Abstract
Objectives
Alterations in the mechanical loading environment in joints may have both beneficial and detrimental effects on articular cartilage and subchondral bone and subsequently influence the development of osteoarthritis (OA). We used an in vivo tibial loading model to investigate the adaptive responses of cartilage and bone to mechanical loading and to assess the influence of load level and duration.
Methods
We applied cyclic compression of 4.5 and 9.0N peak loads to the left tibia via the knee joint of adult (26-week-old) C57Bl/6 male mice for 1, 2, and 6 weeks. Only 9.0N loading was utilized in young (10-week-old) mice. The changes in articular cartilage and subchondral bone were analyzed by histology and microcomputed tomography.
Results
Loading promoted cartilage damage in both age groups, with increased damage severity dependent upon the duration of loading. Metaphyseal bone mass increased in the young mice, but not in the adult mice, whereas epiphyseal cancellous bone mass decreased with loading in both young and adult mice. Articular cartilage thickness decreased, and subchondral cortical bone thickness increased in the posterior tibial plateau in both age groups. Both age groups developed periarticular osteophytes at the tibial plateau in response to the 9.0N load, but no osteophyte formation occurred in adult mice subjected to 4.5N peak loading.
Conclusion
This non-invasive loading model permits dissection of temporal and topographical changes in cartilage and bone and will enable investigation of the efficacy of treatment interventions targeting joint biomechanics or biological events that promote OA onset and progression.
Keywords: Mechanical loading, cartilage, subchondral bone, osteoarthritis, mice
Osteoarthritis (OA) affects nearly 27 million individuals in the United States and is the major cause of disability in the adult population (1). Both systemic and environmental factors, including ethnicity, nutritional factors, genetic background, sex, obesity, and joint injury contribute to the development of OA (2). In particular, the onset and development of OA is greatly influenced by alterations in the local mechanical loading environment in the joint that may result from prior joint injuries, occupational activities involving heavy lifting or repeated joint loading, or obesity (2, 3).
Loading environment plays both beneficial and detrimental roles in the properties of articular cartilage and the risk for the eventual development of OA. Dynamic compressive loading of bovine cartilage explants increases proteoglycan uptake and synthesis (4, 5), and regular moderate running routines in hamsters and rats prevent both naturally occurring and injury-related cartilage degeneration (6, 7). Evidence from animal and clinical studies established mild to moderate exercise as a recommended non-pharmacological intervention that both prevents and alleviates OA symptoms in patients (8–10).
Mechanical loading can also be detrimental to maintaining healthy articular cartilage and preventing the initiation and progression of OA symptoms. A single injurious compression load at a high strain rate or repeated compressive cyclic loading decreases cartilage stiffness and cell viability of chondrocytes and increases aggrecanase expression in bovine cartilage explants (11–13). Clinically, lifting heavy objects in certain occupations, increased body mass, and repetitive physical loading are associated with hip and knee OA (3, 14, 15). The reduction in body weight with an exercise regime effectively reduces pain and increases joint mobility in OA patients (16).
Despite the correlation between the loading environment and the risk for the development of OA in humans, limited studies have investigated the effects of defined mechanical loads and age on the development of OA-like structural alterations in animal models (17). Animal models that initiate OA through surgical disruption are confounded by the adverse effects of the surgical insult and the post-surgical healing response, as well as the inability to rigorously control the mechanical loading environment within the affected joint (18, 19).
Our studies were undertaken to establish the effects of mechanical loading at defined magnitudes on the structural organization and composition of articular cartilage and subchondral bone. Histological and radiological changes were assessed in mouse knee joints in adult mice subjected to in vivo tibial loading at defined magnitudes (4.5N and 9.0N) for durations of 1, 2, and 6 weeks and at 9.0N peak load in young mice. We hypothesized that the alterations in the cartilage and subchondral bone would depend on the magnitude and duration of the load and that the responses to the high peak load would be influenced by the age of the mice.
