Abstract
Multiple sclerosis (MS) is an autoimmune, demyelinating disease and as such, the gold standard of treatment is to selectively suppress the pathogenic autoimmune response without compromising the entire arm of the adaptive immune response. One target of this strategy lying upstream of the pathologic adaptive immune response is the local, innate immune signaling that initiates and drives autoimmunity and sterile injury. High-mobility group box 1 protein (HMGB1) is a ubiquitous nuclear protein that when released from necrotic cells, such as damaged oligodendrocytes in MS lesions, drives pro-inflammatory responses. Here we demonstrate that HMGB1 drives neuroinflammatory responses in experimental autoimmune encephalomyelitis (EAE), a murine model for MS, and that inhibition of HMGB1 signaling ameliorates disease. Specifically i.v. injection of an HMGB1 neutralizing antibody in the C57BL/6 model of chronic EAE or SJL/J model of relapsing-remitting EAE ameliorated clinical disease prophylactically or during ongoing disease, blocked T cell infiltration of the central nervous system, and inhibited systemic CD4+ T cell responses to myelin epitopes. Additionally, lymphocytes from EAE mice restimulated in vitro in the presence of recombinant HMGB1 exhibited increased proliferation and pro-inflammatory cytokine production, an effect that was blocked by anti-HMGB1 antibody. Similarly recombinant HMGB1 promoted proliferation and pro-inflammatory cytokine production of human PBMCs stimulated in vitro, and anti-HMGB1 antibody blocked this effect. These findings indicate that HMGB1 contributes to neuroinflammatory responses that drive EAE pathogenesis and that HMGB1 blockade may be a novel means to selectively disrupt the pro-inflammatory loop that drives MS autoimmunity.
Keywords: HMGB1, innate immunity, neuroinflammation, autoimmune disease, EAE, tolerance
1. Introduction
Multiple sclerosis (MS2) in its various forms can be characterized as a chronic, inflammatory demyelinating disease of the central nervous system (CNS) driven by an aberrant host autoimmune attack. There are currently eight FDA-approved therapies initially focused on broad-based immunosuppression and numerous emerging therapies undertaking a more targeted immunomodulatory strategy such as preventing leukocyte migration into the CNS or inducing epitope-specific tolerance in encephalitogenic T cells [1,2]. All of these strategies though aim to modulate the pathogenic immune response downstream of the initial oligodendrocyte damage and innate immune signaling that sustains the local CNS inflammatory response. An avenue that is currently being explored for treatment of acute tissue injury and autoimmunity in other organs targets the intrinsic alarm signals or damage-associated molecular pattern molecules (DAMPs) driving the local inflammatory response [3,4]. This approach has the distinct advantage of targeting sterile immunity or autoimmunity without compromising host immune responses to pathogens.
High-mobility group box 1 protein (HMGB1) is a highly conserved protein found in the nucleus of almost all eukaryotic cells that, by loosely binding DNA, functions as a non-specific transcriptional regulator and nucleosome stabilizer [5,6]. More recently HMGB1 has been shown to play a secondary role as a cytokine implicated in the pathogenesis of sepsis and other inflammatory diseases [7-9]. HMGB1 can be passively released from necrotic cells or actively secreted by monocytes, tissue-resident macrophages and dendritic cells [10-12]. This dual role: contributing to nuclear homeostasis intracellularly and serving as an extracellular alarm for tissue damage, has positioned HMGB1 as a prototypical DAMP protein [13]. Acute tissue injury including cerebral ischemia is associated with release of HMGB1 and other innate immune signaling molecules [14,15], and HMGB1 has been shown to have pleiotropic downstream effects [16]. HMGB1 binds promiscuous receptors RAGE (receptor for advanced glycation end-products), TLR2, and TLR4 to promote cytokine production by inflammatory cells and facilitate T cell proliferation [17,18]. HMGB1 can also stimulate leukocyte recruitment via these receptors expressed on endothelial cells and has been shown to complex directly with CXCL12 to enhance migration through CXCR4 [19].
Little is known about the contribution of HMGB1 to the initiation of MS or its role in driving progressive autoimmune disease. HMGB1 is found in the nuclei of endogenous cells in the CNS [20], and extracellular HMGB1 as well as the receptors RAGE, TLR2, and TLR4 are upregulated in the cerebrospinal fluid (CSF) of MS patients [21]. Furthermore microglia and macrophages expressing cytosolic HMGB1 are increased in active lesions of MS patients as well as the associated animal model experimental autoimmune encephalomyelitis (EAE) [21]. The present study was designed to assess the contribution of HMGB1 to the initiation and progression of inflammatory processes in EAE and explore HMGB1 blockade as a novel therapeutic approach for EAE amelioration. We demonstrate increased systemic HMGB1 in EAE mice, and that an anti-HMGB1 antibody successfully ameliorates chronic or relapsing-remitting EAE when delivered either prophylactically or during ongoing disease. This amelioration is associated with defective systemic T cell activation and decreased T cell recruitment to the CNS. We further demonstrate that HMGB1 promotes proliferation and cytokine production of lymphocytes from EAE mice and human peripheral blood mononuclear cells (PBMCs) stimulated in vitro. These data indicate an important role for HMGB1 in initiating and directing ongoing neuroinflammation and may suggest a novel target for interfering with the inflammatory cascade that drives MS pathology.
2. Materials and Methods
2.1 Mice
SJL/J and C57BL/6 mice were purchased from Harlan Laboratories (Bethesda, MD). The mice were housed under specific pathogen-free conditions in the Northwestern University Center for Comparative Medicine Barrier Facility (Chicago, IL). All protocols were approved by the Northwestern University Animal Care and Use Committee.
2.2 Peptides
All synthetic peptides were obtained from Genemed Synthesis (San Francisco, CA) including MBP84-104 (VHFFKNIVTPRTPPPSQGKGR, >95.09% purity), MOG35–55 (MEVGWYRSPFSRVVHLYRNGK, >98.16%), OVA323–339 (ISQAVHAAHAEINEAGR, >98.69%), PLP139–151 (HSLGKWLGHPDKF, >97.1%), and PLP178–191 (NTWTTCQSIAFPSK, >96.77%).
2.3 Induction of experimental autoimmune encephalomyelitis
Mice were immunized with peptides in adjuvant as previously reported [22,23]. Briefly, for chronic EAE (C-EAE), C57BL/6 mice were subcutaneously injected with 100 μl of an emulsion of complete Freund's adjuvant (CFA) containing 200 μg Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) and 200 μg MOG35–55 or OVA323–339 distributed in three spots on the flank. Mice also received 200 ng pertussis toxin in 200 μl PBS (List Biological Laboratories, Campbell, CA) i.p. on the day of priming and two days later. For relapsing-remitting EAE (R-EAE), SJL/J mice were injected with CFA emulsion containing 50 μg PLP139–151 or OVA323–339 as the priming peptide and no pertussis toxin. Initial disease symptoms were usually observed between 10 and 15 days post-immunization. Mice were monitored daily for clinical symptoms of EAE after disease onset, and paralyzed animals were provided easier access to food and water. Mice were scored on a scale of 0–5 as follows: 0, no abnormality; 1, limp tail or hind limb weakness; 2, limp tail and hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; and 5, moribund. Results are plotted as the mean daily clinical score by experimental group ± S.D.
2.4 HMGB1 ELISA
C-EAE mice were sacrificed at onset or peak of disease and blood was collected by cardiac puncture into Microtainer serum separation tubes (BD, Franklin Lakes, NJ). Blood samples were centrifuged for 15 minutes at 5000g, and serum was transferred to new tubes for storage at -80°. For HMGB1 quantification serum samples were thawed, and HMGB1 was assayed by ELISA (IBL International, Hamburg, Germany) according to the manufacturer's instructions. Results are expressed as means ± S.D. of treatment groups or animals with equivalent disease scores.
2.5 HMGB1 neutralization in vivo
In C-EAE mice 100 μg of anti-HMGB1 antibody (clone 3B1, IgG2a, purified by affinity chromatography on a Protein G column; OncoImmune, Ann Arbor, MI) or isotype control antibody in 100 μl sterile PBS was injected i.v. prophylactically (7 days post-immunization (p.i.)) or after established clinical disease (14 days p.i.). In R-EAE mice, 100 μg of anti-HMGB1 antibody, selected as a result of a dose-response study (Supplementary Fig. 1), or isotype control in 100 μl PBS was injected i.v. prophylactically (7 days p.i.) or at primary disease remission (~19 days p.i.).
