To the Editor:
Heterozygous gain-of-function mutations in the coiled-coil domain of STAT1 were recently identified as a cause of chronic mucocutaneous candidiasis (CMC) (1, 2). As with STAT3 mutations in hyper-IgE syndrome, the candidal susceptibility associated with gain-of-function STAT1 mutations appears secondary to TH17 cell deficiency (3). The mechanistic link between constitutive STAT1 activity and diminished TH17 cells has yet to be clearly defined. Here we present a kindred with a novel gain-of-function STAT1 mutation associated with a complex clinical phenotype including candidiasis, humoral immunodeficiency, overexpression of programmed cell death protein ligand 1 (PD-L1) and increased B-cell apoptosis.
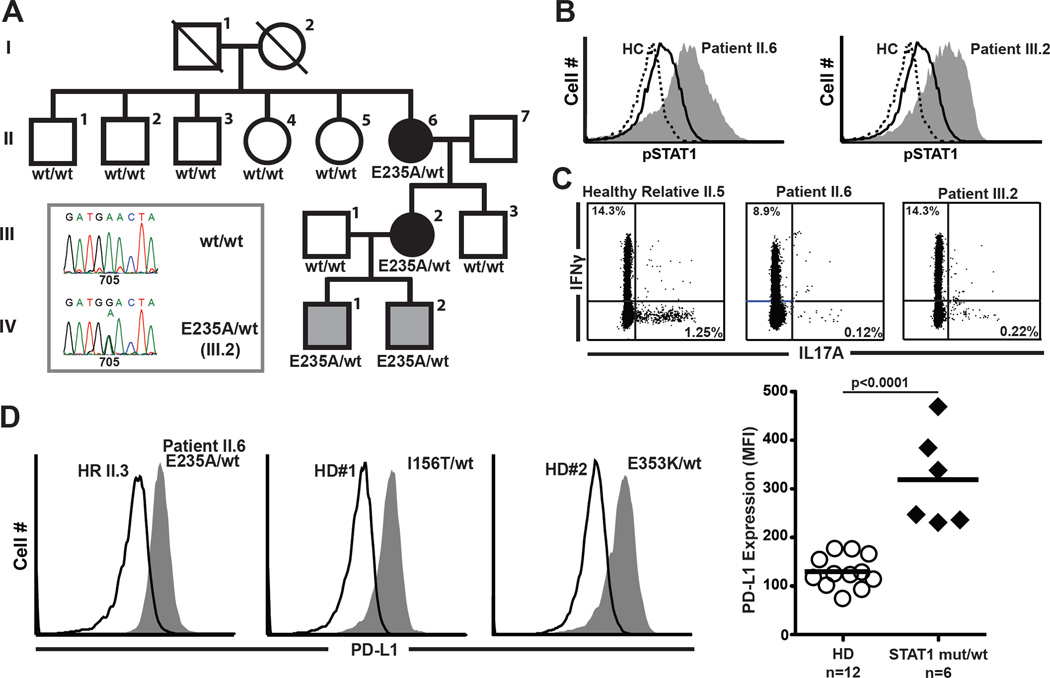
We have identified four related individuals each heterozygous for a novel E235A missense mutation in a highly conserved segment of the coiled-coil domain of STAT1 (Fig 1, A). To our knowledge this is the first mutation described in exon 9 of the STAT1α gene locus to be associated with CMC. The mutation is not present in unaffected adult family members. The index patient (II.6) is a 60-year-old woman with CMC and progressive antibody deficiency. Beginning in infancy, the patient experienced candidal infections at sites including the oral cavity, esophagus, vagina, skin and nails. Later, as a young woman, the patient was diagnosed with IgG2 subclass deficiency that progressed to frank hypogammaglobulinemia (IgG 514, IgA 7, IgM<10 mg/dl) and B-cell lymphopenia (2 CD20+ cells/µl, nml range 97–440) requiring IVIG. Over her lifetime, the patient suffered recurrent pulmonary infections from P. aeruginosa, S. pneumonia, Serratia species, M. avium and respiratory syncytial virus that resulted in severe bronchiectasis requiring lobectomy. Consistent with published reports of patients with gain-of-function STAT1 mutations, our index patient (II.6) has experienced HPV+ squamous cell carcinoma of palate, basal cell carcinoma, shingles and fibromuscular dysplasia with carotid and celiac/splenic artery dissection.
FIG 1.
