Abstract
Prenatal cocaine exposure (PCE) is associated with arousal dysregulation, and alterations of amygdala activity in response to emotional arousal were previously reported. However, voluntary regulation of emotional affect, enabling appropriate neural response to different streams of stimuli, must also engage prefrontal regions, yet PCE impact on these prefrontal mechanisms has not been investigated. Recent neuroimaging studies have shown the involvement of ventral prefrontal cortex (vPFC) in the modulation of amygdala reactivity and the mediation of effective emotion regulation. Based on these findings, using functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI), the present study compared functional activations of vPFC as well as its structural connectivity with amygdala between groups of PCE and control adolescents. In a working memory task with emotional distracters, the PCE adolescents exhibited less capability of increasing their vPFC activation in response to increased memory load, which corresponded with their less suppressed amygdala activation. Reduced structural connectivity between vPFC and amygdala were also observed from DTI measurement in the PCE group. In addition, correlations between amygdala activation and (i) vPFC activation, as well as (ii) amygdala-vPFC structural connectivity, were observed in the control but not in the PCE group. These data complemented previous findings of PCE impact on amygdala activity and extended our understanding of the neurobiological mechanisms of PCE effect on arousal dysregulation reported in human and animal studies.
Keywords: prenatal cocaine exposure, functional magnetic resonance imaging, diffusion tensor imaging, ventral prefrontal cortex, amygdala
1. Introduction
Arousal dysregulation is one of the major findings of previous investigations of neurodevelopmental effects of prenatal cocaine exposure (PCE). This long-term effect has been reported at different stages of postnatal development including neonates (Dipietro et al., 1995; Karmel and Gardner, 1996), infants (Bendersky and Lewis, 1998; Coles et al., 1999; Bard et al., 2000; Schuetze and Eiden, 2006; Eiden et al., 2009b; Eiden et al., 2009a), young children (Bandstra et al., 2001; Dennis et al., 2006; Bada et al., 2007; Kable et al., 2008; Chaplin et al., 2009) and adolescents (Li et al., 2009; Lester et al., 2010; Li et al., 2011). Specifically, children with PCE often exhibit a reduced threshold in response to perceived stress or emotionally salient stimuli, which in turn may affect available attention resource to cognition and behavior (Mayes et al., 1998; Mayes, 2002).
Our previous functional MRI study (Li et al., 2009) revealed a likely neurobiological mechanism associated with dysregulation of emotional arousal in PCE adolescents. With a working memory task paradigm, which had emotionally arousing pictures as the task-irrelevant distracters, the data of this previous study showed that high memory load suppressed the amygdala response to emotional stimuli in the control but not in the PCE adolescents. In other words, the non-exposed participants exhibited reduced emotional response in a situation of more attentional demand, while this capacity of suppressing emotional distraction was diminished in the PCE adolescents. Although this previous finding is suggestive, it is still incomplete because the amygdala, while playing a key role in emotional processing, does not operate in isolation. Given the extensive neural connections between prefrontal regions and amygdala (Price, 2003; Stein et al., 2007; Ghashghaei et al., 2007; Cohen et al., 2008; Roy et al., 2009; Bracht et al., 2009) as well as the importance of this circuit in emotion and behavior regulation (Davidson et al., 2000; Bachevalier and Loveland, 2006; Quirk and Beer, 2006; Banks et al., 2007), the impact of PCE on emotional arousal is expected to also involve alterations of prefrontal activity and alterations of prefrontal-amygdala neural connectivity.
Previous human and animal studies have reported convergent evidence showing a central role of ventral prefrontal cortex (vPFC) in emotion regulation and amygdala inhibition (see (Quirk and Beer, 2006) for review). Also termed orbito-, inferior-, or ventromedial-prefrontal cortex in different studies, the region of vPFC usually involves Brodmann areas of 10, 11, 32, 46 and 47; and it often shows opposing activity to amygdala, functionally considered as suppressing or reappraising negative emotion (Ochsner et al., 2002; Rosenkranz et al., 2003; Kim et al., 2003; Hariri et al., 2003; Levesque et al., 2004; Lzquierdo and Murray, 2005; Urry et al., 2006). Neural connections between vPFC and amygdala have also been reported repeatedly in human neuroimaging studies examining functional connectivity (Banks et al., 2007; Roy et al., 2009), effective connectivity (Stein et al., 2007), and structural connectivity (Cohen et al., 2008; Bracht et al., 2009). In addition, the strength of these connections is correlated with variations in trait anxiety, which is closely associated with affect regulation and amygdala activity (Kim and Whalen, 2009; Chepenik et al., 2010).
Given that (i) alterations of amygdala activation have been observed in adolescents with PCE, and (ii) there are close functional and structural associations between amygdala and vPFC, we hypothesized in the present study that functional activation of vPFC and its neural connections with amygdala are also altered by PCE. Specifically, because high working memory load does not suppress amygdala activation in the PCEs (Li et al., 2009), and vPFC is assumed to exert inhibitive influence on amygdala, it was hypothesized that (1) PCE participants would exhibit less increase of vPFC activation than controls in conditions of high memory load, in which amygdala activations are typically suppressed; (2) PCE participants would exhibit reduced white matter integrity in fiber pathways connecting amygdala and vPFC; and (3) measurements of vPFC activation and amygdala-vPFC connection would correlate with amygdala activations in the controls, but these correlations may be diminished in the PCE group.
To test these hypotheses, the present study further analyzed the neuroimaging data that we previously collected (Li et al., 2009; Li et al., 2011) in studying long-term effect of PCE. To test the hypothesis (1), the vPFC and amygdala activations were examined by defining regions of interests (ROIs) according to the emotion contrast in the task paradigm (see details described in Method section). To test the hypothesis (2), the vPFC-amygdala neural connectivity was examined with diffusion tensor imaging (DTI) data by a probabilistic tractography approach (Behrens et al., 2003; Behrens et al., 2007). Finally, to test the hypothesis (3), individual activations of the amygdala were respectively correlated with (i) the activations of the vPFC and (ii) the vPFC-amygdala neural connectivity.
2. Method
2.1 Participants
Participants were adolescents (12–18 Y.O.) recruited from birth cohorts originally identified as part of two longitudinal studies of PCE effect on development (Coles et al., 1992; Brown et al., 1998). Both cohorts were drawn from a low income, predominantly African-American population with infants delivered at an urban hospital during 1987–1994. The present study involved 76 participants from these cohorts with 70% of them providing usable imaging data for both fMRI and DTI. For those who did not provide imaging data in both modalities, the majority of the data losses were on the fMRI side due to excessive head motion, failure to follow task instructions and scanner malfunction (Li et al., 2009). The fMRI and DTI sample used in the present study respectively included 56 (23 control vs. 33 PCE) and 73 (31 control vs. 42 PCE) participants.