2.6 Immunohistochemistry
Primary antibodies used for immunohistochemistry on CNS sections included rabbit polyclonal anti-HMGB1 [DyLight 549] (1:100; Novus Biologicals, Littleton, CO), mouse anti-PLP (1:200; AbD Serotec, Raleigh, NC), rat anti-CD45 (1:100; Millipore, Billerica, MA), rat anti-TLR2 [biotinylated] (1:50; eBioscience, San Diego, CA), rat anti-TLR4 [biotinylated] (1:50; eBioscience), and rat anti-RAGE (10 μg/ml; R&D Systems, Minneapolis, MN). Isotypes used were mouse IgG2a, rat IgG, and rat IgG2b (eBioscience). Secondary antibodies used included FITC-conjugated anti-mouse (1:200; Jackson ImmunoResearch, West Grove, PA), Cy3-conjugated anti-rat (1:300, Jackson ImmunoResearch), and Cy3-conjugated streptavidin (Life Technologies, Grand Island, NY). Mice were anesthetized with 50 mg/kg Nembutal, transcardially perfused with 30 ml PBS followed by 30 ml 4% paraformaldehyde in PBS. Brains and spinal cords were dissected, fixed overnight in 4% paraformaldehyde, then cryoprotected in 30% sucrose for 48 h. Tissue was embedded in OCT (Miles Laboratories) and frozen on dry ice. Eight μm-thick coronal sections were cut on a Leica CM1850 cryostat (Leica Microsystems, Richmond, VA), mounted on Superfrost Plus electrostatically charged slides (Fisher Scientific, Pittsburgh, PA), and stored at -80°. For staining sections were thawed and blocked with 5% normal donkey serum, 0.1% Triton X-100 in PBS for 1 h at room temperature. Sections were then stained with primary antibodies overnight at 4°. Sections were washed in PBS and incubated in secondary antibodies for 1 h at room temperature. Sections were then washed and coverslipped with Vectashield Hard Set mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Slides were examined and images acquired using a Leica DM5000B epifluorescent microscope with Image-Pro software (Media Cybernetics, Bethesda, MD). At least 6 sections (1:8 series) per animal per group were examined at ×10 and ×20 magnification.
2.7 Isolation of CNS-infiltrating and resident mononuclear cells
Mice were anesthetized with 50 mg/kg Nembutal and transcardially perfused with 30 ml PBS. Brains and spinal cords were dissected, minced with scissors, and digested with 1ml Accutase (Millipore) each for 30 min at 37°. 2 ml 10% FBS in HBSS was added to each sample, and tissue was manually triturated with a transfer pipette. Samples were transferred to 100 μm cell strainers (BD) and pushed through using syringe plungers. The cell strainers were thoroughly rinsed with 10% FBS in HBSS and cell suspensions were centrifuged. To purify cells from myelin debris, cells were resuspended in 40% Percoll (Amersham, Piscataway, NJ) and centrifuged at 650 × g without brake for 25 min at room temperature. The myelin top layer was aspirated and mononuclear cells were resuspended in flow cytometry buffer (2% FBS, 2 mM EDTA in PBS).
2.8 Flow cytometric analysis
Cells were counted using a Coulter Counter (Beckman Coulter, Brea, CA) and 106 cells per sample were stained for analysis as previously described [22]. Briefly, Fc receptors were blocked using anti-mouse CD16/32 (0.25 μg; eBioscience). Live cells were labeled with Calcein Violet AM (Life Technologies). Cells were then stained with anti-CD45 (V500, 0.2 μg), anti-mouse CD4 (FITC, 0.2 μg), and anti-mouse CD11b (PerCP-Cy5.5, 0.2 μg) from BD Biosciences; anti-mouse CD3 (APC-eFluor780, 0.2 μg), anti-mouse CD11c (PE, 0.2 μg), anti-mouse CD80 (APC, 0.5 μg), and anti-mouse CD44 (PE-Cy7, 0.2 μg) from eBioscience; or respective isotype controls for 1 h at 4°. For oligodendrocyte analysis anti-galactocerebroside (GalC; Millipore) and anti-O1 (R&D Systems) antibodies were conjugated with PE and APC-Cy7, respectively, using Lightning-Link Antibody Labeling Kits (Novus Biologicals). Cell suspensions were stained with 1 μg anti-GalC and 0.5 μg anti-O1 for 1 h at 4°. Samples were run on a Canto II flow cytometer with FACS DIVA software (BD Biosciences) and analyzed using FlowJo software (Treestar FlowJo, Ashland, OR). Results are plotted by individual animal with means ± S.D. reflecting treatment groups. Back-calculated total cell numbers are indicated on graphs above data points.
2.9 Delayed type hypersensitivity
Delayed type hypersensitivity (DTH) was performed via ear challenge. For each mouse baseline thickness of both ears was measured using a Mitutoyo model 7326 μm (Schlesinger's Tools, Brooklyn, NY). Ears were then subcutaneously injected with 10 μg of either PLP139-151, PLP178-191, or MBP86-104 peptides in 10 μl PBS using a 100 μl Hamilton syringe fitted with a 30-gauge needle. 24 h after injection of peptide, ear thickness was re-measured and mean ear swelling was determined by subtracting baseline ear thickness and normalizing to measurements from age-matched naïve mice. Results are plotted by individual animal with means ± S.D. reflecting treatment groups.
2.10 Lymphocyte restimulation in vitro
R-EAE mice were sacrificed 10 days after immunization with PLP139-151 and draining inguinal lymph nodes were harvested. Lymphocytes were isolated using a 100 μm cell strainer. Cells were counted and plated in 96 well flat-bottomed microtiter plates at a density of 106 cells per well in a total volume of 200 μl HL-1 medium (BioWhittaker; 1% penicillin/streptomycin, 1% glutamine). Cells were cultured at 37 ° and stimulated with 20 μg/ml PLP139–151 or irrelevant peptide. 0.1, 1, or 10 μg/ml recombinant mouse (r)HMGB1 (eBioscience) was added to wells in the presence or absence of 10 or 100 μg/ml anti-HMGB1 (OncoImmune). At 72 h 50 μl of medium was transferred to a new plate and frozen for later cytokine analysis. Wells were pulsed with 1 μCi/well [3H]TdR and cultured for an additional 24 h. Plates were then harvested and uptake was detected using a TopCount-NXT microplate scintillation counter (Packard). For cytokine quantification, media samples were diluted 1:3 and interrogated by multiplex cytokine assays (Millipore) for IFN-γ, IL-10, IL-17, GM-CSF and TNFα production according to manufacturer's instructions. Results from proliferation and cytokine production assays are expressed as means ± S.D. of triplicate wells.
2.11 Flow cytometric analysis of cultured lymphocytes
Lymphocytes were isolated from R-EAE mice and stimulated in the presence or absence of rHMGB1 and anti-HMGB1 antibody as detailed in 2.10. After 96 h in culture, cells were lifted, stained, and assayed by flow cytometry as detailed in 2.8. Antibodies used for staining included CD11b (APC-Cy7, 0.2 μg) from BD Biosciences; and CD4 (PE-Cy5, 0.2 μg), CD8 (PerCP, 0.2 μg), CD11c (PE-Cy7, 0.2 μg), CD40 (PE, 0.2 μg), CD80 (APC, 0.5 μg), and PD-1 (PE, 0.2 μg) from eBioscience. Results are expressed as means ± S.D. of triplicate wells.