Materials and Method
Mechanical Loading Conditions
To test the effects of load duration on the joint changes, we subjected the left tibiae of twenty-one young (10-week-old) C57Bl/6 male mice (Jackson Laboratories, Bar Harbor, ME) to cyclic compressive loading for 1, 2, and 6 weeks at a 9.0N peak load. We also loaded the tibiae of forty-two adult (26-week-old) C57Bl/6 male mice at 4.5N and 9.0N peak loads for 1, 2, and 6 weeks. A load level of 9.0N generates 1200με in the tibial mid-shaft of 10-week-old mice based on in vivo strain gauging (20). In vivo tibial loading was applied for 1200 cycles at 4 Hz for 5 days per week at each peak load under general anesthesia (2% Isoflurane, 1.0 L/min, Webster). The applied loading was based on protocols demonstrated previously to have an anabolic effect on the tibial metaphysis in growing and adult mice (Supp Figure 1) (20–22). The left limb was loaded, and the right limb served as the non-loaded control. In preliminary studies in our laboratory, we found that metaphyseal bone mass and architecture of the non-loaded control (right) limbs were not affected by loading of the left limbs. After the specified duration, the mice were euthanized, and the intact knee joints were dissected and fixed in 10% formalin overnight. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
Cartilage and Subchondral Bone Assessment
After tissue fixation overnight, intact joints in PBS were scanned by microcomputed tomography (microCT) with an isotropic voxel resolution of 10 μm (μCT35, Scanco: 55 kVp, 145 μA, 600 ms integration time, no frame averaging) to assess bone morphological changes. A 0.5mm aluminum filter reduced the effects of beam hardening. Knee joints were then decalcified in EDTA for 2 weeks, dehydrated in an ethanol gradient, and embedded in paraffin. Serial coronal sections (6 μm thick) were obtained from posterior to anterior using a rotary microtome (Leica RM2255, Germany). Safranin O/Fast green staining was performed on sections at 90 μm intervals to assess cartilage morphology. Cartilage degeneration was assessed in the tibial plateau using a modified murine cartilage histological scoring system (23).
Bone morphology from microCT was assessed in two regions: the metaphysis (distal to the growth plate) extending 10% of the total bone length, excluding primary spongiosa and cortical bone; and the epiphysis (proximal to the growth plate) excluding cortical bone. Trabecular bone from both regions was isolated by manually contouring inside the cortical shell. The global threshold was set at 3100 HU to segment mineralized tissue. For each region, cancellous bone volume fraction, trabecular thickness and separation, and tissue mineral density were measured. Also, localized thickness measurements of subchondral cortical bone (Sub. Pl.) and cartilage were performed using Osteomeasure (OsteoMetrics, USA) on representative Safranin O/Fast green stained sections previously used for histological scoring. Six different regions of interest were defined by dividing the tibial plateau into medial and lateral halves, each of which was then further divided into anterior, middle, and posterior regions. A single representative section from each of six regions of each joint from all animals was chosen to measure localized thickness. Five linear projections from the cartilage surface to the boundary between the cartilage and subchondral bone, including calcified cartilage, were used to measure cartilage thickness. The projection was extended into the subchondral bone to measure the subchondral bone thickness (24).
Statistics
In adult mice, the effects of loading, load level, and duration were detected using a 3-factor repeated measure ANOVA with interactions (JMP Pro 9.02, SAS Institute Inc), with loading as the intra-group variable, and load level and duration as inter-group variables. In young mice, the effects of loading and duration were detected using a 2-factor repeated measure ANOVA with interactions with loading as the intra-group variable and duration as inter-group variable. Post-hoc means comparison tests with Bonferroni correction were performed only when interaction effects were significant. P-values < 0.05 indicated significance. All values are presented as mean ± SD.
