selected volitional factors
| All women | Intercourse ≥2 times per week | Timing intercourse | Non-hormonal last method of birth control | Non-hormonal last BC, intercourse ≥2/week, and timing intercourse | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Age (y) | Preg/total | % a | Preg/total | % a | Preg/total | % a | Preg/total | % a | Preg/total | % a |
| Females | ||||||||||
| 20–24 | 314/466 | 78% | 237/345 | 79% | 132/190 | 78% | 91/124 | 81% | 37/46 | 91% |
| 25–29 | 996/1355 | 83% | 692/917 | 84% | 402/542 | 82% | 345/467 | 83% | 112/143 | 87% |
| 30–34 | 625/791 | 87% | 369/460 | 88% | 276/334 | 90% | 285/356 | 87% | 79/93 | 90% |
| 35–40 | 140/208 | 72% | 83/123 | 73% | 72/102 | 78% | 78/111 | 76% | 26/37 | 75% |
| Males | ||||||||||
| 20–24 | 135/198 | 80% | 112/151 | 85% | 61/87 | 81% | 44/61 | 86% | 16/20 | 90% |
| 25–29 | 747/1046 | 81% | 535/728 | 82% | 312/429 | 81% | 260/351 | 81% | 89/116 | 85% |
| 30–34 | 817/1048 | 86% | 505/651 | 85% | 328/410 | 87% | 314/405 | 85% | 89/106 | 88% |
| 35–39 | 283/393 | 81% | 179/240 | 83% | 131/171 | 83% | 144/185 | 86% | 52/62 | 90% |
| ≥40 | 93/135 | 86% | 50/75 | 82% | 50/71 | 83% | 37/56 | 75% | 8/15 | 64% |
|
| ||||||||||
| Total | 2075/2820 | 83% | 1381/1845 | 84% | 882/1168 | 84% | 799/1058 | 84% | 254/319 | 88% |
| 95% CI | 81%–85% | 81%–86% | 81%–86% | 81%–87% | 83%–93% | |||||
From life-table analysis that accounts for censoring events (e.g., loss to follow-up).
Table 3 shows the unadjusted and fully-adjusted associations for fecundability in relation to female and male age, derived from a proportional probabilities regression model. With adjustment for confounders, a woman’s age had comparatively little effect except for a marked decline in fecundability in the oldest age category, 35–40 yrs, to 0.77 (95% CI: 0.62—0.97) relative to women aged 20–24. Male partner’s age appeared showed the same pattern as females with an increase in fecundability from age 20–24 to age 30–34, followed by a drop at older ages, but the drop was small, declining to 0.95 for men aged 35–39 (95% CI: 0.77—1.19) relative to men aged 20–24. We also fit these models after removing terms for cycle regularity and menstrual cycle length, to address the concern that these variables were mediating factors and therefore should not be controlled. The results were essentially unchanged when these variables were removed.
Table 3.
Modeled fit between age and time to pregnancy, with fecundability ratios and 95% confidence intervals, using separate models for females and for males.
| Age (y) | Pregnancies | Cycles | Unadjusted model
|
Adjusted modela
|
||
|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | |||
| Females | ||||||
| 20–24 | 314 | 2035 | 1.00 | (ref.) | 1.00 | (ref.) |
| 25–29 | 996 | 5734 | 1.12 | 0.99, 1.25 | 1.03 | 0.90, 1.18 |
| 30–34 | 625 | 3115 | 1.26 | 1.12, 1.43 | 1.05 | 0.89, 1.23 |
| 35–40 | 140 | 948 | 0.97 | 0.81, 1.16 | 0.77 | 0.62, 0.97 |
| Males | ||||||
| 20–24 | 135 | 916 | 1.00 | (ref.) | 1.00 | (ref.) |
| 25–29 | 747 | 4453 | 1.13 | 0.95, 1.33 | 1.05 | 0.87, 1.26 |
| 30–34 | 817 | 4186 | 1.27 | 1.08, 1.50 | 1.09 | 0.90, 1.32 |
| 35–39 | 283 | 1680 | 1.12 | 0.93, 1.36 | 0.96 | 0.77, 1.19 |
| ≥40 | 93 | 597 | 1.04 | 0.82, 1.33 | 0.95 | 0.73, 1.25 |
Note: FR = fecundability ratio, CI = confidence interval. Unadjusted model controls for cycle number.