2.11 Human PBMC culture and analysis
Buffy coat isolates from human blood were purchased from LifeSource (Chicago, IL). Samples were diluted 1:1 with sterile PBS, layered on 15ml Lymphocyte Separation Medium (Cellgro, Manassas, VA) in a 50 ml conical tube, and centrifuged at 400 × g without brake for 30 min at room temperature. The plasma top layer was aspirated, and the lymphocyte-containing interface was transferred to a new tube. Cells were washed twice with sterile PBS, counted, and plated in 96 well U-bottomed microtiter plates at a density of 105 cells per well in a total volume of 200 μl X-VIVO 20 medium (Lonza, Allendale, NJ). Cells were stimulated with 0.5 μg/ml anti-human CD3 (eBioscience) and cultured at 37 °. 100 or 500 ng/ml human rHMGB1 (R&D Systems) was added to wells in the presence or absence of 10 or 100 μg/ml anti-HMGB1 (OncoImmune). At 48 h 50 μl of medium was transferred to a new plate and frozen for later cytokine analysis. Wells were pulsed with 1 μCi/well [3H]TdR and cultured for an additional 24 h. Plates were then harvested and counted. For cytokine quantification, media samples were diluted 1:3 and interrogated by multiplex cytokine assays (Millipore) for IFN-γ production according to manufacturer's instructions. Results from proliferation and cytokine production assays are expressed as means ± S.D. of triplicate wells.
2.12 Statistical analyses
Comparisons of ELISA results and DTH responses between groups of mice were analyzed by Student's two-tailed t test. Flow cytometric quantification between groups of mice were analyzed by one-way ANOVA or Student's two-tailed t test. In vitro proliferation, cytokine production, and flow cytometric quantification results between groups were compared by one-way ANOVA. Disease courses in mice were compared by two-way ANOVA followed by Tukey's post hoc tests. p values of < 0.05 were considered significant.
3. Results
3.1 Serum HMGB1 levels are elevated in EAE
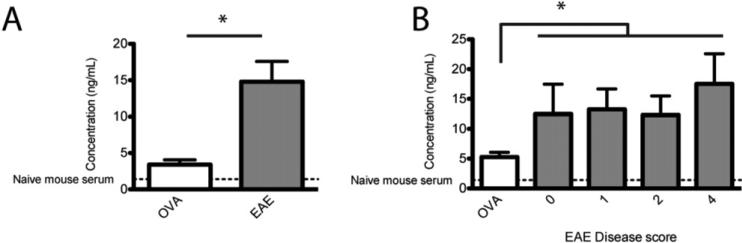
HMGB1, released from necrotic cells or secreted by activated macrophages in response to injury or inflammation, is now recognized as a classic DAMP molecule. HMGB1 has been shown to exacerbate autoimmune diabetes and neutralization via anti-HMGB1 antibody is effective in inhibiting disease progression [24]. While transcripts for HMGB1 and the known receptors RAGE, TLR2, and TLR4 have been shown to be upregulated in CSF from MS patients [21], no one has examined HMGB1 as a potential systemic biomarker in EAE or as a potential therapeutic target. We first asked whether HMGB1 could be used as a serum biomarker for EAE clinical disease. C57BL/6 mice immunized with MOG35-55/CFA were sacrificed at onset or peak of clinical disease, and serum HMGB1 was quantified by ELISA. Compared to OVA323-339/CFA-primed control mice, C-EAE mice had significantly elevated levels of HMGB1 in serum (Fig. 1A, p < 0.05). OVA-primed as well as C-EAE mice had significantly higher levels of serum HMGB1 compared to naïve mice suggesting that immunization with the CFA emulsion itself partly contributed to the effect, but mice with active C-EAE had significantly higher serum concentrations. We next asked whether HMGB1 in peripheral blood correlated with clinical disease score and could thus be used as a biomarker of disease progression. Again we observed higher serum concentrations of HMGB1 in C-EAE compared to OVA-primed control mice (p < 0.05), but there was no significant difference between mice with different disease scores (Fig. 1B). These data indicate that HMGB1 levels in serum increase with the onset of C-EAE but that levels do not reflect clinical disease scores throughout subsequent disease progression.
Figure 1. Serum HMGB1 is elevated during EAE.
C57BL/6 mice, 6-8 wks of age, were immunized with 200 μg MOG35-55 (experimental) or OVA323–339 (control) emulsified in CFA and scored daily for EAE clinical disease. Mice were sacrificed at onset or peak of clinical symptoms, and blood was collected via cardiac puncture. Serum levels of HMGB1 were determined by ELISA on pooled samples from mice at peak disease (A) and individual animals grouped by clinical disease score (B). Data represent ≥ 5 mice/group from three separate experiments. Asterisks denote significance (p < 0.05).
3.2 HMGB1 contributes to EAE pathogenesis
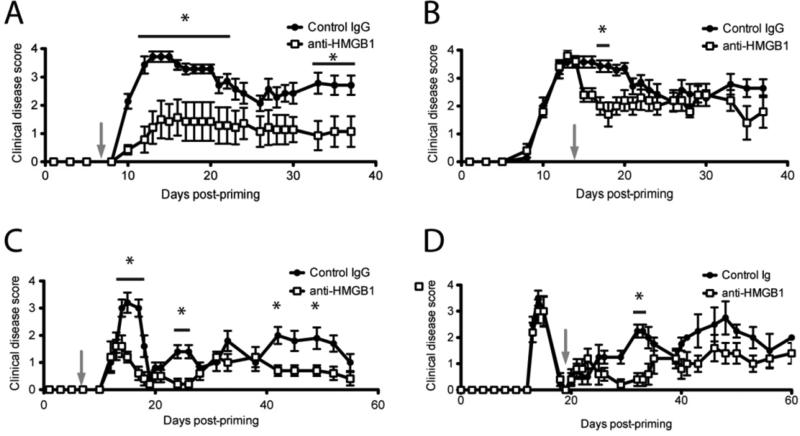
Since extracellular HMGB1 acts as a mediator of inflammation and, as we have shown, is elevated systemically during EAE, we hypothesized that disrupting HMGB1 function may inhibit development of or ameliorate progression of EAE clinical disease. We first investigated multiple doses of anti-HMGB1 neutralizing antibody delivered i.v. for any effect on EAE clinical disease. R-EAE was induced in SJL/J mice with PLP139-151/CFA, and 10, 50, 100, or 200 μg anti-HMGB1 was administered i.v. seven days after induction. 50 and 100 μg of anti-HMGB1 significantly ameliorated clinical disease with 100 μg producing maximum improvement (Suppl. Fig. 1). We thus used 100 μg anti-HMGB1 per mouse for all further studies. This antibody dose was shown to neutralize serum HMGB1 in vivo by ELISA of isolated serum (data not shown). To examine effects in the chronic model of EAE, we induced C-EAE in C57BL/6 mice then injected 100 μg anti-HMGB1 or isotype control antibody i.v. to functionally block HMGB1 activity and monitored mice for clinical disease. anti-HMGB1 administered prophylactically to C-EAE (7 days p.i.) significantly blocked disease induction (Fig. 2A, p < 0.05). This effect was maintained upwards of 35 days after C-EAE induction. To test the effectiveness of anti-HMGB1 in blocking ongoing C-EAE disease, we administered 100 μg anti-HMGB1 or control antibody 14 days p.i. when mice were already showing severe symptoms of C-EAE. Here HMGB1 neutralization with a single injection of mAb again significantly ameliorated clinical disease progression (Fig. 2B, p < 0.05). Unlike prophylactic treatment though, the effect was transient and anti-HMGB1-treated mice were indistinguishable from control mice beyond 20 days p.i. (7 days post treatment). We next investigated a role for HMGB1 in R-EAE that mimics the relapsing-remitting disease course commonly manifested in a subset of MS patients. Clinical disease remission and relapses are typical and predictable in this model. R-EAE was induced in SJL/J mice with PLP139-151/CFA, and prophylactic injection of 100 μg anti-HMGB1 (7 days p.i.) significantly improved clinical disease compared to control-treated animals, an effect that was maintained for upwards of 40 days post-immunization (Fig. 2C, p < 0.05). To test effectiveness in ongoing R-EAE, 100 μg anti-HMGB1 or control antibody was administered at the first clinical disease remission (~19 days post-immunization). Anti-HMGB1 prevented the first disease relapse (p < 0.05), but mice proceeded to relapse thereafter (Fig. 2D). Taken together these results suggest that HMGB1 is a critical mediator of C-EAE and R-EAE but not the sole factor driving disease pathogenesis. Further we can ameliorate EAE persistently by blocking HMGB1 activity with a single dose of neutralizing antibody administered prophylactically or acutely during ongoing disease.