Results
Articular Cartilage Matrix Changes
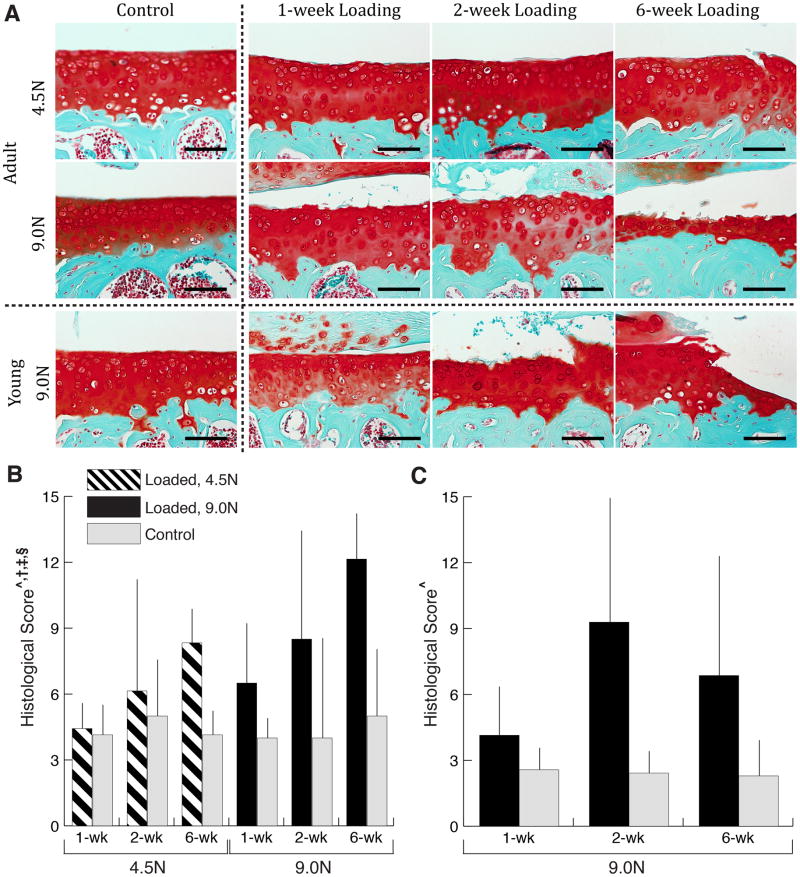
Mechanical loading induced cartilage matrix changes, including cartilage fibrillation, fragmentation, and erosion in both young and adult mice (Fig. 1A). As load duration increased, damage in the cartilage matrix increased, visualized by loss of Safranin O staining and cartilage thinning. The high (9.0N) load level induced more dramatic changes than the low (4.5N) load level in adult mice. After 6 weeks of loading at 9.0N, almost the entire cartilage thickness was lost in the adult mice based on Safranin O staining, while only slight cartilage surface fibrillation was apparent in joints loaded with 4.5N. The high peak load produced marked cartilage thinning in the young mice but the changes were less severe than those observed in the adult mice. We, therefore, did not examine young mice subjected to the low peak load.
Figure 1.
Qualitative (A) and quantitative (B, C) measures of cartilage matrix changes. Cartilage damage is evident following mechanical loading in both young and adult mice, and was exacerbated with longer durations and higher level of loading in adult mice (A, Scale bar = 100 μm). Safranin O/Fast Green staining of the medial side of articular cartilage in young and adult mice loaded for 1-, 2- and 6-weeks and at two load levels (4.5N and 9.0N) for adult mice. Control images represent the non-loaded contralateral limb at 6-week duration at each respective age and load level. Loading increased histological scores in both adult (B) and young (C) mice. Duration of loading increased the histological score in adult mice. Load-induced increases in histological score depended on load level and duration in adult mice. Data presented as mean ± SD.
^loading, †duration, ‡loading*duration, §loading*load level effect, p<0.05 by repeated measures two-way ANOVA for young mice and repeated measures three-way ANOVA for adult mice.
The quantitative cartilage scoring confirmed the observations of the histological staining (Fig. 1B,C). At both ages the histological score increased with loading: 2.8-fold in young and 1.8-fold in adult mice (loaded and control limbs pooled individually at all time points). Both longer duration of loading and higher load level increased the histological score in adult mice. In young mice, the response to high peak load was delayed and not apparent until the 6-week time point.
Cartilage thickness changes varied with location in the tibial plateau. In young mice, cartilage thinning was apparent at the posterior aspect of the lateral and medial tibial plateau (Fig. 2A, Supp Table 2). However, cartilage thickness did not change or increased in the loaded joint at the anterior aspect of the lateral plateau. Similar changes in cartilage thickness occurred in the adult mice with loading (Fig. 2B, Supp Table 1) including increased cartilage thinning at the posterior aspect of the lateral tibial plateau, and were promoted by higher load level and longer duration of loading.