Information about maternal cocaine and other drug use was obtained both from abstraction of medical records and from the birth mother’s self-report at recruitment in the immediate postpartum period using the Addiction Severity Index (McLellan et al., 1985) and the Drug Grid (Coles et al., 1992). The Addiction Severity Index allows assessment of the degree of addiction as well as the social and medical impact of the mother’s substance use. The Drug Grid quantifies the use of 16 licit and illicit drugs by quantity/frequency as well as timing of use. Maternal self-report was confirmed using EMIT urine screens taken postpartum from mothers and infants. Participating mothers were free of major medical conditions as well as ant abuse, drugs for seizure disorders, warfarin, insulin, benzodiazepines, antipsychotic drugs or any other teratogenic drugs, or any addictive substances other than cocaine, alcohol and marijuana. Infants were either healthy full term or preterm (30+ weeks) without major medical complications. For neuroimaging, potential adolescent participants were excluded from the source longitudinal cohort if they had contraindications for MRI or were pregnant, claustrophobic or extremely obese. More details regarding PCE determination and participant screening were reported in our previous publications (Coles et al., 1992; Brown et al., 1998; Li et al., 2011). The adolescents’ demographic information at the time of imaging and at birth is shown in Table 1. Their maternal characteristics at the time of recruitment are shown in Table 2.
Table 1.
Characteristics of Adolescents at the Time of Imaging and at Birth
| Variable | fMRI sample | DTI sample | ||||
|---|---|---|---|---|---|---|
| CON (N=23)a |
PCE (N=33)a |
p Valueb |
CON (N=31)a |
PCE (N=42)a |
p Valueb |
|
| Adolescent Variables | ||||||
| Age, M (SD) | 14.61 (2.3) | 14.64 (2.0) | .962 | 14.3 (2.5) | 14.4 (2.0) | .782 |
| Gender, No. (%) | .019 | .174 | ||||
| Female | 15 (65.2) | 11 (33.3) | 16 (51.6) | 15 (35.7) | ||
| Male | 8 (34.8) | 22 (66.7) | 15 (48.4) | 27 (64.3) | ||
| Total monthly household income - $, M (SD) (NfMRI=53, NDTI=70) |
1898 (1284) | 1221 (922) | .030 | 2042 (1239) | 1349 (1123) | .017 |
| Handedness, No. (%) | .918 | .500 | ||||
| Right | 20 (87.0) | 29 (87.9) | 26 (83.9) | 36 (85.7) | ||
| Left | 3 (13.0) | 4 (12.1) | 5 (16.1) | 6 (14.3) | ||
| Full-Scale IQ - WASI, M (SD) | 88.8 (8.4) | 87.0 (11.4) | .497 | 85.7 (9.9) | 86.3 (12.6) | .831 |
| Verbal IQ - WASI, M (SD) | 90.7 (9.5) | 86.6 (12.6) | .182 | 87.3 (10.2) | 85.8 (13.0) | .588 |
| Performance IQ - WASI, M (SD) | 89.3 (9.5) | 89.8 (11.2) | .855 | 87.0 (11.7) | 89.1 (12.5) | .483 |
| Birth Variables | ||||||
| Birthweight (g) (M, SD) | 2959 (783) | 2883 (644) | .693 | 3060 (694) | 2796 (652) | .100 |
| Gestational Age, No. (%) | .179 | .554 | ||||
| Fullterm | 17 (73.9) | 29 (87.9) | 28 (90.3) | 36 (85.7) | ||
| Preterm (30–36 weeks of gestational age) | 6 (26.1) | 4 (12.1) | 3 (9.7) | 6 (14.3) | ||
If data for a variable are not available for some participants, the N used for the analysis is noted next to the variable name.
Chi-square analyses completed for categorical variables; Independent sample t-tests completed for continuous variables.
CON: control, PCE: prenatal cocaine exposure
Table 2.
Maternal Characteristics at the Time of Birth
| Variable | fMRI sample | DTI sample | ||||
|---|---|---|---|---|---|---|
| CON (N=23)a |
PCE (N=33)a |
p Valueb |
CON (N=31)a |
PCE (N=42)a |
p Valueb |
|
| Age, M (SD) | 26.3 (5.2) | 28.2 (4.3) | .138 | 26.2 (5.1) | 28.4 (4.2) | .051 |
| Education, No. (%) (NfMRI=51, NDTI=68) |
.006 | .014 | ||||
| High school not completed | 2 (9.1) | 13 (44.8) | 5 (16.7) | 17 (44.7) | ||
| High school graduate or more | 20 (90.9) | 16 (55.2) | 25 (83.3) | 21 (55.3) | ||
| Monthly income, No. (%) (NfMRI=51, NDTI=68) |
.773 | .457 | ||||
| ≤$600 | 20 (90.9) | 27 (93.1) | 27 (90.0) | 36 (94.7) | ||
| >$600 | 2 (9.1) | 2 (6.9) | 3 (10.0) | 2 (5.3) | ||
| Marital status, No. (%) | .179 | .123 | ||||
| Married | 6 (26.1) | 4 (12.1) | 9 (29.0) | 6 (14.3) | ||
| Single, divorced, separated, widowed | 17 (73.9) | 29 (87.9) | 22 (71.0) | 36 (85.7) | ||
| Other substance use in pregnancy, M (SD) | ||||||
| Tobacco - cigarettes/week (NfMRI=52, NDTI=69) |
9.1 (32.0) | 61.1 (50.1) | .000 | 8.1 (27.9) | 58.6 (51.3) | .000 |
| Alcohol - oz. of absolute alcohol/week (NfMRI=54, NDTI=72) |
0.0 (0.1) | 1.0 (1.8) | .004 | 0.0 (0.1) | 1.1 (1.8) | .002 |
| Marijuana - joints/week (NfMRI=54, NDTI=71) |
0.0 (0.0) | 1.3 (2.9) | .016 | 0.0 (0.0) | 1.3 (2.7) | .012 |
If data for a variable are not available for some participants, the N used for the analysis is noted next to the variable name.
Chi-square analyses completed for categorical variables; Independent sample t tests completed for continuous variables.
CON: control, PCE: prenatal cocaine exposure
Before the imaging session, urine specimens for 731 adolescents were tested to identify metabolites of seven drugs: amphetamines, barbiturates, benzodiazepines, marijuana, cocaine, opiates, and phencyclidine. Of the 511 tests completed, only 10 of them were positive (6 PCE and 2 control tests were positive for marijuana, 2 PCE tests were positive for amphetamine) and no group differences were noted. Children’s reported social history was examined for evidence of stable custody arrangements and history of physical or sexual abuse. Of 7 items related to stability and trauma (years at current address, changes in house-hold composition in the last year, stability in custody, protective services involvement, reported abuse/neglect, school discipline problems and legal problems), 3 items, the number of changes in care-giving, protective service involvement, and school discipline problems were higher in PCE youth. For care-giving, 10 PCE children had more than one caregiver versus 1 in the contrast group (Fisher’s Exact Test, 1-sided, p= 0.01); for protective service involvement, 12 PCE children had Division of Family and Children Service record versus 1 in the contrast group (p= 0.002); for school records, 18 PCE children had school discipline problems noted versus 5 in the contrast group (p = 0.01).