Figure 2. HMGB1 contributes to EAE pathogenesis.
For C-EAE, C57BL/6 mice, 6-8 wks of age, were immunized with 200 μg MOG35–55/CFA s.c., received 200 ng pertussis toxin i.p. on days 0 and 2, and scored daily for clinical disease using a standard five-point scale. C-EAE mice were injected i.v. with 100 μg anti-HMGB1 neutralizing antibody or isotype control antibody in PBS 7 days (A) or 14 days (B) after disease induction (grey arrows). For R-EAE, SJL/J mice, 6-8 wks of age, were immunized with 50 μg PLP139-151/CFA s.c. and scored daily for clinical disease using a standard five-point scale. R-EAE mice were injected i.v. with 100 μg anti-HMGB1 neutralizing antibody or isotype control antibody 7 days after disease induction (C) or at disease remission, 19 days after induction (D) (grey arrows). Data are representative of two experiments of ≥ 5 mice per group. Main effects of treatment were tested for by two-way ANOVA. Asterisks denote a significant difference in mean clinical score for the indicated days by Tukey's post hoc tests (p < 0.05).
3.3 HMGB1 neutralization prevents CD4+ T cell infiltration and pathology in the CNS and inhibits systemic T cell responses
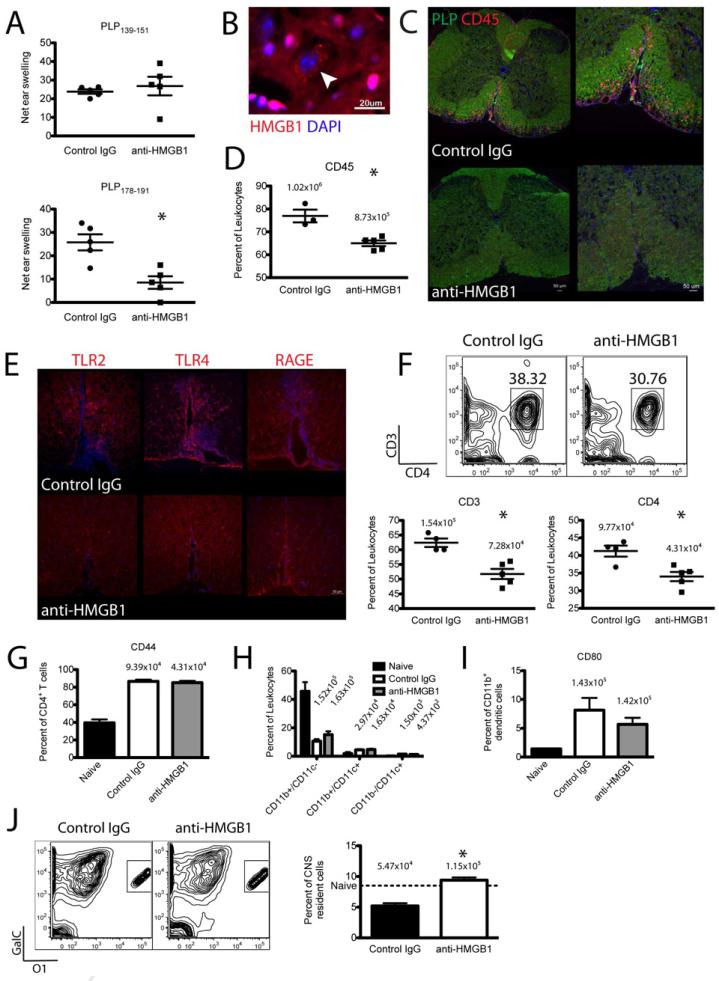
Since HMGB1 neutralization inhibited EAE clinical disease, we next asked whether antibody injection directly altered immune responses in EAE mice. We therefore induced R-EAE in SJL/J mice and at primary disease remission injected 100 μg anti-HMGB1 i.v. Seven days later when control-treated mice had relapsed we examined immune cell infiltration and activation in the CNS and systemic CD4+ T cell responses. First, to confirm HMGB1 expression in the CNS, we stained mouse brain sections for HMGB1 and observed the protein in most cells of the CNS (Fig. 3B). HMGB1 was primarily localized to endogenous cell nuclei with a few sparse cells expressing HMGB1 in the cytoplasm (arrowhead), and we observed no HMGB1 in extracellular spaces. This supported a primary role for HMGB1 in the CNS in maintaining nuclear homeostasis in resident cells. Next we looked for local effects of HMGB1 neutralization in the CNS of EAE mice. During clinical disease R-EAE mice typically show mass inflammatory cell infiltration in the CNS (red CD45 staining), most notably the white matter of the ventral spinal cord indicated by robust green proteolipid protein (PLP) staining (Fig. 3C-upper panel). R-EAE mice injected with anti-HMGB1 at primary remission showed dramatically less CD45+ hematopoietic cell infiltration in the ventral spinal cord compared to control-treated mice seven days later (Fig. 3C-lower panel). This was quantified by flow cytometry in repeat experiments showing significantly fewer CD45+ cells in the CNS of anti-HMGB1 treated mice (Fig. 3D, p < 0.05). Investigating the HMGB1 receptors in the CNS of R-EAE mice, TLR2, TLR4, and RAGE were expressed in the spinal cord but were primarily localized to perivascular sites of EAE lesions (Fig. 3E-upper panel). Mice injected with anti-HMGB1 at disease remission showed dramatically less TLR2 and TLR4 expression than control-treated mice but comparable RAGE staining (Fig. 3E-lower panel). We next sought to further characterize infiltrating immune cells by flow cytometry and observed significantly less CD3+ and CD4+ T cells in the CNS of anti-HMGB1 treated mice compared to controls (Fig. 3F, p < 0.05). There was no defect in activation of infiltrating CD4+ T cells as the percentage expressing the memory T cell marker CD44+ was equivalent between anti-HMGB1 and control-treated mice (Fig. 3G). HMGB1 receptors TLR2, TLR4, and RAGE are known to be expressed by infiltrating antigen presenting cells (APCs), so we also examined these populations following HMGB1 neutralization. There were no differences in the numbers of CD11b+/CD11c- macrophages, CD11b-/CD11c+ myeloid dendritic cells, or CD11b+/CD11c+ plasmacytoid dendritic cells between anti-HMGB1 and control-treated mice (Fig. 3H). Additionally there was no difference in costimulatory molecule CD80 expression by CD11b+ macrophages/microglia (Fig. 3I). These results suggested HMGB1 neutralization specifically inhibits CD4+ T cell infiltration in the CNS of EAE mice and TLR2/TLR4 upregulation in EAE lesions but gives no indication if anti-HMGB1 treatment alters immune responses systemically. To investigate this we used DTH assays to assess CD4+ T cells responses to myelin epitopes in vivo at the primary disease relapse. Anti-HMGB1 treatment did not affect systemic responses to the priming epitope PLP139-151, but significantly impaired responses to spread epitope, PLP178-191, responses that only develop during the primary relapse (Fig. 3A, p < 0.05). This suggested that HMGB1 neutralization has no effect on established CD4+ T cell responses but that impaired T cell infiltration and/or expansion in the CNS prevented the spread of T cell responses to endogenous myelin epitopes. Finally we wanted to assess the functional outcome of this impaired T cell infiltration and epitope spreading by quantifying mature oligodendrocytes in the CNS. R-EAE mice treated with control antibody at remission demonstrated a significant loss of GalC+/O1+ oligodendrocytes at disease relapse, whereas anti-HMGB1 treatment significantly protected against oligodendrocyte loss (Fig. 3J, p < 0.05). Taken together these results suggest that HMGB1 neutralization ameliorates EAE by inhibiting CD4+ T cell infiltration, TLR2/TLR4 upregulation in lesions, epitope spreading to novel myelin peptides, and secondarily oligodendrocyte loss in the CNS.
Figure 3. HMGB1 neutralization inhibits T cell infiltration and activation early after treatment.