Model is adjusted for cycle number, age of the female or male partner (yr), vigorous physical activity (y/n), BMI, vocational training (y/n), alcohol consumption (y/n), current smoking (y/n), pack-years of smoking (ever smokers), partner smoking (y/n), partner BMI, parity, cycle length (days), cycle regularity (y/n), intercourse frequency (no./wk), last method of contraception (barrier/hormonal/other), and methods used to time intercourse (basal body temp/LH ovulation test kit/other).
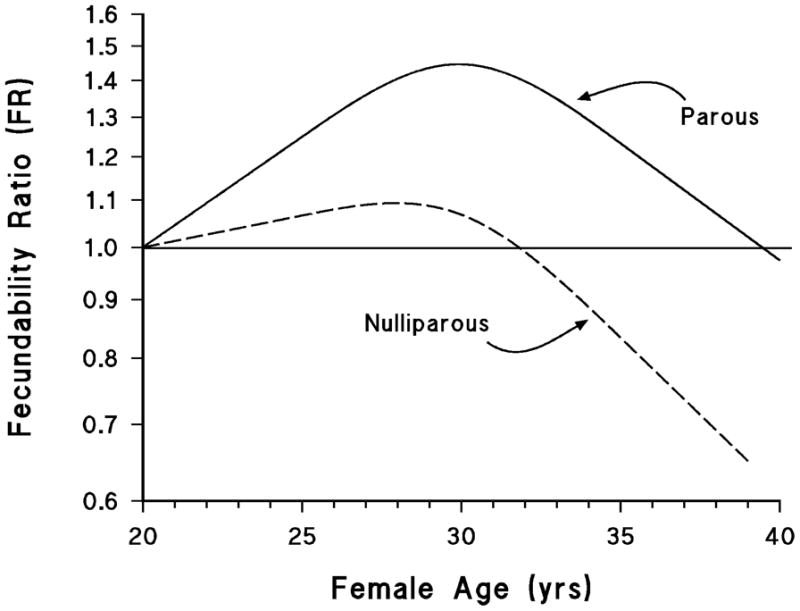
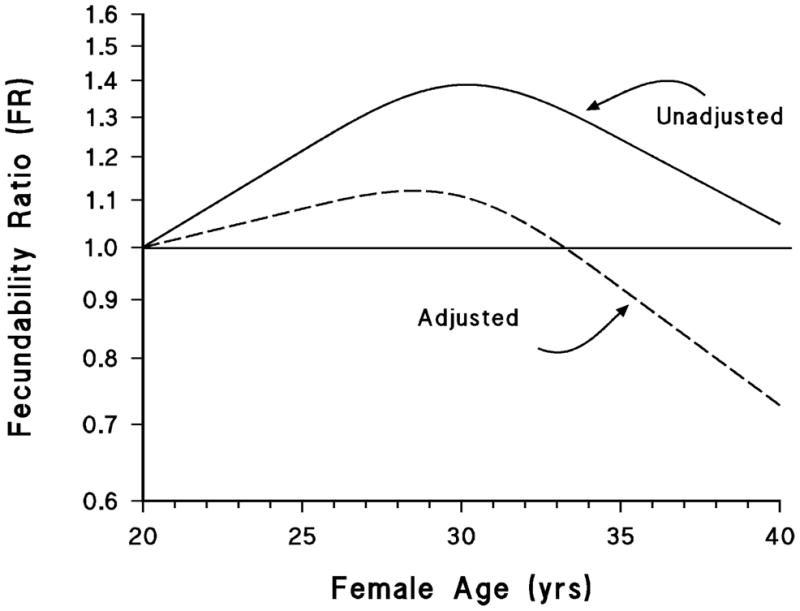
These results involve collapsing data into a few relatively broad age categories; to avoid the loss of information from collapsing the data, and to depict the age trend visually, we also plotted smoothed curves using a restricted cubic spline regression model. The first panel of figure 1 shows the trend in fecundability by female age with and without adjustment for other predictors of fecundability. With adjustment, fecundability rises only slightly until around age 30, after which there is a gradual decline. In the bottom panel of figure 2, the results are shown separately for parous and nulliparous women. For parous women the curve is an inverted “U,” with fecundability at age 40 similar to fecundability at age 20, and much higher between these ages. For nulliparous women, however, fecundability does not appear to increase much between age 20 and 30 and then declines so that by age 40 it is substantially below the value at age 20.
figure 1.