Figure 2.
Localized cartilage thickness for young (A) and adult (B) mice. Safranin O/Fast Green images (Scale bar = 100 μm) represent control and loaded limbs at 9.0N for 6-week duration at each respective age. Cartilage thinned with loading on the posterior side of the lateral tibial plateau in young mice. Adult mice also underwent cartilage thinning with loading posteriorly on the lateral plateau. Higher load level and longer duration of loading increased load-induced thinning of laterial-posterior tibial plateau cartilage. Data presented as mean ± SD.
^loading, †duration, ‡load level, §loading*duration, ¶loading*load level, #loading*duration*load level effect, p<0.05 by repeated measures two-way ANOVA for young mice and repeated measures three-way ANOVA for adult mice. Groups with different letters are significantly different by post-hoc means comparisons with Bonferroni correction.
Epiphyseal and Metaphyseal Bone Adaptation
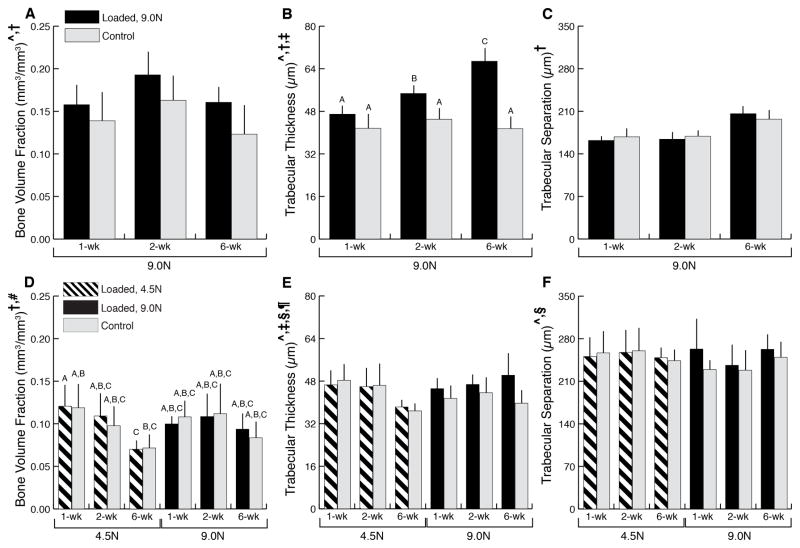
In addition to cartilage matrix changes, we observed alterations in the epiphyseal cancellous bone in response to in vivo tibial loading. In both adult and young mice, loading at 9N decreased epiphyseal bone mass with a greater decrease observed in the adult mice compared to the young mice (14% versus 4.9%, respectively) (Fig. 3). The decreased bone mass was attributable to the increased trabecular separation, which translated to decreased trabecular number in adult mice. In the adult mice, the decreased bone mass was dependent on the load with greater decrease observed in the higher load group (Fig. 3D–F).
Figure 3.
Epiphyseal cancellous bone microCT measurements from young (A–C) and adult (D–F) mice. The decreased bone mass was attributable to the increased trabecular separation, which translated to decreased trabecular number, in adult mice. Trabecular thickness was not altered by loading in both adult and young mice. Data presented as mean ± SD.
^loading, †duration, ‡loading*duration effect, p<0.05 by repeated measures two-way ANOVA for young mice and repeated measures three-way ANOVA for adult mice. Groups with different letters are significantly different by post-hoc means comparisons with Bonferroni correction.
In contrast to the epiphyseal bone, metaphyseal bone mass increased by 20% with loading in young mice (Fig. 4A–C). The increased cancellous bone mass was due to trabecular thickening, which increased by 32%. Also, longer duration of loading further increased the trabecular thickness. No change in metaphyseal bone mass was present in adult mice (Fig. 4D–F); loading promoted trabecular thickening, which was counteracted by the increase in trabecular separation that inversely related to lower number of trabeculae.
Figure 4.
Metaphyseal cancellous bone microCT measures for young (A–C) and adult (D–F) mice. Loading increased cancellous mass in young mice through thickening of trabeculae. In adult mice cancellous mass was not altered with loading. Data presented as mean ± SD.