R-EAE was induced in SJL/J mice by immunization with 50 μg PLP139-151/CFA s.c. and 100 μg anti-HMGB1 neutralizing antibody or isotype control antibody was injected i.v. at disease remission (day 19 p.i.). PLP139-151- and PLP178-191-specific CD4+ T cell responses were assessed by DTH assays five days later (A). Spinal cords were harvested for immunohistochemical or flow cytometric analysis seven days after antibody injection (26 days p.i.). For IHC, 8 μm-thick coronal sections were cut from fixed tissue, mounted onto slides, and stained for HMGB1 with DAPI counterstaining (B); PLP and CD45 (C); and TLR2, TLR4, and RAGE (E). Fluorescent images were acquired at ×10 (C-left panels) and ×20 magnification (B,C-right panels, E). HMGB1 was found in a majority of cells in the CNS localized to the nucleus with a few cells expressing cytoplasmic HMGB1 (B, arrowhead). Anti-HMGB1-treated mice showed less CD45+ immune cell infiltration (C) and less TLR2 and TLR4 expression (E) in the PLP-rich white matter of the ventral spinal cord than control-treated mice. For flow cytometry, spinal cords were dissociated, single cells were purified, and cells were stained for CD45 to distinguish resident CNS cells from immune infiltrates (D). T cells were stained for CD3, CD4, and CD44 expression (F,G), APCs were stained for CD11b, CD11c, and CD80 expression (H,I), and mature oligodendrocytes were stained for GalC and O1 expression (J). Data are representative of two experiments of ≥ 5 mice per group. Back-calculated absolute cell numbers are indicated in plots above data points. Asterisks denote a significant difference in DTH responses or flow cytometric quantification between treatment groups by Student's t-tests (p < 0.05).
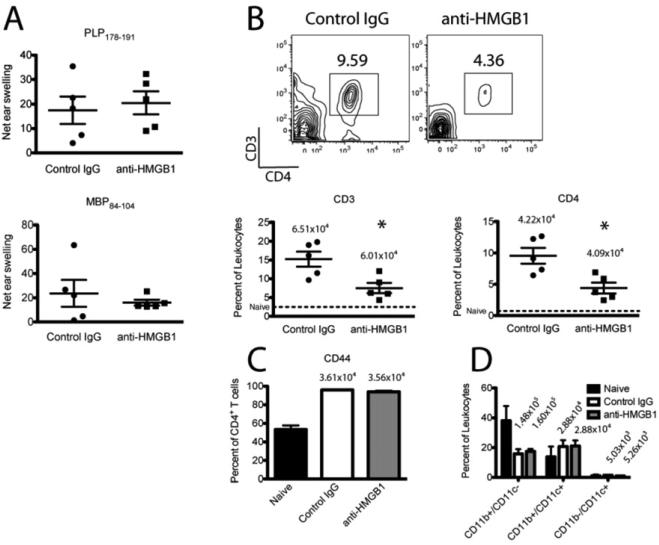
Following our observation that HMGB1 neutralization during established disease only ameliorated C-EAE and R-EAE clinical disease scores acutely, we next asked whether the immune modulatory effects were also short-lived. To test this R-EAE mice were injected with 100 μg anti-HMGB1 at primary remission, and immune infiltration and systemic T cell responses were assessed during the third relapse (~78 days post-immunization). Anti-HMGB1 treated mice showed significantly less CD3+ and CD4+ T cells in the CNS compared to control-treated mice (Fig. 4B, p < 0.05). There was no difference in the percentage of CD4+ T cells expressing CD44 between anti-HMGB1 and control treated mice (Fig. 4C). Examining APC populations we found no significant difference in the percentages of CD11b+/CD11c- macrophages, CD11b-/CD11c+ myeloid dendritic cells, or CD11b+/CD11c+ plasmacytoid dendritic cells between anti-HMGB1 and control treated mice (Fig. 4D). Furthermore there was no difference in the percentage of CD11b+ macrophages expressing the costimulatory molecule CD80 (data not shown). Finally we used DTH assays to examine systemic CD4+ T cell responses to PLP178-191 and MBP84-104, two myelin epitopes against which T cell responses develop only after primary remission. There was no difference in T cell responses to PLP178-191 or MBP84-104 between anti-HMGB1 and control-treated mice (Fig. 4A). These results suggest anti-HMGB1 transiently ameliorates established clinical disease but that suppression of T cell infiltration in the CNS of EAE mice is long-lasting. Despite the sustained interference with T cell trafficking, disease pathology progresses as there are no significant defects in local T cell activation, APC proliferation or activation, or epitope spreading.
Figure 4. HMGB1 neutralization results in sustained inhibition of CNS T cell infiltration/expansion.
R-EAE was induced in SJL/J mice by immunization with 50 μg PLP139-151/CFA s.c., and 100 μg anti-HMGB1 neutralizing antibody or isotype control antibody was injected i.v. at disease remission (day 19 p.i.). PLP178-191- and MBP84-104-specific CD4+ T cell responses were assessed by DTH assays 75 days p.i. (A). Spinal cords were harvested for flow cytometric analysis at the third relapse (78 days p.i.), dissociated, and single cells were purified. T cells were stained for CD3, CD4, and CD44 expression (B,C), and APCs were stained for CD11b and CD11c expression (D). Data are representative of two experiments of ≥ 5 mice per group. Asterisks denote a significant difference in DTH responses or flow cytometric quantification between treatment groups by Student's t-tests (p < 0.05).
3.4 HMGB1 promotes EAE lymphocyte restimulation in vitro
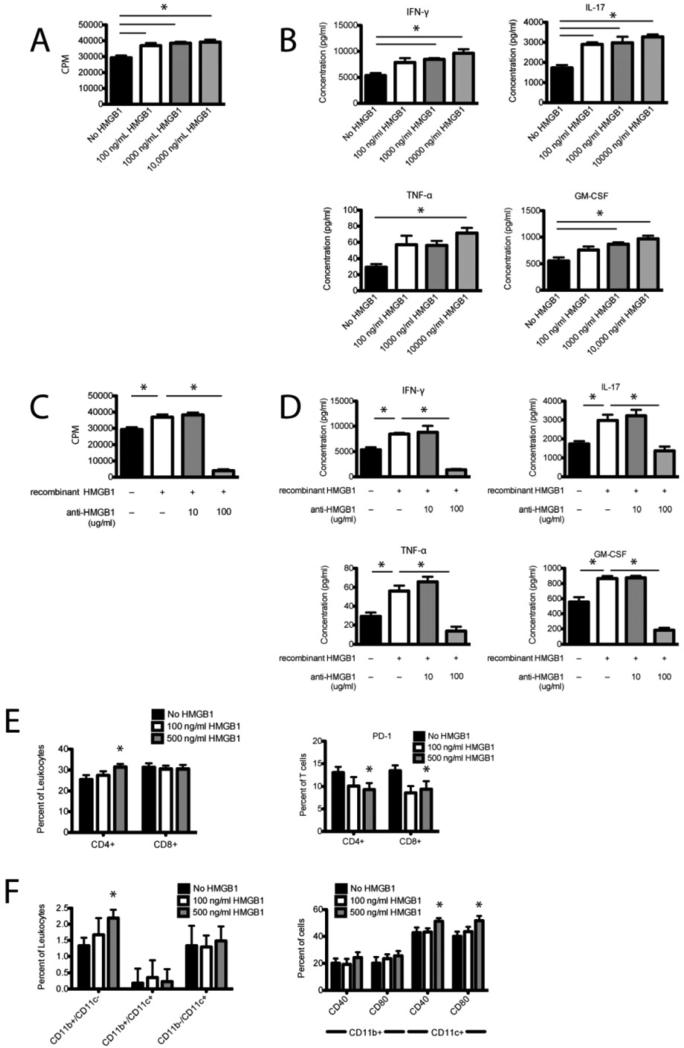
We next undertook a series of in vitro studies to further investigate the role of HMGB1 in inflammatory cellular responses. Lymphocytes were isolated from the draining inguinal lymph nodes of PLP139-151/CFA-primed R-EAE mice 10 days after immunization and restimulated in vitro with PLP139-151 in the presence or absence of increasing concentrations of rHMGB1 and/or anti-HMGB1 antibody. rHMGB1 significantly enhanced lymphocyte proliferation in response to the myelin epitope in a dose-dependent manner (Fig. 5A, p < 0.05) without inducing apoptosis (data not shown). Production of pro-inflammatory TH1 cytokines (IFN-γ and TNF-α) and TH17 cytokines (IL-17 and GM-CSF) were also significantly increased in the presence of rHMGB1 in a dose-dependent manner (Fig. 4B, p < 0.05). rHMGB1 had no effect on production of the TH2 cytokine IL-10 suggesting HMGB1 signaling specifically enhances TH1/TH17 cell function (data not shown). We repeated restimulation assays in the presence or absence of anti-HMGB1 at concentrations previously reported to inhibit pro-inflammatory cytokine production without inducing cytotoxicity [9]. We found that 100 g/ml anti-HMGB1 significantly inhibited the increase in lymphocyte proliferation (Fig. 4C, p < 0.05) and production of pro-inflammatory cytokines IFN-γ, TNF-α, IL-17, and GM-CSF (Fig. 5D, p < 0.05). We further characterized the cellular effects of rHMGB1 using flow cytometric analysis of lymphocytes isolated from R-EAE mice cultured with PLP139-151 peptide in the presence of increasing concentrations of rHMGB1. We observed an increase in the percentage of CD4+ T cells in culture but no change in the percentage of CD8+ T cells with addition of 500 ng/ml rHMGB1 (Fig. 5E, p < 0.05). Additionally, CD4+ and CD8+ T cells both showed decreased expression of the T cell regulatory marker PD-1 (Fig. 5E, p < 0.05), suggesting HMGB1 stimulated CD4+ T cell expansion and decreased TCR inhibition. Examining APC populations, rHMGB1 increased the percentage of CD11b+/CD11c- macrophages in cultures but had no effect on the percentage of CD11b-/CD11c+ myeloid dendritic cells or CD11b+/CD11c+ plasmacytoid dendritic cells (Fig. 5F, p < 0.05). Moreover rHMGB1 enhanced APC function of CD11b-/CD11c+ myeloid dendritic cells demonstrated by increased expression of costimulatory molecules CD40 and CD80 (Fig. 5F, p < 0.05). Taken together these data suggest rHMGB1 promotes EAE lymphocyte expansion and function specifically by stimulating CD4+ T cell and macrophage proliferation, promoting dendritic cell activation, and decreasing T cell regulation.