Participating families consented for the imaging study according to a protocol approved by Emory University’s Institutional Review Board. Adolescents provided written assent and adults, including both teens and caregivers, informed consent, to participate.
2.2 Experimental task design
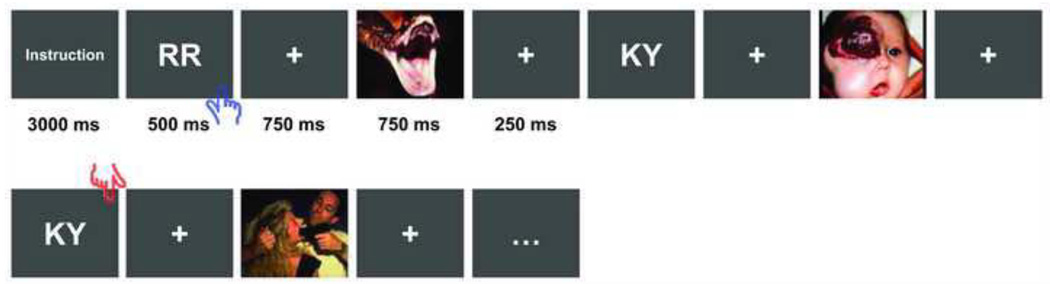
The experimental task and paradigm have been described in details in our previous reports (Li et al., 2009; Li et al., 2011). Briefly, an N-back working memory task was used in the present block-design fMRI experiment. With a list of letter pairs sequentially presented, the task required the participants to press a response button either when seeing “RR” (0-back condition, low memory load), or when the displaying letter pair exactly matched the last one displayed (1-back condition, high memory load). There were also emotionally neutral and negative pictures embedded in the letter list as task-irrelevant distracters. These pictures were selected from the International Affective Picture System (Lang et al., 1997) with the arousal score being 3.2 (SD=0.8) and 5.7 (SD=0.8), respectively. According to different permutations of the memory load and emotional distraction, there were 4 experimental conditions: Neutral_0-back (Nu0), Neutral_1-back (Nu1), Negative_0-back (Ng0) and Negative_1-back (Ng1). Task blocks of these 4 conditions, with each repeated 6 times, were pseudo-randomly distributed in 2 fMRI scans. A schematic diagram of the experimental paradigm is shown in Figure 1. Participants were instructed to only focus on the memory task and ignore all the distracting pictures.
Figure 12.
Schematic diagram of the experimental paradigm. Each task block began with an instruction asking subjects to either perform the 0-back or 1-back memory task. The letter pairs were interleaved by fixation crosses and distracter pictures (duration labeled). These pictures were either neutral or negative (only negative picture shown here) within each fMRI block. The blue/red hands indicate the display on which a button response is required for the 0-back/1-back task.
2.3 MRI data acquisition
The neuroimaging data were acquired using a 3T MRI scanner (Siemens Medical Solutions, Malvern, PA).For fMRI, a T2*-weighted echo-planar imaging sequence was used with the following parameters:120 volumes, matrix = 64 × 64, 30 axial slices without gap, thickness =3 mm, TR / TE = 3000 ms/30 ms, flip angle = 90°, FOV = 192 × 192 cm2. For DTI, a diffusion-weighted, single-shot, spin-echo echo-planar imaging sequence was used with the following parameters: 12 directions, b = 0, 1000 s/mm2, TR/TE/ FOV = 6500 ms/90 ms/ 220 mm, flip angle = 90°, matrix = 128×128, 34 axial slices without gap, thickness =3.5 mm, 6 averages. This DTI sequence used a dual spin-echo technique combined with bipolar gradients (Alexander et al., 1997) which minimizes the geometric distortion induced by eddy currents. High resolution (256 × 256) 3D T1-weighted images were also collected from each subject for anatomical reference and stereotaxic transformation.
2.4 Imaging data analysis
AFNI (http://afni.nimh.nih.gov) was generally used in the present imaging data analysis. In addition, FSL (http://www.fmrib.ox.ac.uk/fsl/) was used for probabilistic fiber tracking with DTI data.
The fMRI data were analyzed at the individual level with the following processing pipeline: slice timing correction, volume registration, anatomy-to-EPI co-registration, signal percent change normalization, scan-run concatenation, spatial smoothing (FWHM = 5 mm), and multiple regression. The “anatomy-to-EPI co-registration” used a newly developed approach of weighted local Pearson correlation (Saad et al., 2009), which improved the function-to-anatomy image alignment than our previous studies of the same dataset (Li et al., 2009; Li et al., 2011). The multiple regression analysis used task regressors modeling the 4 experimental conditions and nuisance regressors modeling linear signal drifts and head motion. The task regressors were generated by convolving respective boxcar stimulation functions with an impulse response function [y =tb × exp(−t/c), b and c are constants] (Cohen, 1997). Once the regression coefficients (beta values) of the 4 task conditions were obtained, they were transformed into the Talairach space (Talairach and Tournoux, 1988) and fed into a 2 (high and low memory load) × 2 (neutral and negative emotion) repeated measures ANOVA. This ANOVA, with the data from 46 (23 PCE + 23 control) participants as the inputs, was used to determine the ROIs for the subsequent data analysis. It used equal number of PCE and control subjects (10 PCEs randomly dropped) to ensure the defined ROIs would not be biased by dominant contribution from either group. With a threshold of significance (p<0.001/voxel and 82 mm3 cluster, p<0.05 corrected) applied in the resultant activation map, 2 clusters in bilateral amygdala (centroidleft= 21.9, 5.1, −9.2, centrioldright = −23.9, 6.1, −9.7; volumeleft = 679 mm3, volumeright = 1170 mm3) that showed a positive emotion main effect (higher activation in negative than in neutral condition) were defined as the amygdala ROIs. Because vPFC is assumed to exert inhibitory influence on amygdala, the ROIs of vPFC were defined as clusters with a negative emotion main effect (higher activation in neutral than in negative condition) in the ventral prefrontal cortex. The ANOVA revealed 3 such clusters with 2 small ones in the left hemisphere (centroid1 = 18.2, −38.3, −9.2, centriold2 = 27.4, −55.1, −1.3; volume1 = 295 mm3, volume2 = 265 mm3) and a large one in the right hemisphere (centroid = −21.3, −54.2, −3.5, volume = 1695 mm3); therefore, the right cluster was defined as the ROI of right vPFC and the 2 left clusters were combined to form the ROI of left vPFC. To examine the PCE effect on activations, regression coefficients of the 4 experimental conditions were extracted from the ROIs of vPFC and amygdala, respectively, and then fed into a 2 (high and low memory load) × 2 (negative and neutral emotion) × 2 (left and right hemisphere) × 2 (PCE and control group) ANOVA. The major effect of interest in this analysis was the group × memory interaction, where the high memory load was hypothesized to increase the vPFC activation and suppress the amygdala activation in the controls but less so in the PCE group.