Association between age and fecundability, fitted by restricted cubic splines. Reference level for FR is 20 years. All curves used 3 knots located at 25, 30, and 35 years, except for the male curves, which have an additional knot at age 40. In the first two panels, the unadjusted curve controls for cycle number only, and the adjusted curve controls for cycle number, partner’s age, vigorous physical activity, BMI, vocational training, alcohol intake, current smoking, pack-years of smoking (ever smokers), partner smoking, partner BMI, cycle length, cycle regularity, parity, intercourse frequency, last method of contraception, and methods used to time intercourse. In the third panel, both curves control for cycle number, female age, vigorous physical activity, BMI, vocational training, alcohol intake, current smoking, pack-years of smoking (ever smokers), partner smoking, partner BMI, cycle length, cycle regularity, parity, intercourse frequency, last method of contraception, and methods used to time intercourse.
Because nulliparous women comprise gravid and nulligravid women, we also fit the curves examining fecundability ratio by age for these two groups separately (not shown). The former group comprised only 306 women out of the 1939 nulliparous women, and consequently that curve had wide confidence bands and the regression model did not converge with all covariates included. With control for a smaller subset of covariates, we found that the curve for the nulligravid group moved closer to that for parous women in figure 3, and the nulliparous but gravid group showed an earlier and much steeper decline in fecundability with age, although this finding was based on comparatively few women.
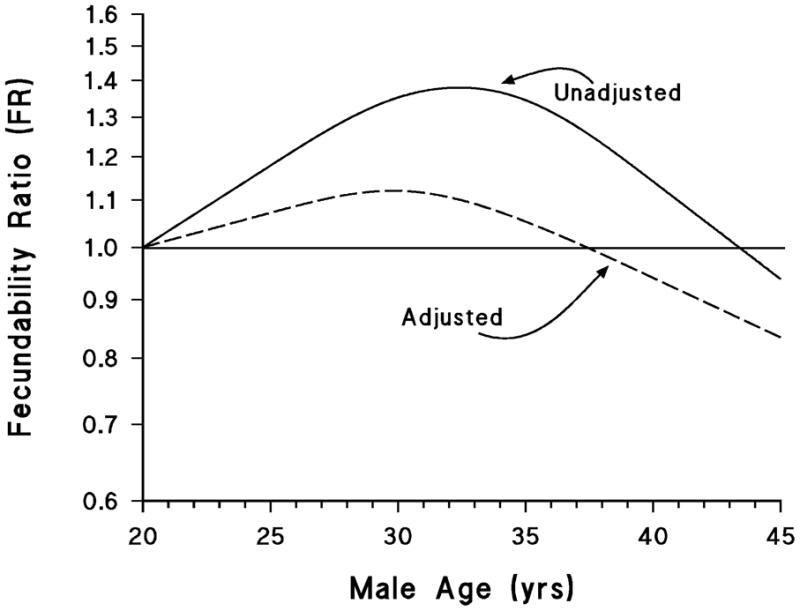
The central panel of figure 1 summarizes the data on the relation between fecundability and age of male partner. The changes with age resemble those of females, but when confounders are controlled, the change appears much smaller than for females, dropping about 20% from the peak at age 30 to age 45. In all these analyses, age of partner has been controlled. Most couples, however, will experience the compounding effect of a simultaneous advance in both partners’ ages, and therefore the decline in fecundability after age 30 will be considerably steeper than is depicted in any of these figures. As an illustration, we estimated the fecundability ratio for couples in which both partners were either age 37 or above or in the age range 27–33 (essentially comparing couples around age 40 with couples around age 30), using the same model as in table 3. The fecundability ratio for the older couples was 0.58 compared with the younger couples (95% CI 0.42—0.79).