Figure 5. HMGB1 enhances EAE lymphocyte re-stimulation in vitro.
Inguinal draining lymph nodes were harvested from R-EAE mice 10 days p.i. and dissociated. Lymphocytes were re-stimulated in vitro with the priming peptide, PLP 139-151, for 72 h, and proliferation was assayed by cellular incorporation of [3H]TdR (A,C), cytokine production in the medium by multiplex cytokine assay (B,D), and activation of cells by flow cytometry (E,F). Lymphocyte proliferation (A) and production of TH1/TH17 cytokines: IFN-γ, TNF-α, IL-17, and GM-CSF (B) was determined in the presence of increasing concentrations of recombinant HMGB1. To verify the specificity of HMGB1 in stimulating lymphocyte function, 10 or 100 μg/ml anti-HMGB1 antibody was added to replicate cultures and proliferation (C) and IFN-γ, TNF-α, IL-17, and GM-CSF cytokine production (D) were assessed. For flow cytometric analysis of lymphocyte function, T cells were stained with phenotypic markers CD4 and CD8, and regulatory marker PD-1 (E). APCs were stained with phenotypic markers CD11b and CD11c, and functional markers: CD40 and CD80 (F). Data are representative of three experiments. Asterisks denote a significant difference in proliferative responses, cytokine production in the medium, and flow cytometric quantification by one-way ANOVA followed by Tukey's post hoc tests (p < 0.05).
3.5 HMGB1 promotes human PBMC stimulation in vitro
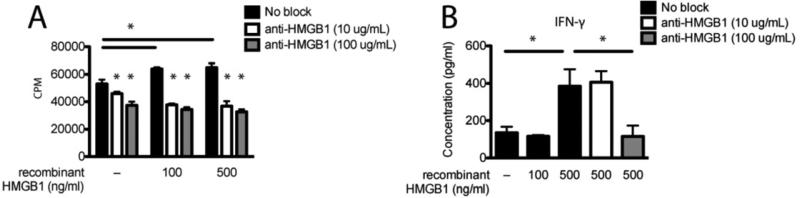
Finally we sought to determine whether rHMGB1 and anti-HMGB1 had similar effects on the proliferation and activation of human lymphocytes. To test this we isolated PBMCs from human blood and stimulated them in vitro with anti-CD3 in the presence of increasing concentrations of rHMGB1 and/or anti-HMGB1. rHMGB1 significantly enhanced T cell proliferation (Fig. 6A, p < 0.05). Furthermore anti-HMGB1 significantly abolished this increase in proliferation. Examining cytokine production in the culture medium, we found that 500 ng/ml rHMGB1 stimulated IFN-γ production and that anti-HMGB1 significantly abrogated this effect (Fig. 6B, p < 0.05). These results, consistent with the HMGB1-mediated stimulation of lymphocyte function from EAE mice, indicate rHMGB1 enhances pro-inflammatory responses in human PBMCs.
Figure 6. HMGB1 enhances human PBMC stimulation in vitro.
Human PBMCs were isolated from blood by density gradient centrifugation and stimulated in vitro with 0.5 μg/ml anti-CD3 in the presence of 100 or 500 ng/ml recombinant HMGB1 with or without 10 or 100 μg/ml anti-HMGB1 antibody. After 48 h 50 μl medium was removed from wells for IFN-γ analysis by multiplex cytokine assay (B), and cultures were pulsed with 1 μCi/well [3H]TdR for proliferation analysis (A). After 24 h cultures were harvested and counted. Data are representative of three experiments. Asterisks denote a significant difference in proliferative responses and cytokine production in the medium by one-way ANOVA followed by Tukey's post hoc tests (p < 0.05).
4. Discussion
The cytokine functions of HMGB1 were initially described in a murine model of sepsis where HMGB1 was identified as a late mediator of LPS-induced death and HMGB1-specific antibodies were shown to rescue mice [9,25]. Since that time the pathophysiological role of HMGB1 has been explored in a number of animal models of sterile injury. HMGB1 is enhanced following paracetamol-induced liver damage [26], ischemia-reperfusion damage of the heart [27], acute lung and kidney injury [28,29], hemorrhage [30], and burns [31]. Increased HMGB1 has also been detected in patients and animal models of autoimmune disease including diabetes [24], rheumatoid arthritis [32], and systemic lupus erythematosus [33] indicating its immune functions are not solely restricted to acute injuries. These observations prompted us to investigate whether HMGB1 increased in the blood during inflammatory demyelinating disease and thus could be used as a serum biomarker of disease-associated inflammation. We observed elevated HMGB1 in the serum of C-EAE mice showing motor deficits consistent with the observation of Andersson et al. that HMGB1 mRNA is increased in the CSF of rats with MOG-induced EAE [21]. When we examined systemic HMGB1 levels broken down by specific disease score though, we found that HMGB1 did not correlate with the severity of clinical disease suggesting HMGB1 reflects overall inflammatory demyelinating disease but is not sensitive enough for tracking inflammation in vivo over time. In the future it should prove interesting to assess protein levels of HMGB1 in the CNS and CSF through an EAE disease course to see if levels more local to the site of inflammation mimic disease scores.