The DTI data analysis followed the pipelines suggested by FSL’s Diffusion Toolbox (http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_pipeline.html). After eddy current correction and diffusion tensor fitting, individual’s DTI data were fed into a process of Markov Chain Monte Carlo sampling to build up distributions on diffusion (2 directions) parameters (Behrens et al., 2007). With the diffusion parameters estimated, fiber tracking was performed probabilistically between each of the 4 ROI pairs, namely, vPFCleft-amygdalaleft, vPFCleft-amygdalaright, vPFCright-amygdalaleft, and vPFCright-amygdalaright. This was a voxel-wise repetitive sampling process, each time computing a streamline connecting the 2 ROIs of vPFC and amygdala. With 5000 such samplings performed, the posterior distribution of the connection was established. Because of inter-subject variability in the spatial distribution of tracts, we defined the vPFC-amygdala pathways by voxels that had non-zero tract estimates in at least 60% of the total DTI sample (Cohen et al., 2008). To avoid group bias, the total DTI sample used here consisted of equal numbers of PCE and control participants (31 control and 31 PCE) with 11 PCEs randomly dropped. This fiber tracking procedure only revealed pathways within hemisphere, namely, vPFCleft-amygdlaleft and vPFCright-amygdalaright; therefore, subsequent analysis only focused on these 2 pathways. To perform the PCE vs. control comparison of the white matter integrity on these vPFC-amygdala pathways, fractional anisotropy (FA) values were extracted from each pathway in each one of the participants and submitted to a 2 (PCE and control group) × 2 (left and right hemisphere) ANOVA.
Because the number of male and female adolescents was not evenly distributed in the PCE and control groups, and the effect of PCE in humans is often confounded by many other factors (e.g. multi-drug exposure, socioeconomic status) that are very difficult to match between groups (Hurt et al., 2009; Hackman et al., 2010; Ackerman et al., 2010), statistical control (via nuisance covariates) of the following 7 confounding factors were included in all the ANOVA analyses of fMRI and DTI data that involved a PCE vs. control group effect: (i) gender, (ii) prenatal alcohol exposure (ounces of absolute alcohol used weekly during pregnancy), (iii) prenatal marijuana-tobacco exposure (amount used during pregnancy in units of joints/cigarette per week), (iv) change in care-giving (yes or no), (v) protective service involvement (yes or no), (vi) notice of school discipline problems (yes or no) and (vii) total monthly household income ($). The marijuana–tobacco was a combined variable derived by principle component factor reduction because their uses were highly correlated in our samples (Pearson correlation, for fMRI sample R=0.29, p= 0.04; for DTI sample, R=0.24, p = 0.05).
3. Results
The results of group comparison on behavioral performances of the working memory task were described in detail in our previous report (Li et al., 2009). Briefly, the memory performance was decreased with higher memory load and/or negative emotional distraction. However, the PCE and control groups were not significantly different in task performance as the task paradigm was deliberately designed to minimize behavioral group difference.
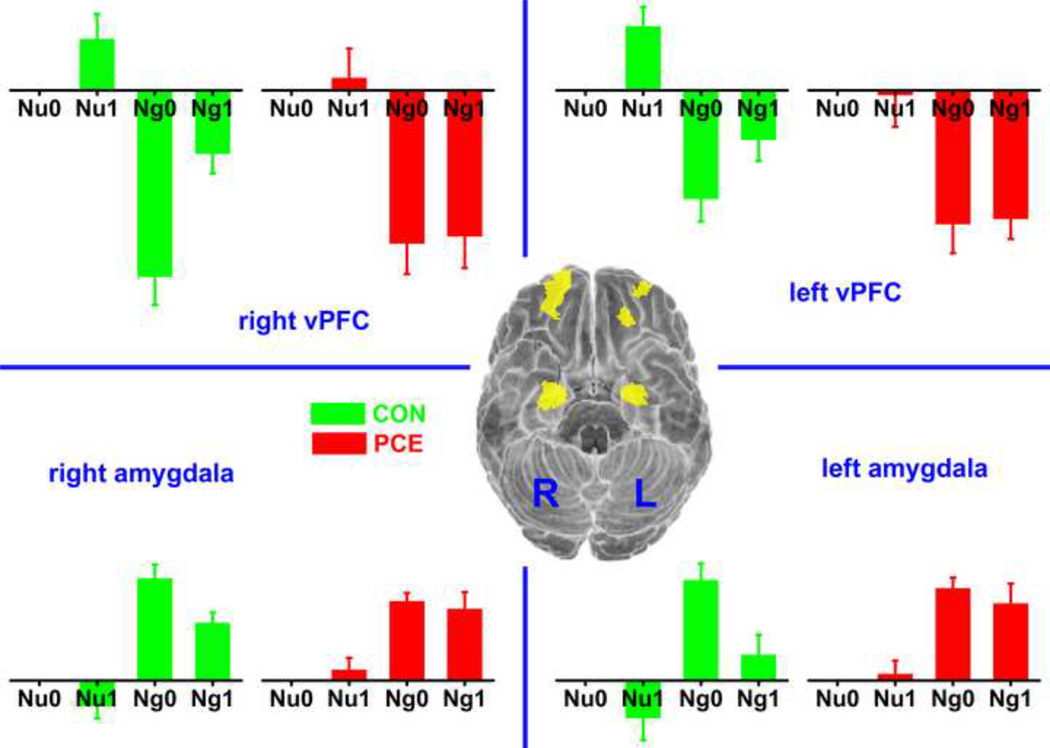
In the bilateral ROIs of amygdala, significant emotion (F1,54= 165, p < 0.001) and memory (F1,54 = 7.2, p = 0.01) effects were observed in the fMRI data. Namely, amygdala activation was increased by higher emotional arousal and suppressed by higher memory load. As we reported previously (Li et al., 2009), the group difference in amygdala activation appeared in the memory effect. When the memory load increased from 0- to 1-back, the amygdala activation decreased in the control but much less so in the PCE group (Figure 2, bottom portion). This memory × group effect was significant without statistically controlling the 7 confounding factors (F1,54= 6.4, p = 0.02), but not significant when those covariates were considered in the ANOVA (F1,36 = 1.0, p = 0.32). However, instead of considering a binary measurement of PCE (with or without exposure), we also tested the effect of an alternative factor, the frequency of maternal cocaine use (more than once per day, once per day, 3–4 times per week, 1–2 times per week, 2–3 times per month, less than once per month, or never used). When the values of memory main effect (1-back minus 0-back) were put into a hierarchical regression analysis with the 7 confounding factors as the 1st-step regressors and frequency of maternal cocaine use as the 2nd-step regressor, the frequency of maternal cocaine use provided additional explanation of the data variance (R2 change = 0.10, p=0.04).Thus, PCE contributed to the group difference of memory effect in the amygdala, but the effect of poly-drug exposure and other socioeconomic factors were also considerable.
Figure 2.