Discussion
We found that women had slightly higher fecundability than estimates reported in earlier studies (1–5), a difference that may at least partially stem from differences in the populations studied. In our study, peak fecundability was approximately 29–30 years among parous women and 27–28 years among nulliparous women. Among parous women, age was associated with increasing fecundability until age 30 years, after which it decreased; among nulliparous women, there was little increase in fecundability after age 20 and a marked decline starting around age 28 years. The effect of male age was more modest, but also was associated with a decline beginning near age 30 years. Our cohort is relatively young, with few participants from the oldest categories of reproductive age, and therefore our findings may not be applicable for the oldest couples seeking to conceive.
Previous studies have examined fecundability in women receiving donor insemination, whereas the current study prospectively followed a cohort of women trying to conceive naturally, and is restricted to women who were just beginning to attempt pregnancy. Our study most closely resembles that of Howe et al. (3), a prospective cohort study in which data also were stratified by parity. In that study, the effect of age on fecundability was modest among parous women until age 37, after which it declined appreciably, but for nulliparous women there was a strong and consistent decrease in fecundability in each age category above 25–27 (the youngest age group in study). Howe et al. reported peak fecundability for parous women at age 28–29 years (3). Therefore, the results from these two prospective studies show remarkably similar parity-specific age patterns. Both studies indicate that the age-fecundability fertility decline is stronger in nulliparous women
The validity of our findings depends ultimately on the data quality and completeness of follow up. Our study population was recruited via the internet. Although study participants are volunteers, the same is true for essentially all population-based cohort studies and all randomized trials. If the age-related decline that we report differed between Danish internet users and others, our results might have limited generalizability, but we see no reason why age-related declines in fecundability would differ according to whether a woman had internet access. Other factors, such as reproductive history, could have affected participation and be related to fecundability; if these factors were also associated with age, they could have distorted the estimates of fecundability ratios by age. Because women who were included in this analysis began their attempts to conceive up to three months before we began to follow them, women who became pregnant very soon after attempting to conceive were less likely to be enrolled. This selective enrollment also could have distorted our findings.
We found that older women more often employed timing of intercourse to improve their chances for conception, but there was nevertheless a decreasing frequency of intercourse with increasing age, even among this population of women who were trying to conceive. Among women who had both a decreased frequency of intercourse and more careful timing of intercourse, there was only a modest net effect on fecundability, which offset some but not all of the age-related decline.
The decline in fecundability that we observed is steeper for women than for men, but begins at approximately the same age for both. Thus, for committed couples who are aging together, there is a compounded effect of their separate age-related declines. For example, if both partners are the same age and the declines are considered independent, then their fecundability at age 40 is nearly half of what it is at age 30.
Acknowledgments
We acknowledge the contributions of the study staff and all the women who participated in the Snart Gravid study. We specifically thank Ms. Tina Christensen for her support with data collection and media contact, Dr. Donna Baird for her feedback on questionnaire development, and Mr. Thomas Jensen for his assistance with website design. We also thank Ms. Kristen Hahn and Ms. Rose Radin for their general assistance with the manuscript.
Funding: This work was supported by the U. S. National Institute of Child Health and Human Development (R21-050264) and the Danish Medical Research Council (271-07-0338). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributors
All authors were involved in the conception, design, and implementation of the Snart Gravid study, as well as the data analysis presented here and the interpretation of the results. KJR wrote the first draft of the manuscript; AHR managed the data, LAW ran the data analyses, and all authors contributed to revisions and approved the final manuscript.
We have no conflicts of interest to declare.
Competing interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization other than the NICHD for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Kenneth J. Rothman, Boston University School of Public Health, 715 Albany Street, Boston, MA 02118, USA, and Distinguished Fellow at RTI International, Research Triangle Park, North Carolina.
Dr. Lauren A. Wise, Boston University School of Public Health, and Senior Epidemiologist at the Slone Epidemiology Center of Boston University.
Dr. Henrik T. Sørensen, Department of Clinical Epidemiology, Institute of Clinical Medicine, Aarhus University Hospital.
Mr. Anders H. Riis, Department of Clinical Epidemiology, Institute of Clinical Medicine, Aarhus University Hospital.