HMGB1 is ubiquitously expressed in most cells throughout mammalian tissues where it functions as a non-specific transcriptional regulator enhancing DNA binding of transcription factors and other DNA-binding proteins. HMGB1 transcription has been shown to be under control of various factors including estrogen, progesterone, p53, p73a, CTF2, and CTF/NF-1 [34]. In addition injury-induced upregulation of HMGB1 has been shown to depend on JAK/STAT signaling [34]. Interestingly the pro-inflammatory properties of extracellular HMGB1 are only evidenced when released from necrotic cells. Apoptotic cells induce a caspase-dependent oxidation of HMGB1, neutralizing its cytokine activity, and effectively allowing the immune system to distinguish between sterile injury requiring an immune response and programmed cell death [35]. In the CNS HMGB1 is constitutively present in neurons, oligodendrocytes, astrocytes, and microglia [20,36], and a neuroinflammatory role for HMGB1 has been demonstrated for numerous CNS insults. HMGB1 is upregulated following brain ischemia and spinal cord injury [15,37] and in one study is directly correlated with neuronal death [37]. Elevated HMGB1 has also been observed in animal models of neurodegenerative disease including Alzheimer's disease [38], Huntington's disease [39], and amyotrophic lateral sclerosis [40]. In 2008 Andersson et al. were the first to investigate a role for HMGB1 in the pathophysiology of MS demonstrating increased numbers of macrophages with cytoplasmic HMGB1 in active lesions [21]. In the rat MOG-induced EAE model, cytoplasmic HMGB1 was found in active lesions in the spinal cord as well as expression of the receptors RAGE, TLR2, and TLR4. Based on these findings, we hypothesized that HMGB1 passively released from necrotic oligodendrocytes or secreted by activated microglia or infiltrating leukocytes contributes to EAE inflammation and could be targeted for therapeutic intervention. Indeed HMGB1-neutralizing antibody has been shown to alleviate clinical disease and pathology in animal models of diabetes and arthritis [24,41]. We found that blocking HMGB1 function with a single injection of monoclonal antibody was most effective in preventing C-EAE and R-EAE disease and to a lesser extent effective in ameliorating established disease. This is consistent with the preservation of mature oligodendrocytes we observed by flow cytometry. Further investigation into repeated administration of anti-HMGB1 antibody will be insightful as our data now suggest that HMGB1 is crucial to disease induction but perhaps only partially contributes to the inflammatory loop that perpetuates disease. Nonetheless administration during ongoing disease was temporarily effective suggesting HMGB1 blockade may be suitable for treating acute MS attacks especially in patients fighting concomitant infections or with latent viral infections where broad-based immunosuppression is not feasible.
We were next interested in elucidating the mechanism(s) underlying the HMGB1 contribution to inflammatory demyelination. HMGB1 as well as other DAMP molecules released from necrotic cells have been shown to bind the promiscuous receptors TLR2, TLR4, and RAGE and specifically promote the recruitment of inflammatory cells across endothelial barriers to sites of injury [42]. HMGB1 binding RAGE on endothelial cells induces cell activation as well as increased expression of vascular cell-adhesion molecule 1 (VCAM1), intercellular adhesion molecule 1 (ICAM1) and endothelial-cell selectin (E-selectin) [8]. Recently a RAGE-independent mechanism for HMGB1 promoting inflammatory cell recruitment by forming a heterocomplex with CXCL12 to directly recruit CXCR4+ monocytes was demonstrated [19]. Examining HMGB1 receptors in EAE mice we observed robust expression of TLR2, TLR4, and RAGE in EAE lesions. Interestingly TLR2 and TLR4 but not RAGE expression were dramatically reduced following HGMB1 neutralization suggesting HMGB1 at least partly contributes to local the local inflammatory response by inducing TLR2 and TLR4 upregulation. Given the multiple mechanisms for HMGB1 regulating leukocyte migration, it was not surprising to find that HMGB1 neutralization at disease remission inhibited infiltration of CD45+ inflammatory cells, CD3+ and CD4+ T cells specifically, in the CNS of R-EAE mice seven days later. We were more surprised to find that reduced immune cell infiltration was still detected by 56 days after injection even though clinical scores were no longer distinguishable from control-treated mice. This may suggest that chronic disease EAE is due to neurodegeneration more so than continuous inflammatory damage, and repeat administrations of anti-HMGB1 antibody may be an effective way to restrict encephalitogenic T cell infiltration and ameliorate disease in a more sustained manner. Interestingly HMGB1 neutralization had no effect on numbers of infiltrating macrophages or DC populations early or late after antibody injection. In addition we observed no changes in APC expression of the costimulatory molecule CD80 or T cell expression of the memory marker CD44 in the CNS suggesting impaired T cell infiltration rather than local effects on immune-mediated inflammation may be the causal basis for improved disease scores. Targeted intracerebroventricular injection of anti-HMGB1 may produce more robust effects on local inflammatory responses and long-term clinical outcomes, and we plan to explore this fully in the future.
Since our CNS analysis suggested anti-HMGB1 effects on EAE disease may occur in the periphery, we assessed systemic CD4+ T cell responses to myelin epitopes by DTH after treatment at disease remission. Responses to the spread epitope PLP178-191 were indeed inhibited during R-EAE relapse suggesting a defect in priming of antigen-specific T cells. HMGB1 released by necrotic cells has been termed an “endogenous immune adjuvant” [43], and there is a sizeable body of literature on HMGB1 enhancing monocyte function, specifically DC maturation and macrophage activation. In addition to RAGE, HMGB1 is known to bind and/or signal through DAMP-associated receptors TLR2, TLR4, and recently TLR9 [44-46]. HMGB1 has been shown to enhance the maturation of DCs [43], and HMGB1-treated DCs stimulated the proliferation and Th1 polarization of alloreactive T cells [47]. This is consistent with our in vitro data showing that recombinant HMGB1 promoted the proliferation and pro-inflammatory cytokine production of T cells from draining lymph nodes of EAE mice restimulated in culture. Our flow cytometric data provide further mechanistic data in that CD40 and CD80 costimulatory molecule expression was increased on DCs and regulatory PD-1 expression was decreased on CD4+ T cells in the presence of HMGB1. Other researchers have suggested that highly purified recombinant HMGB1 has weak pro-inflammatory effects but that HMGB1 forms stable complexes with various pro-inflammatory molecules to enhance their function [48,49]. Thus we think it likely that the mitogenic effects of HGMB1 in our in vitro experiments was dependent on prior activation of the lymphocytes in vivo via EAE priming. The exact nature and extent of HMGB1 forming complexes with pro-inflammatory substrates to enhance innate immunity will be interesting to examine in the future.
Finally, to verify if HMGB1 has an equivalent role in human leukocyte function, we undertook a series of in vitro experiments examining human PBMC proliferation and activation in the presence of HMGB1. Recombinant HMGB1 was previously shown to enhance proliferation of purified human CD4+ T cells stimulated with anti-CD3 in vitro [18]. Similarly we found that HMGB1 enhanced proliferation and pro-inflammatory cytokine production of human PBMCs stimulated in vitro. This is encouraging support for continued study of the mechanistic actions of HMGB1 in stimulating pro-inflammatory function of human leukocytes and anti-HMGB1 antibody for regulating human autoimmune diseases.
Taken together our data suggest HMGB1 robustly mediated leukocyte activation in vitro but the mechanism in vivo was primarily through modulation of leukocyte recruitment. This could be due to limited access of anti-HMGB1 to the CNS, but the biochemistry of HMGB1 should also be considered. A recent study by Venereau et al. suggested that HMGB1 exists in mutually exclusive redox states that independently orchestrate chemoattraction and activation [16]. Within injured muscle HMGB1 is initially released extracellularly in a reduced form and in this state mediates leukocyte recruitment. Later HMGB1 is disulfide-bonded promoting cytokine secretion by leukocytes. In EAE the redox state of HMGB1 might likewise change through disease course (and consequently downstream function). In future EAE studies it will be critical to examine the biochemistry of HMGB1 throughout the EAE disease course in addition to the site of action of the neutralizing antibody.
5. Conclusions
We have shown that HMGB1 is a critical component to the pathogenesis of autoimmune demyelinating disease and that neutralization is an effective means to inhibit clinical disease and encephalitogenic lymphocyte infiltration and systemic T cell activation. To our knowledge this is the first evidence of a direct role for HMGB1 in driving neuroinflammatory responses in EAE and the first investigation of the underlying mechanisms. Further mechanistic analyses should provide insight into the pro-inflammatory loop that drives EAE and indeed MS autoimmune demyelination, as well as the therapeutic feasibility for HMGB1 neutralization in the clinical setting.
Supplementary Material
Supplementary Figure 1. Anti-HMGB1 amelioration of EAE is antibody dose-dependent. SJL/J mice, 6-8 wks of age, were immunized with 50 μg PLP139-151/CFA s.c. and scored daily for clinical disease using a standard five-point scale. R-EAE mice were injected i.v. with 200, 100, 50, or 10 μg anti-HMGB1 neutralizing antibody or 200 μg isotype control antibody 7 days after disease induction (n = 4-5 per group). 50 and 100 μg anti-HGMB1 significantly ameliorated clinical disease (Mann Whitney U test, p < 0.05). 200 μg-treated mice showed a dramatic remission but proceeded to relapse suggesting the high dose of antibody may have induced an immune response against the treatment itself causing the antibody to be more rapidly cleared from the circulation.