^loading, †duration, ‡loading*duration, §loading*load level, ¶load level*duration, #load*duration*load level effect, p<0.05 by repeated measures two-way ANOVA for young mice and repeated measures three-way ANOVA for adult mice. Groups with different letters are significantly different by post-hoc means comparisons with Bonferroni correction.
The adaptive pattern of epiphyseal subchondral cortical bone in response to load differed from the pattern observed in the epiphyseal cancellous bone (Fig. 5, Supp Tables 1 and 2). In contrast to the decreased cancellous bone in response to load, both young and adult mice exhibited thickening of the subchondral cortical bone at the posterior-lateral aspect of the tibial plateau. On the anterior side, age-dependent subchondral cortical bone changes occurred. In young mice at the end of the 6-week loading duration, subchondral cortical bone thinned while in adult mice, the thickness did not change.
Figure 5.
Localized subchondral cortical bone thickness in young (A) and adult (B) mice. Safranin O/Fast Green images (Scale bar = 100 μm) represent control and loaded limbs at 9.0N for 6-week duration at each respective age. The subchondral bone thickened with loading at the posterior aspect of the lateral tibial plateau in young mice. Load level and duration affected load-induced thickening of the subchondral plate on the lateral side. Adult mice also underwent subchondral plate thickening with loading at the posterior aspect of the lateral tibial plateau. Data presented as mean ± SD.
^loading, †duration, ‡loading*duration, §duration*load level effect, p<0.05 by repeated measures two-way ANOVA for young mice and repeated measures three-way ANOVA for adult mice. Groups with different letters are significantly different by post-hoc means comparisons with Bonferroni correction.
Osteophyte formation
Mechanical loading also induced periarticular osteophyte formation at the tibial joint margins in the 9.0N-loaded limbs of both young and adult mice (Fig. 6). After 1 and 2 weeks of loading, cartilage was present in the marginal periarticular tissue, which then mineralized after 6 weeks of loading. The 4.5N load level did not induce formation of any cartilaginous tissue after 1 or 2 weeks or any mineralized tissue after 6 weeks of loading in adult mice.
Figure 6.

Mechanical loading induced osteophyte formation in both young and adult mice at the 9.0N load level. The lower load level did not induce osteophyte formation in adult mice. Safranin O/Fast Green staining of the medial side of the knee joint in young and adult mice loaded for 1-, 2-, and 6-weeks at two different load levels (only for adult mice). Control images represent non-loaded contralateral limbs at 6-weeks of load duration at each respective age and load level. Scale bar 200 μm.
Discussion
Noninvasive mechanical loading induced cartilage matrix damage, epiphyseal bone adaptation, and osteophyte formation in both age groups, recapitulating the morphological and anatomic features of OA in both cartilage and bone. Metaphyseal bone adaptation to mechanical loading occurred only in the young mice. In addition to gross morphological changes in cartilage, cartilage thickness decreased and subchondral cortical bone thickness increased in the posterior-lateral aspect of the tibial plateau in response to loading in both age groups, while at the anterior tibial plateau, age-dependent subchondral cortical thickness changes occurred. Cartilage and bone adaptation to loading depended on the load level applied and duration and varied by anatomic location.
Cyclic compressive loading of mouse knee joints induced marked alterations in the cartilage matrix, including fibrillation and thinning as well as altered Safranin O staining that reflected compositional changes in proteoglycan content. Similar cartilage changes were reported previously in vivo with tibial loading and long duration treadmill running (17, 25), but subchondral bone changes were not assessed. Compared to treadmill running, tibial loading applies compression to the knee in a flexed loading position and does not replicate the full range of motion experienced during locomotion. In addition to the load-induced cartilage alterations, we demonstrated that the high load magnitude resulted in differential joint tissue responses in young and adult mice, including the extent of cartilage damage, subchondral cortical bone adaptation, and osteophyte formation. In an injurious model of tibial impact loading that noninvasively induced ACL injury in young mice (26), the cartilage and bone adaptive changes were more severe than in our adult high load group and included considerable ectopic bone formation. In contrast to our data for moderate load levels, the observed benefits of exercise activities in rodent models for short duration (6, 7) suggest that either the in vivo loads engendered with exercise are lower than 4.5N or our model is not replicating the beneficial aspect of exercise loading.