Activation pattern in the 4 ROIs of bilateral vPFC and amygdala. The ROIs are shown through bottom view in the central brain with the left (L) and right (R) hemispheres labeled. ROI activations in different experimental conditions are shown with the bar graphs in corresponding quadrants. All the bars, presenting regression coefficients, are plotted in the same scale with the neutral_0-back condition as the baseline. CON: control; PCE: prenatal cocaine exposure; Nu: neutral; Ng: negative: 0: 0-back; 1: 1-back.
In the bilateral ROIs of vPFC, the fMRI data exhibited a pattern of emotion main effect opposite to that of the amygdala. Namely, the activations of bilateral vPFC were decreased in the negative condition versus in the neutral (F1,54= 198, emotion effect p < 0.001). This opposite emotion effect in the vPFC and amygdala was determined by the ROI definition as the vPFC should generally exert a suppressive effect on the amygdala. Corresponding to the PCE adolescents’ less-suppressed amygdala activation in higher memory load, the ROIs of bilateral vPFC also exhibited a significant memory × exposure effect (figure 2, top portion). Namely, when the memory load increased from 0-back to 1-back, the activations of vPFC were increased in the control but much less so in the PCE group (without covariates control, F1,54= 8.8, p = 0.01; 7 covariates controlled, F1,36 = 6.0, p = 0.02). This observation is consistent with the concept of vPFC to amygdala inhibition. For the control adolescents, higher memory load led to increased vPFC activations and decreased emotional responses in amygdala. However, this inhibitory mechanism was impaired in the PCE group with an absence of memory effect in both the vPFC and amygdala.
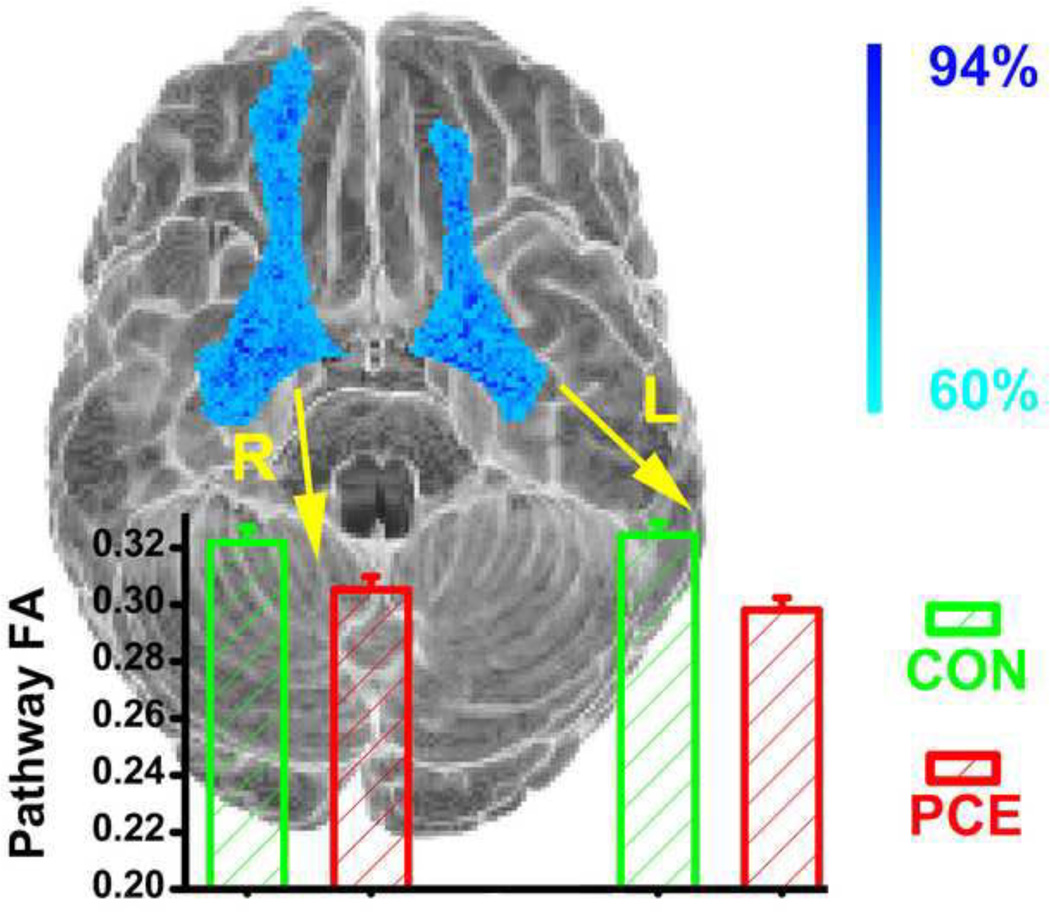
Another aspect of evidence indicating PCE-associated impairment in vPFC-amygdala communication came from the DTI data. As described above in the method section, the probabilistic fiber tracking revealed robust (non-zero tract estimates in more than 60% of the participants) white matter tracts connecting the vPFC and amygdala within hemisphere. They correspond to the uncinate fasciculus described by Bracht and colleagues (Bracht et al., 2009), which connect the limbic structures (e.g. hippocampus and amygdala) with orbitofrontal cortex. These two pathways are graphically shown in Figure 3 a long with the group comparison of the FA values extracted from them. In both of the pathways, the PCE adolescents exhibited a lower FA value than the control group (without covariates control, F1,71= 12, p = 0.001;7 covariates controlled, F1,53 = 6.8, p = 0.01) indicating a PCE-associated reduction of white matter connectivity that mediates the vPFC-amygdala communication. There was neither hemisphere main effect (without covariates control, F1,71= 0.63, p = 0.43; 7 covariates controlled, F1,53 = 1.6, p = 0.21) nor hemisphere × group interaction (without covariates control, F1,71 = 2.9, p = 0.10; 7 covariates controlled, F1,53 = 1.7, p = 0.19) noticed in the present DTI data. In addition, the observed DTI group difference in the amygdala-vPFC pathway does not simply reflect a global FA reduction in the PCE group as the mean FA value in the entire brain was not significantly different between the two groups (FAPCE=0.37, FAcontrol=0.36; group t-test, t71 = 1.3, p = 0.19).
Figure 3.
Group comparison of the vPFC-amygdala structural connectivity. White matter pathways defined by the probabilistic fiber tracking are shown with the cyan-blue color scale, which represents the percentage of subjects with a non-zero tract estimates in that voxel. FA values, respectively extracted from the left and right pathways are compared between the two groups by the bar graph. CON: control, PCE: prenatal cocaine exposure.