Gain-of-function STAT1 mutations increase PD-L1 expression. A, Pedigree with patients (black) and carriers (gray) heterozygous at STAT1 nucleotide 705. B, Increased STAT1 phosphorylation in IL-21 treated T-cells from patients (filled), compared to controls (solid) and unstimulated (dashed). C, Reduced TH17-cell frequencies in E235A STAT1 patients. D, PD-L1 overexpression on CD3+CD4+CD62L+CD45RO− cells from subjects with STAT1 mutations (filled) versus controls (solid).
The index patient’s daughter (III.2) is a 30-year-old female with CMC, B-cell lymphopenia (6 CD20+ cells/µl) and IgG2 subclass deficiency manifesting in adolescence. Despite antifungal therapy, the patient experienced candidal infections at sites including the vagina, skin and nails. She has also experienced non-candidal infections including pneumonia, otitis media, sinusitis and chronic bronchitis. The third and forth persons carrying the E235A allele are children of patient III.2, two males ages 6 weeks and 24 months (IV.1 and IV.2). They have not yet manifested symptoms of immunodeficiency.
Stimulation of T cells from patients II.6 and III.2 with IL-21 significantly increased phosphorylation of STAT1 compared to a healthy control (Fig 1, B). Furthermore, stimulation of patient PBMCs with PMA/ionomycin demonstrated diminished IL-17 secreting CD4+ cells compared to a related healthy control (Fig 1, C). Hence the E235A mutation confers to STAT1 a gain-of-function and is associated with TH17-cell deficiency.
A remarkable feature of our kindred is overexpression of PD-L1 on the surface of naïve CD4+ T cells. All four family members carrying the E235A allele had higher PD-L1 staining compared to members without it (Fig 1, D). To investigate if overexpression of PD-L1 was a common feature to gain-of-function STAT1 mutations, we obtained PBMCs from two additional patients carrying either the I156T or the E353K missense STAT1 mutation. Both patients, unrelated to our kindred, revealed a similar increase in PD-L1 expression on their naïve T cells (Fig 1, D). Recent data from mice demonstrate that the expression of PD-L1 on undifferentiated naïve T cells prevents commitment to the TH17 lineage through a PD-1/PD-L1 interaction. In this context, PD-L1 expression is dependent on IL27/IL27R binding and STAT1 (4). Accordingly, constitutively active STAT1 molecules in subjects carrying gain-of-function STAT1 mutations may be responsible for increased PD-L1 expression on naïve T cells thereby discouraging differentiation into TH17 cells.
As previously described, the clinical phenotype of patients with gain-of-function STAT1 mutations is quite broad and can include candidiasis, anti-thyroid autoimmunity, squamous cell carcinoma and vascular anomalies. In this issue two reports show broader phenotypes to gain-of-function STAT1 mutations with an IPEX-like autoimmune syndrome in one report, and disseminated coccidioidomycosis and histoplasmosis in the other. Here, we report a not yet appreciated feature associated with gain-of-function STAT1 mutations: humoral immunodeficiency.
STAT1’s function as a key modulator of cell death is thoroughly described. Perhaps the best illustration of this is growth arrest of the STAT1-negative U3A fibroblast line upon transfection with wild type STAT1α and treatment with interferon-γ (5). The interferon-γ receptor requires STAT1 for intracellular signaling. Moreover, transfection of the same cell line with constitutively activated STAT1 initiates caspase mediated apoptosis (6). Related experiments implicate STAT1 activation during apoptosis in B-cell lymphoma cells (7).