Alterations in the composition and structural integrity of cartilage matrix in response to loading also have been demonstrated in vitro. For example, high load levels and long durations of cyclic loading increase chondrocyte death in the superficial zones of bovine cartilage explants (27) and acute impact loading increases proteoglycan release and chondrocyte death (28–30). We also noted in our study that proteoglycan loss and cartilage thinning (Fig. 1) increased with mechanical loading and continued throughout the study period.
Our study did not permit us to define whether the cartilage alterations that we observed depended on the changes in the subchondral bone. However, our findings with limbs loaded for 6 weeks at 9.0N in both young and adult mice demonstrate that the anatomic sites of subchondral cortical bone thickening correspond to the regions of cartilage thinning, suggesting that each tissue responds to the local mechanical environment and to reciprocal changes in the other tissue. Other studies have shown that cartilage degeneration is reduced when subchondral bone turnover and associated increases in cortical thickness are suppressed by alendronate treatment in OA animal models, providing evidence that subchondral bone properties can affect the state of articular cartilage (31, 32). Clinical observations confirm the anatomic association of cartilage damage and subchondral bone sclerosis and increased bone mineral density in the hip and knee joints of OA patients (33). Radin and Rose (34) suggested that the increased stiffness in subchondral bone tissue and the associated changes in the shape and contour of the subchondral plate may adversely affect the capacity of the adjacent articular cartilage to adapt to mechanical loads. Consistent with our findings, recent studies in surgical and spontaneous animal models have shown a close relationship between the process of cartilage damage and subchondral bone histomorphometry (35, 36). Our results demonstrating that regions of increased cortical subchondral thickness in the posterior aspect of tibial plateau correspond with sites of cartilage thinning provide evidence that both tissues respond to the local loading environment. They cannot, however, establish that the changes in the bone are responsible for the alterations in the cartilage or vice versa.
Thickening of the subchondral cortical bone in the loaded limb also may have contributed to the decreased epiphyseal cancellous bone mass, by stress shielding of the underlying epiphyseal bone. In contrast to our results, other studies showed that epiphyseal cortical bone mass decreases at the onset of early OA and subsequently increases at later stages (37, 38). These apparently inconsistent observations may reflect differences in the time of tissue sampling after induction of mechanical alterations, but also may be related to additional features of these models, including, for example, surgical insult or chemically induced inflammation (18, 19).
The subchondral cortical bone on the posterior aspect of the tibial plateau that was thickened with loading corresponds to the location where the femoral condyles contact the plateau in the flexed loading position, indicating that loading is likely the primary cause of the subchondral bone changes. Loading in the posterior tibial plateau during flexion of the knee has been documented in studies of human kinematics in vivo (39, 40). Similar to flexion kinematics in human knees, mouse femurs translate to the posterior tibial plateau due to the presence of the posterior cruciate ligament (41). As a result, flexed knees in our loading model likely experience cyclic compression at the posterior tibial plateau, the location of subchondral cortical bone thickening.
In addition to subchondral cortical and cancellous bone changes, the loaded joints formed periarticular osteophytes. Clinically, osteophytes are a radiographic and anatomic hallmark of OA. Osteophytes are believed to form in response to local mechanical influences at the joint margins but their role in joint load bearing is unclear. With in vivo tibial loading, osteophytes formed at the joint margins only at the high (9N) load level and not in limbs subjected to the moderate (4.5N) load, at least up to 6 weeks. Similar results were reported in models of severe joint instability (42). However, these results contrast with studies reporting osteophyte formation with reduced levels of loading. For example, immobilization of rabbit knees resulted in cartilage degeneration and osteophyte formation (43, 44). Further evidence indicating that joint immobilization can adversely affect cartilage homeostasis is provided by the observation that patients with limited mobility due to ankle arthrodesis or spinal cord injury have increased incidence of cartilage-associated OA features (45, 46).