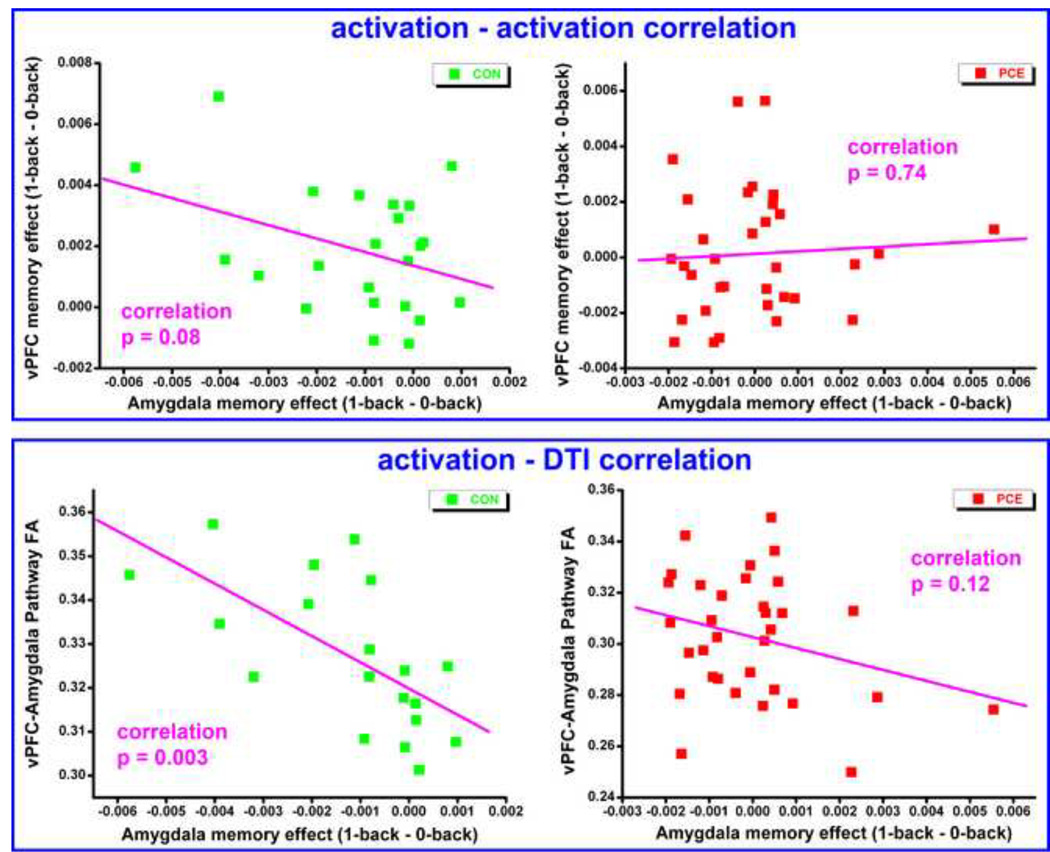
To further explore the vPFC-amygdala interactions, correlations between amygdala suppression and (i) vPFC activation, as well as (ii) vPFC-amygdala structural connectivity were examined in both of the participating groups. The suppression of amygdala responses was represented by the memory effect, calculated as {[(Nu1-Nu0) + (Ng1 - Ng0)]left_amygdala+ [(Nu1-Nu0)+ (Ng1- Ng0)]right_amygdala}/4. This value was generally negative across subjects as it reflected cognitive suppression of emotional responses. Correspondingly, the memory effect of the vPFC was calculated the same way, but was generally positive across subjects as it reflected increased vPFC effort with high memory load. The index of vPFC-amygdala structural connectivity was calculated as the mean FA values in the 2 pathways shown in Figure 3. Because not all the subjects provided imaging data in both modalities, the correlation of fMRI-DTI only used the 19 control and 33 PCE subjects who were the intersection of the fMRI and DTI samples. As shown in Figure 4, suppressions of amygdala activation were negatively correlated with the vPFC activation (top portion) and vPFC-amygdala structural connectivity (bottom portion) in the control group. In other words, more suppression of the amygdala response (more negative value on horizontal axis) corresponded to a higher vPFC activation (R=−0.37, p = 0.08) and a stronger vPFC-amygdala structural connection (R=−0.64, p = 0.003). However, neither of these correlations were notices in the PCE group (R=0.06, p = 0.74; and R=−0.28, p=0.12) suggesting an impaired interaction between the vPFC and amygdala. To test the statistical significance of these group differences on correlation coefficients, an iterative permutation process was performed. Specifically, in 10000 repetitive iterations, the element orders in both of the correlation vectors were randomized and the corresponding correlation coefficients were calculated. With this randomization, the resultant correlation coefficients simply reflect by-chance correlations, thus the probabilistic null distribution of the correlation coefficients, as well as their group differences, were established empirically. Then the p value of the original group difference is determined by its position in this empirically established distribution. Using this method, the group difference of fMRI-fMRI correlation was significant (p=0.02), and the group difference of the fMRI-DTI correlation was marginally significant (p=0.06).
Figure 4.
Correlations of amygdala suppression with vPFC activation (top portion) and with vPFC-amygdala structural connectivity (bottom portion). Each dot in the plot represents data from one participant. CON: control, PCE: prenatal cocaine exposure.
4. Discussion
With multimodal MRI techniques, the present study examined PCE associated alteration of functional activations in the ventral prefrontal cortex as well as the alteration of its structural connections with amygdala. Based on the well-established model of vPFC to amygdala inhibition (Urry et al., 2006; Quirk and Beer, 2006; Banks et al., 2007), the present results augment our previous findings of PCE impact on amygdala activity (Li et al., 2009); and these observations, taken together, extend our understanding of the neurobiological mechanisms underlying PCE associated arousal dysregulation.
Previous behavioral and animal studies of PCE impact on neurodevelopment have suggested that, in prenatally exposed individuals, the mediation of arousal by prefrontal cortex may be affected because the threshold for activation in response to perceived stress or emotionally salient stimuli is altered by the exposure (see (Mayes, 2002) for review). As the amygdala is considered the critical region in generation and experience of stress and negative emotion (Aggleton, 1993; Phelps, 2006; Holzel et al., 2010), our previous finding of unsuppressed amygdala activation in the PCE adolescents (Li et al., 2009) may be a neural correlate of this altered threshold in response to stress or emotional arousal. However, the specific prefrontal mechanism involved in arousal regulation and how it would be affected by PCE are still unclear. The present data reveals part of this prefrontal mechanism by showing altered vPFC activation and its structural connection with amygdala in the exposed adolescents. When the cognitive demand increases requiring more suppression of the task-irrelevant emotional arousal, the control subjects increased their vPFC activation to suppress their emotional response in the amygdala while the PCE subjects did not. In addition, impact of PCE on this pathway of arousal regulation is manifested as reduced white matter integrity. The compromise of this inhibitory pathway is consistent with the model of neural “switch” for the long-term effects of PCE (Mayes, 2002). Namely, the brain keeps a balance between executive and automatic functions with a “switch” controlling the dominance of automatic responses in situations of danger and extreme stress. Under challenging, novel, or stressful conditions, the exposed children, as well as exposed animals (Johns et al., 1992; Romano and Harvey, 1998; Campbell et al., 2000), usually activate this switch too early. The balance is biased favoring the automatic function in exposed individuals as their frontal activations associated with suppression of automatic responses are impaired; and/or their white matter pathways associated with delivering of this suppressing signal are damaged.