Highlights.
HMGB1 contributes to the pathogenesis of EAE.
HMGB1 neutralizing antibody ameliorated EAE.
HMGB1 neutralization blocked CNS T cell infiltration and systemic T cell responses.
Recombinant HMGB1 enhances in vitro activation of CD4+ T cells from EAE mice.
Recombinant HMGB1 promotes activation of human PBMCs.
Acknowledgments
We thank the Northwestern Immunobiology Center Flow Cytometry Core Facility and all members of the Miller laboratory for helpful comments and discussion.
Abbreviations used in this paper
- CNS
central nervous system
- DAMP
damage-associated molecular pattern
- DTH
delayed-type hypersensitivity
- EAE
experimental autoimmune encephalomyelitis
- HMGB1
high-mobility group box 1 protein
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- PLP
proteolipid protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported in part by NIH Grant NS-026543.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J. Immunol. 2011;187:2405–17. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Podojil JR, Miller SD. Molecular mechanisms of T-cell receptor and costimulatory molecule ligation/blockade in autoimmune disease therapy. Immunol. Rev. 2009;229:337–55. doi: 10.1111/j.1600-065X.2009.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seong S-Y, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–78. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 4.Zeh HJ, Lotze MT. Addicted to death: invasive cancer and the immune response to unscheduled cell death. J. Immunother. 2005;28:1–9. doi: 10.1097/00002371-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Arcaroli J, Yum H-K, Yang H, Wang H, Yang K-Y, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol. 2003;284:C870–9. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- 6.Stros M, Ozaki T, Bacikova A, Kageyama H, Nakagawara A. HMGB1 and HMGB2 cell-specifically down-regulate the p53- and p73-dependent sequence-specific transactivation from the human Bax gene promoter. J. Biol. Chem. 2002;277:7157–64. doi: 10.1074/jbc.M110233200. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 8.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 10.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–95. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 12.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–16. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 13.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Ann. Rev. Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J. Immunol. 2000;165:2950–54. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 15.Kim J-B, Sig Choi J, Yu Y-M, Nam K, Piao C-S, Kim S-W, et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 2006;26:6413–21. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 2012;209:1519–28. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castiglioni A, Canti V, Rovere-Querini P, Manfredi AA. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res. 2011;343:189–99. doi: 10.1007/s00441-010-1033-1. [DOI] [PubMed] [Google Scholar]
- 18.Sundberg E, Fasth AER, Palmblad K, Harris HE, Andersson U. High mobility group box chromosomal protein 1 acts as a proliferation signal for activated T lymphocytes. Immunobiol. 2009;214:303–09. doi: 10.1016/j.imbio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 2012;209:551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang P, Schachner M, Shen Y-Q. HMGB1 in development and diseases of the central nervous system. Mol. Neurobiol. 2012;45:499–506. doi: 10.1007/s12035-012-8264-y. [DOI] [PubMed] [Google Scholar]
- 21.Andersson A, Covacu R, Sunnemark D, Danilov AI, Dal Bianco A, Khademi M, et al. Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J. Leuk. Biol. 2008;84:1248–55. doi: 10.1189/jlb.1207844. [DOI] [PubMed] [Google Scholar]
- 22.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides preferentially polarize CD4+ TH-17 cells in relapsing EAE. Nat. Immunol. 2007;8:172–80. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 23.Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur. J. Immunol. 2008;38:2706–17. doi: 10.1002/eji.200838137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han J, Zhong J, Wei W, Wang Y, Huang Y, Yang P, et al. Extracellular high-mobility group box 1 acts as an innate immune mediator to enhance autoimmune progression and diabetes onset in NOD mice. Diabetes. 2008;57:2118–27. doi: 10.2337/db07-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G-Y, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–25. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–26. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Gong Q, Zhong S, Wang L, Guo H, Xiang Y, et al. Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia-reperfusion injury. Nephrol. Dial. Transplant. 2011;26:469–78. doi: 10.1093/ndt/gfq466. [DOI] [PubMed] [Google Scholar]
- 29.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am. J. Resp. Crit. Care Med. 2004;170:1310–16. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 30.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol. Med. 2006;12:105–14. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang W-H, Yao Y-M, Shi Z-G, Yu Y, Wu Y, Lu L-R, et al. The significance of changes in high mobility group-1 protein mRNA expression in rats after thermal injury. Shock. 2002;17:329–33. doi: 10.1097/00024382-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Kokkola R, Sundberg E, Ulfgren A-K, Palmblad K, Li J, Wang H, et al. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis & Rheum. 2002;46:2598–603. doi: 10.1002/art.10540. [DOI] [PubMed] [Google Scholar]
- 33.Urbonaviciute V, Fürnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J. Exp. Med. 2008;205:3007–18. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. Journal of internal medicine. 2004;255:332–43. doi: 10.1111/j.1365-2796.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- 35.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J-B, Lim C-M, Yu Y-M, Lee J-K. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J. Neurosci. Res. 2008;86:1125–31. doi: 10.1002/jnr.21555. [DOI] [PubMed] [Google Scholar]
- 37.Kawabata H, Setoguchi T, Yone K, Souda M, Yoshida H, Kawahara K-i, et al. High mobility group box 1 is upregulated after spinal cord injury and is associated with neuronal cell apoptosis. Spine. 2010;35:1109–15. doi: 10.1097/BRS.0b013e3181bd14b6. [DOI] [PubMed] [Google Scholar]
- 38.Takata K, Kitamura Y, Tsuchiya D, Kawasaki T, Taniguchi T, Shimohama S. High mobility group box protein-1 inhibits microglial Abeta clearance and enhances Abeta neurotoxicity. J. Neurosci. Res. 2004;78:880–91. doi: 10.1002/jnr.20340. [DOI] [PubMed] [Google Scholar]
- 39.Goula A-V, Berquist BR, Wilson DM, Wheeler VC, Trottier Y, Merienne K. Stoichiometry of base excision repair proteins correlates with increased somatic CAG instability in striatum over cerebellum in Huntington's disease transgenic mice. PLoS Gen. 2009;5:e1000749. doi: 10.1371/journal.pgen.1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo Coco D, Veglianese P, Allievi E, Bendotti C. Distribution and cellular localization of high mobility group box protein 1 (HMGB1) in the spinal cord of a transgenic mouse model of ALS. Neurosci. Let. 2007;412:73–77. doi: 10.1016/j.neulet.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 41.Schierbeck H, Lundbäck P, Palmblad K, Klevenvall L, Erlandsson-Harris H, Andersson U, et al. Monoclonal anti-HMGB1 (high mobility group box chromosomal protein 1) antibody protection in two experimental arthritis models. Mol. Med. 2011;17:1039–44. doi: 10.2119/molmed.2010.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 1995;270:25752–61. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 43.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–30. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JS, Svetkauskaite D, He Q, Kim J-Y, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–77. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 45.Tian J, Avalos AM, Mao S-Y, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 46.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J. Immunol. 2005;175:7661–68. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 47.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J. Immunol. 2004;173:307–13. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 48.Bianchi ME. HMGB1 loves company. J. Leuk. Biol. 2009;86:573–76. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- 49.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin). J. Leuk. Biol. 2007;81:49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Anti-HMGB1 amelioration of EAE is antibody dose-dependent. SJL/J mice, 6-8 wks of age, were immunized with 50 μg PLP139-151/CFA s.c. and scored daily for clinical disease using a standard five-point scale. R-EAE mice were injected i.v. with 200, 100, 50, or 10 μg anti-HMGB1 neutralizing antibody or 200 μg isotype control antibody 7 days after disease induction (n = 4-5 per group). 50 and 100 μg anti-HGMB1 significantly ameliorated clinical disease (Mann Whitney U test, p < 0.05). 200 μg-treated mice showed a dramatic remission but proceeded to relapse suggesting the high dose of antibody may have induced an immune response against the treatment itself causing the antibody to be more rapidly cleared from the circulation.