Alterations in articular cartilage and subchondral bone reported in mice were observed in other OA murine models with altered joint loading environments, such as treadmill running, destabilization of medial meniscus (DMM), collagenase injection, or articular fracture (19). Loss of proteoglycan content and cartilage fibrillation due to mechanical loading in our study is similar to cartilage changes in mice after 18 months of treadmill running or 4 weeks post-DMM surgery (25, 47). Decreased epiphyseal cancellous mass was observed at early time points in mice injected with collagenase (37). However, treadmill running, DMM surgery, and collagenase injection showed limited osteophyte formation in mice, whereas our controlled loading protocol consistently induced osteophyte formation. The location and characteristics of osteophytes were similar to mice injected with TGF-β, suggesting that loading may induce signaling that promotes TGF-β and induces osteophyte formation in our model (48).
The metaphyseal cancellous bone changes that we observed are consistent with findings from previous studies in young male mice, indicating the robustness and reproducibility of our model (20, 49). Unlike our findings in young mice, metaphyseal bone mass in adult mice did not increase with loading in our study. A previous report demonstrated increased cancellous bone mass in response to mechanical loading in adult female mice of a similar age, but the response to loading was reduced compared to that in young female mice (22). Thus, in the adult male mice used in our study, the loading responses in the metaphyseal cancellous region may reflect gender differences or the effects of aging that may contribute to reduced mechano-responsiveness.
Further studies are needed to identify both the mechanical and biological mechanisms underlying the load-induced alterations. Additional mechanical characterization involving different loading protocols, stiffness mapping, joint displacements, and contact stress mapping during loading could provide insights into the underlying mechanisms responsible for the observed changes in joint tissues. Also, protein and gene expression patterns in chondrocytes of mechanically loaded joints should be related to tissue level responses to define the biological mechanisms. The robustness and repeatability of our model, which permits the use of controlled, non-invasive mechanical loading protocols, provides a unique experimental system for defining the relationships between mechanical loading and biological responses that influence cartilage, bone, and other joint tissues in animal models of OA. Elucidating mechanical and biomolecular pathways may lead ultimately to the development of non-pharmacological and pharmacological interventions to treat OA in clinical settings.
Supplementary Material
Acknowledgments
We thank Drs. Cornelia Farnum, Larry Bonassar, Mathias Bostrom, and Chris Hernandez for valuable input. Lyudmila Lukashova, Antonia Hille, Daniel Brooks, and the Cornell CARE staff provided experimental assistance. This work was supported by National Institutes of Health Grants R01-AG028664 and P30-AR046121 (MCHM), R01-AG022021 and RC4-AR060546 (MBG), and NSF GRF (FCK).
Footnotes
Conflict of interest
All authors state that they have no conflict of interest related to this work.
Contributor Information
Frank C. Ko, Email: fck9@cornell.edu.
Cecilia Dragomir, Email: DragomirC@hss.edu.
Darren A. Plumb, Email: PlumbD@hss.edu.
Steven R. Goldring, Email: GoldringS@hss.edu.
Timothy M. Wright, Email: WrightT@hss.edu.
Mary B. Goldring, Email: GoldringM@hss.edu.