Dr. Ellen M. Mikkelsen, Department of Clinical Epidemiology, Institute of Clinical Medicine, Aarhus University Hospital.
Dr. Elizabeth E. Hatch, Boston University School of Public Health, 715 Albany Street, Boston, MA 02118, USA.
References
- 1.Schwartz D, Mayaux MJ. Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. Federation CECOS. N Engl J Med. 1982;306:404–406. doi: 10.1056/NEJM198202183060706. [DOI] [PubMed] [Google Scholar]
- 2.van Noord-Zaadstra BM, Looman CWN, Alsbach H, Habbema JDF, te Velde ER, Karbaat J. Delaying childbearing: effect of age on fecundity and outcome of pregnancy. BMJ. 1991;302:1361–1365. doi: 10.1136/bmj.302.6789.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howe G, Westoff C, Vessey M, Yeates D. Effects of age, cigarette smoking and other factors on fertility: findings in a large prospective study. BMJ. 1985;290:1697–1700. doi: 10.1136/bmj.290.6483.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen U, Yan S. The age pattern of fecundability: an analysis of French Canadian and Hutterite birth histories. Biodemography and Social Biology. 2000;47:34–50. doi: 10.1080/19485565.2000.9989008. [DOI] [PubMed] [Google Scholar]
- 5.Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389–1394. doi: 10.1126/science.3755843. [DOI] [PubMed] [Google Scholar]
- 6.Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Selection bias in determining the age dependence of waiting time to pregnancy. Am J Epidemiol. 2000;152:565–572. doi: 10.1093/aje/152.6.565. [DOI] [PubMed] [Google Scholar]
- 7.Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum Reprod Update. 2007;13:209–223. doi: 10.1093/humupd/dml056. [DOI] [PubMed] [Google Scholar]
- 8.Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Van Steirteghem A, Cohen J, Crosignani PG, Devroey P, Diedrich K, Fauser BC, Fraser L, Glasier A, Liebaers I, Mautone G, Penney G, Tarlatzis B for the ESHRE Capri Workshop Group. Fertility and ageing. Hum Reprod Update. 2005;11:261–276. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- 9.Hassan MAM, Killick SR. Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertil Steril. 2003;79(Suppl 3):1520–1527. doi: 10.1016/s0015-0282(03)00366-2. [DOI] [PubMed] [Google Scholar]
- 10.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237– 248. doi: 10.1016/s0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 11.Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort profile: the Danish Web-based Pregnancy Planning Study – “Snart-Gravid. Int J Epidemiol. 2009;38:938–943. doi: 10.1093/ije/dyn191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothman KJ, Mikkelsen EM, Riis A, Sorensen HT, Wise LA, Hatch EE. Randomized trial of questionnaire length. Epidemiology. 2009;20:154. doi: 10.1097/EDE.0b013e31818f2e96. [DOI] [PubMed] [Google Scholar]
- 13.Huybrechts KF, Mikkelsen EM, Christensen T, et al. A successful implementation of e-epidemiology: the Danish pregnancy planning study “Snart-Gravid. Eur J Epidemiol. 2010;25:297–304. doi: 10.1007/s10654-010-9431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikkelsen EM, Riis AH, Wise LA, Hatch EE, Rothman KJ, Sørensen HT. Pre-gravid oral contraceptive use and time to pregnancy: A Danish prospective cohort study. Hum Reproduction. 2013:xxx–xxx. doi: 10.1093/humrep/det023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg CR, Baird DD, Wilcox AJRe. Effects of caffeine consumption on delayed conception. Am J Epidemiol. 1996;144:799. doi: 10.1093/oxfordjournals.aje.a009006. author reply 801. [DOI] [PubMed] [Google Scholar]
- 16.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in Medicine. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 17.Li R, Hertzmark E, Spiegelman D. The SAS GLMCURV9 Macro. Boston, MA: Channing Laboratory; 2008. [Google Scholar]
- 18.Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20:1541–1549. doi: 10.1002/sim.689. [DOI] [PubMed] [Google Scholar]
- 19.SAS Institute. SAS/STAT 9.2 user’s guide. SAS Institute; Cary, NC: 2008. [Google Scholar]