Although the specific function of vPFC is assumed to be inhibition of the amygdala in the present study, functional alterations of vPFC may contribute to the PCE impact on arousal regulation in a more general level. The ROIs of vPFC in the present study covers Brodmann area 10, which is also termed anterior or rostral prefrontal cortex in previous studies (Ramnani and Owen, 2004; Burgess et al., 2007). This region is involved in coordination of information processing and transfers when multiple processes are engaged (Ramnani and Owen, 2004) and in coordinating attention between external stimuli and internal thoughts (Burgess et al., 2007). These functions are known to be compromised in general in PCE children (Mayes et al., 1998; Mayes, 2002). For multiple processes, the present study has two independent streams of information input, one being emotional and the other being cognitive. When the processing resource needs to be coordinated in favor of the cognitive stream, the PCE adolescents exhibit reduced capability in doing so. For external and internal attention, it is closely associated with the “default mode” brain network (Buckner et al., 2008) and the autonomic nervous system (Porges, 1992), which are both affected by PCE (Sheinkopf et al., 2007; Li et al., 2011). Functional (and probably structural as well) alteration in vPFC could be central to our understanding of our previous findings of PCE effect in the amygdala and in the “default mode” network.
The observed amygdala-vPFC dysfunction may also contribute to the relatively higher risk for sociobehavioral problems associated with PCE, including antisocial personality, aggression, or delinquency (Bendersky et al., 2006; Bennett et al., 2007; Minnes et al., 2010). These behavioral problems were previously reported to be associated with functional and structural aberrations of the frontal-limbic system, in which amygdala and vPFC both play a critical role (Blair, 2004; Siever, 2008; Vloet et al., 2008). Generally, vPFC is considered to be more involved in the modulation of reactive aggression while amygdala to be more involved in goal directed aggression (Blair, 2004). Exaggerated amygdala and diminished vPFC reactivity to angry faces as well as reduced functional connection between them were reported in individuals with impulsive aggression (Coccaro et al., 2007). In addition, individual differences in the structural integrity of vPFC-amygdala pathway can predict trait anxiety with higher pathway strength associated with lower anxiety (Kim and Whalen, 2009). These data show similarity to the present results observed in PCE adolescents. The corresponding dopaminergic frontal-limbic pathway may be disrupted in the fetal development due to cocaine’s blocking effect on reuptake of monoaminergic neurotransmitters (Mayes, 1999).
Similar to most of the human studies that examine the impact of PCE on neural development and behavior, the main limitation of the present study is that the reported PCE effect may actually represent the effect of a plethora of factors. Because women who use cocaine during pregnancy usually also use other drugs (e.g. alcohol, tobacco and marijuana), and the exposure is often associated with adverse environment in development, the exposed vs. non-exposed group difference may not only reflect the effect of PCE, but also the effect of complicated interactions between poly-drug exposure and socioenviornmental factors (Yumoto et al., 2008). While efforts were made in reducing the proportion of children with other prenatal drug exposure, in matching the exposed and the control groups (Coles et al., 1992; Brown et al., 1998), as well as in statistically controlling 7 confounding factors in the present study, this limitation cannot be completely eliminated. In fact, this limitation is made more acute in neuroimaging studies than in neurobehavioral studies because of the relatively smaller sample size necessitated by the expense and complexity of the study. However, neuroimaging studies offset this shortcoming by attributing behavioral outcomes to specific brain mechanisms in different regions or pathways (Smith et al., 2001; Warner et al., 2006; Avants et al., 2007; Rao et al., 2007; Hurt et al., 2008; Rivkin et al., 2008; Ackerman et al., 2010). As part of our “frombehavior-to-brain” research line on the same cohort of subjects (Coles et al., 1999; Bard et al., 2000; Brown et al., 2004; Kable et al., 2008; Li et al., 2009; Li et al., 2011), the present study extended our understanding of PCE effect on arousal regulation into alterations of the amygdala-vPFC neural network. Similar to the present findings, dopamine-rich frontal and sub cortical structures have been show to be vulnerable to prenatal drug exposure, suggesting that multiple drug exposure may share a profile of developmental neurotoxicity (Derauf et al., 2009). Continued follow-up of the exposed cohorts and the advancing of neuroimaging techniques will allow us to characterize this profile in multiple regions of the brain network and multiple time points of the developmental trajectory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For the remaining 3 adolescents, 1 had no specimen taken and 2 specimens were lost by labs
This figure is reproduced from our previous publication (Li et al., 2009) with permission obtained from the publisher
References
- Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends in Neurosciences. 1993;16:328–333. doi: 10.1016/0166-2236(93)90110-8. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Tsuruda JS, Parker DL. Elimination of eddy current artifacts in diffusion-weighted echo-planar images: the use of bipolar gradients. Magnetic Resonance in Medicine. 1997;38:1016–1021. doi: 10.1002/mrm.1910380623. [DOI] [PubMed] [Google Scholar]
- Avants B, Hurt H, Giannetta J, Epstein C, Shera D, Rao H, Wang J, Gee J. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatric Neurology. 2007;37:275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neuroscience and Biobehavioral Reviews. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, Hammond J, Wright LL, Higgins R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:e348–e359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicology and Teratology. 2001;23:545–559. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard KA, Coles CD, Platzman KA, Lynch ME. The effects of prenatal drug exposure, term status, and care giving on arousal and arousal modulation in 8-week-old infants. Developmental Psychobiology. 2000;36:194–212. [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR Imaging. Magnetic Resonance in Medicine. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bendersky M, Bennett D, Lewis N. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. Journal of Pediatric Psychology. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Developmental Psychology. 1998;34:555–564. [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. Journal of Developmental and Behavioral Pediatrics. 2007;28:467–472. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Bracht T, Tüscher O, Schnell S, Kreher B, Rüsch N, Glauche V, Lieb K, Ebert D, Il'yasov KA, Hennig J, Weiller C, Elst LTv, Saur D. Extraction of prefronto-amygdalar pathways by combining probability maps. Psychiatry Research: Neuroimaging. 2009;174:217–222. doi: 10.1016/j.pscychresns.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Brown JV, Bakeman R, Coles CD, Platzman KA, Lynch ME. Prenatal cocaine exposure: a comparison of 2-year-old children in parental and nonparental care. Child Development. 2004;75:1282–1295. doi: 10.1111/j.1467-8624.2004.00739.x. [DOI] [PubMed] [Google Scholar]