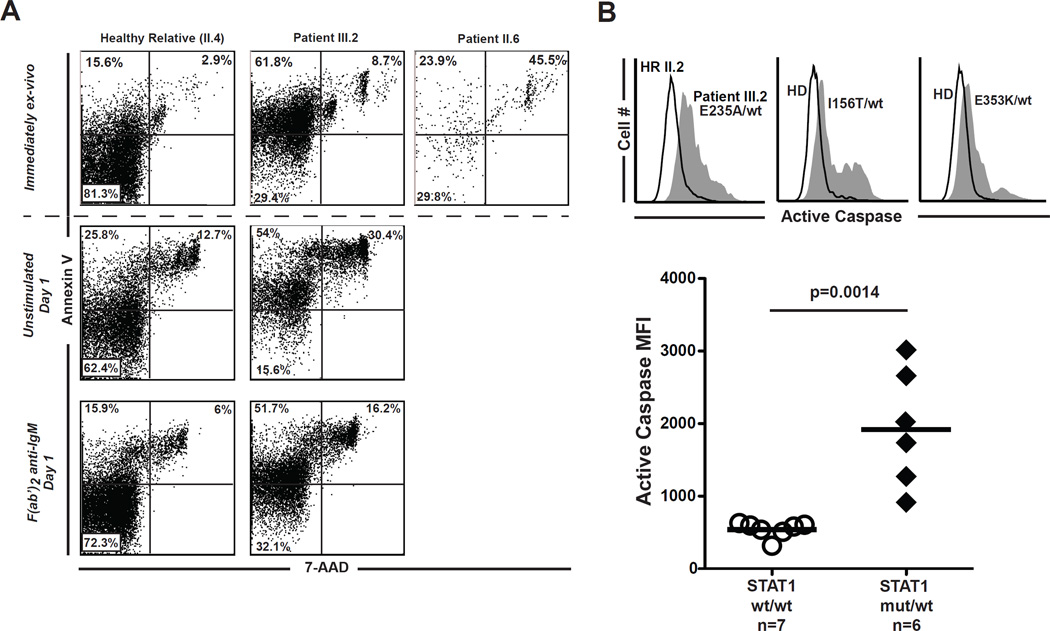
Interestingly, progressive B-cell lymphopenia is a remarkable feature of patients (II.6 and III.2) in our kindred suggesting a defect in cell survival. Indeed, CD19+ B cells from subjects carrying the E235A allele appear apoptotic with increased Annexin V staining (Fig 2, A top row) and elevated caspase activity (Fig 2, B). In culture for 24 hours, B cells from patient III.2 demonstrated even greater Annexin V and considerable 7-AAD staining, evidence of accelerated cell death (Fig 2, A middle row). The patient’s B cells were only partially rescued by stimulation of their B-cell receptors (Fig 2,A bottom row). We also found enhanced caspase activity in B cells from the two additional patients that were heterozygous for either the E353K or the I156T missense STAT1 mutation (Fig 2, B). B cell lymphopenia was a significant finding in the former patient (39 cells/µl) but not the latter (335 cells/ul). Altogether, our data reveal that gain-of-function STAT1 mutations increase B-cell apoptosis. Over time this may result in B-cell lymphopenia and antibody deficiency.
FIG 2.
Gain-of-function STAT1 mutations promote B-cell death. A, Annexin V and 7-AAD staining of CD19+ cells immediately ex-vivo (top row) and after 24 hours in culture with (middle row) or without rescue with F(ab’)2-anti-IgM (bottom row). B, Active caspase staining of CD19+ cells in subjects heterozygous for the indicated STAT1 mutations (filled) vs. related and unrelated controls (solid).
In summary, we identified individuals heterozygous for gain-of-function STAT1 mutation with two unappreciated features. The first is the overexpression of PD-L1 on naïve T cells which provides a general mechanism for how constitutively active STAT1 blocks the development of the TH17 lineage. The second feature, accelerated B-cell apoptosis that may result in progressive B-cell lymphopenia and humoral immunodeficiency, further broadens the clinical phenotype associated with gain-of-function STAT1 mutations.
Acknowledgments
Declaration of all sources of funding: This work was supported by grant number AI061093, AI071087, AI082713 and AI095848 from NIH-NIAID (to E. M.), K12HD0141401-10 from NIH-NICHD (to N.R.) and MO 2160/2-1 from DFG (to H.M.), and by the Division of Intramural Research, NIAID, NIH (to SMH and JDM).
We would like to acknowledge Dr. O’Shea of the NIH for sharing his unpublished manuscript with our group and to thank the patients and their family members for making this work possible.
Abbreviations
- STAT1
Signal transducer and activator of transcription 1
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death protein ligand 1
- CMC
Chronic mucocutaneous candidiasis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All patient samples were collected in accordance with IRB-approved protocols.
References
- 1.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011 Jul 7;365(1):54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. The Journal of Experimental Medicine. 2011 Aug 1;208(8):1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008 Apr 10;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, et al. Interleukin-27 Priming of T Cells Controls IL-17 Production In trans via Induction of the Ligand PD-L1. Immunity. 2012 Jun 29;36(6):1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996 May 3;272(5262):719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 6.Sironi JJ, Ouchi T. STAT1-induced apoptosis is mediated by caspases 2, 3, and 7. J Biol Chem. 2004 Feb 6;279(6):4066–4074. doi: 10.1074/jbc.M307774200. [DOI] [PubMed] [Google Scholar]
- 7.Niitsu N, Higashihara M, Honma Y. Human B-cell lymphoma cell lines are highly sensitive to apoptosis induced by all-trans retinoic acid and interferon-gamma. Leuk Res. 2002 Aug;26(8):745–755. doi: 10.1016/s0145-2126(01)00202-8. [DOI] [PubMed] [Google Scholar]