- Brown JV, Bakeman R, Coles CD, Sexson WR, Demi AS. Maternal drug use during pregnancy: are preterm and full-term infants affected differently? Developmental Psychology. 1998;34:540–554. doi: 10.1037//0012-1649.34.3.540. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relavance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences. 2007;11:290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Bliven TD, Silveri MM, Snyder KJ, Spear LP. Effects of prenatal cocaine on behavioral adaptation to chronic stress in adult rats. Neurotoxicology and Teratology. 2000;22:845–850. doi: 10.1016/s0892-0362(00)00104-5. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Fahy T, Sinha R, Mayes LC. Emotional arousal in cocaine exposed toddlers: prediction of behavior problems. Neurotoxicology and Teratology. 2009;31:275–282. doi: 10.1016/j.ntt.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepenik LG, Raffo M, Hampson M, Lacadie C, Wang F, Jones MM, Pittman B, Skudlarski P, Blumberg HP. Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Research: Neuroimaging. 2010;182:207–210. doi: 10.1016/j.pscychresns.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Weber B. Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. NeuroImage. 2008;39:1396–1407. doi: 10.1016/j.neuroimage.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Coles CD, Bard KA, Platzman KA, Lynch ME. Attentional response at eight weeks in prenatally drug-exposed and preterm infants. Neurotoxicology and Teratology. 1999;21:527–537. doi: 10.1016/s0892-0362(99)00023-9. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Smith I, James ME, Falek A. Effects of cocaine and alcohol use in pregnancy on neonatal growth and neurobehavioral status. Neurotoxicology and Teratology. 1992;14:23–33. doi: 10.1016/0892-0362(92)90025-6. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation - a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Developmental Psychobiology. 2006;42:688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derauf C, Kekatpure M, Neyzi N, Lester B, Kosofsky B. Neuroimaging of children following prenatal drug exposure. Seminars in Cell and Developmental Biology. 2009;20:441–451. doi: 10.1016/j.semcdb.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipietro JA, Suess PE, Wheeler JS, Smouse PH, Newlin DB. Reactivity and regulation in cocaine-exposed neonates. Infant Behavior and Development. 1995;18:407–414. [Google Scholar]
- Eiden RD, McAuliffe S, Kachadourian L, Coles C, Colder C, Schuetze P. Effects of prenatal cocaine exposure on infant reactivity and regulation. Neurotoxicology and Teratology. 2009a;31:60–68. doi: 10.1016/j.ntt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Veira Y, Granger DA. Prenatal cocaine exposure and infant cortisol reactivity. Child Development. 2009b;80:528–543. doi: 10.1111/j.1467-8624.2009.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW. Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt H, Betancourt LM, Malmud EK, Shera DM, Giannetta JM, Brodsky NL, Farah MJ. Children with and without gestational cocaine exposure: a neurocognitive systems analysis. Neurotoxicology and Teratology. 2009;31:334–341. doi: 10.1016/j.ntt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt H, Giannetta J, Korczykowski M, Hoang A, Tang K, Betancourt L, Brodsky N, Shera D, Farah M, Detre J. Functional magnetic resonance imaging and working memory in adolescents with gestational cocaine exposure. The Journal of Pediatrics. 2008;152:371–377. doi: 10.1016/j.jpeds.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Johns JM, Means MJ, Anderson DR, Means LW, McMillen BA. Prenatal exposure to cocaine II: effects on open-filed activity and cognitive behavior in Sprague-Dawley rats. Neurotoxicology and Teratology. 1992;14:343–349. doi: 10.1016/0892-0362(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Lynch ME, Platzman K. Physiological responses to social and cognitive challenges in 8-year-olds with a history of prenatal cocaine exposure. Developmental Psychobiology. 2008;50:251–265. doi: 10.1002/dev.20285. [DOI] [PubMed] [Google Scholar]
- Karmel BZ, Gardner JM. Prenatal cocaine exposure effects on arousal-modulated attention during the neonatal period. Developmental Psychobiology. 1996;29:463–480. doi: 10.1002/(SICI)1098-2302(199607)29:5<463::AID-DEV5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system: technical manual and affective ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention; 1997. [Google Scholar]
- Lester BM, LaGasse LL, Shankaran S, Bada HS, Bauer CR, Lin R, Das A, Higgins R. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. Journal of Pediatrics. 2010;157:288–295. doi: 10.1016/j.jpeds.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux J-M, Bourgouin P, Beauregard M. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Li Z, Coles CD, Lynch ME, Hamann S, Peltier S, LaConte S, Hu X. Prenatal cocaine exposure alters emotional arousal regulation and its effects on working memory. Neurotoxicology and Teratology. 2009;31:342–348. doi: 10.1016/j.ntt.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Lynch ME, Hamann S, Peltier S, Hu X. Increased "default mode" activity in adolescents prenatally exposed to cocaine. Human Brain Mapping. 2011;32:759–770. doi: 10.1002/hbm.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lzquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. European Journal of Neuroscience. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Development and Psychopathology. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicology and Teratology. 2002;24:385–395. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Grillon C, Granger R, Schottenfeld R. Regulation of arousal and attention in preschool children exposed to cocaine prenatally. Annals of the New York Academy of Sciences. 1998;846:126–143. [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O'Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, Queh D. The effects of prenatal cocaine exposure on problem behavior in children 4–10 years. Neurotoxicology and Teratology. 2010;32:443–451. doi: 10.1016/j.ntt.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Porges SW. Vagal tone: a physiologic marker of stress vulnerability. Pediatrics. 1992;90:498–504. [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Annals of the New York Academy of Sciences. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre JA, Hurt H. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120:1245–1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Rivkin MJ, Davis PE, Lemaster JL, Cabral HJ, Warfield SK, Mulkern RV, Robson CD, Rose-Jacobs R, Frank DA. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. 2008 Pediatrics. 2008:741–750. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano AG, Harvey JA. Prenatal cocaine exposure: long-term deficits in learning and motor performance. Annals of the New York Academy of Sciences. 1998;846:89–108. [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. Journal of Neuroscience. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45 doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local pearson correlation. NeuroImage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD. The association between maternal cocaine use during pregnancy and physiological regulation in 4- to 8-week-old infants: an examination of possible mediators and moderators. Journal of Pediatric Psychology. 2006;31:15–26. doi: 10.1093/jpepsy/jsj022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lagasse LL, Lester BM, Liu J, Seifer R, Bauer CR, Shankaran S, Bada H, Das A. Vagal tone as a resilience factor in children with prenatal cocaine exposure. Dev Psychopathol. 2007;19:649–673. doi: 10.1017/S0954579407000338. [DOI] [PubMed] [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. American Journal of Psychiatry. 2008;165:429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Gilbride K, Kuo J, Poland RE, Walot I, Ernst T. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107:227–231. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- Urry HL, Reekum CMv, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vloet TD, Konrad K, Huebner T, Herpertz S, Herpertz-Dahlmann B. Structural and functional MRI findings in children and adolescents with antisocial behavior. Behavioral Sciences and the Law. 2008;26:99–111. doi: 10.1002/bsl.794. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, Garvan CW, Schmalfuss IM, Blackband SJ. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118:2014–2024. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto C, Jacobson SW, Jacobson JL. Fetal substance exposure and cumulative environmental risk in an african american cohort. Child Development. 2008;79:1761–1776. doi: 10.1111/j.1467-8624.2008.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]