Marjolein C.H. van der Meulen, Email: mcv3@cornell.edu.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 3.Kaila-Kangas L, Arokoski J, Impivaara O, Viikari-Juntura E, Leino-Arjas P, Luukkonen R, et al. Associations of hip osteoarthritis with history of recurrent exposure to manual handling of loads over 20 kg and work participation: a population-based study of men and women. Occup Environ Med. 2011;68:734–8. doi: 10.1136/oem.2010.061390. [DOI] [PubMed] [Google Scholar]
- 4.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–36. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 5.Parkkinen JJ, Lammi MJ, Helminen HJ, Tammi M. Local stimulation of proteoglycan synthesis in articular cartilage explants by dynamic compression in vitro. J Orthop Res. 1992;10:610–20. doi: 10.1002/jor.1100100503. [DOI] [PubMed] [Google Scholar]
- 6.Otterness IG, Eskra JD, Bliven ML, Shay AK, Pelletier JP, Milici AJ. Exercise protects against articular cartilage degeneration in the hamster. Arthritis Rheum. 1998;41:2068–76. doi: 10.1002/1529-0131(199811)41:11<2068::AID-ART23>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Galois L, Etienne S, Grossin L, Watrin-Pinzano A, Cournil-Henrionnet C, Loeuille D, et al. Dose-response relationship for exercise on severity of experimental osteoarthritis in rats: a pilot study. Osteoarthritis Cartilage. 2004;12:779–86. doi: 10.1016/j.joca.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905–15. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis. 1999;58:15–9. doi: 10.1136/ard.58.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52:3507–14. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 11.Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001;19:1140–6. doi: 10.1016/S0736-0266(01)00033-X. [DOI] [PubMed] [Google Scholar]
- 12.Thibault M, Poole AR, Buschmann MD. Cyclic compression of cartilage/bone explants in vitro leads to physical weakening, mechanical breakdown of collagen and release of matrix fragments. J Orthop Res. 2002;20:1265–73. doi: 10.1016/S0736-0266(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52:2386–95. doi: 10.1002/art.21215. [DOI] [PubMed] [Google Scholar]
- 14.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 15.Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974–82. doi: 10.1002/art.30498. [DOI] [PubMed] [Google Scholar]
- 16.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 17.Poulet B, Hamilton RW, Shefelbine S, Pitsillides AA. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011;63:137–47. doi: 10.1002/art.27765. [DOI] [PubMed] [Google Scholar]
- 18.Ameye LG, Young MF. Animal models of osteoarthritis: lessons learned while seeking the “Holy Grail”. Curr Opin Rheumatol. 2006;18:537–47. doi: 10.1097/01.bor.0000240369.39713.af. [DOI] [PubMed] [Google Scholar]
- 19.Little CB, Zaki S. What constitutes an “animal model of osteoarthritis”--the need for consensus? Osteoarthritis Cartilage. 2012;20:261–7. doi: 10.1016/j.joca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, et al. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol. 2010;109:685–91. doi: 10.1152/japplphysiol.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–8. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Lynch ME, Main RP, Xu Q, Schmicker TL, Schaffler MB, Wright TM, et al. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone. 2011;49:439–46. doi: 10.1016/j.bone.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18 (Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Cao L, Youn I, Guilak F, Setton LA. Compressive properties of mouse articular cartilage determined in a novel micro-indentation test method and biphasic finite element model. J Biomech Eng. 2006;128:766–71. doi: 10.1115/1.2246237. [DOI] [PubMed] [Google Scholar]
- 25.Lapvetelainen T, Nevalainen T, Parkkinen JJ, Arokoski J, Kiraly K, Hyttinen M, et al. Lifelong moderate running training increases the incidence and severity of osteoarthritis in the knee joint of C57BL mice. Anat Rec. 1995;242:159–65. doi: 10.1002/ar.1092420204. [DOI] [PubMed] [Google Scholar]
- 26.Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JH, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20:773–82. doi: 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Chen CT, Bhargava M, Lin PM, Torzilli PA. Time, stress, and location dependent chondrocyte death and collagen damage in cyclically loaded articular cartilage. J Orthop Res. 2003;21:888–98. doi: 10.1016/S0736-0266(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 28.Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19:242–9. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 29.Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Arch Biochem Biophys. 1995;322:87–96. doi: 10.1006/abbi.1995.1439. [DOI] [PubMed] [Google Scholar]
- 30.Torzilli PA, Grigiene R, Borrelli J, Jr, Helfet DL. Effect of impact load on articular cartilage: cell metabolism and viability, and matrix water content. J Biomech Eng. 1999;121:433–41. doi: 10.1115/1.2835070. [DOI] [PubMed] [Google Scholar]
- 31.Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, et al. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 32.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong le T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234–43. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage. 2004;12 (Suppl A):S10–9. doi: 10.1016/j.joca.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986:34–40. [PubMed] [Google Scholar]
- 35.Botter SM, Glasson SS, Hopkins B, Clockaerts S, Weinans H, van Leeuwen JP, et al. ADAMTS5−/− mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: implications for a link between cartilage and subchondral bone changes. Osteoarthritis Cartilage. 2009;17:636–45. doi: 10.1016/j.joca.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Pastoureau P, Leduc S, Chomel A, De Ceuninck F. Quantitative assessment of articular cartilage and subchondral bone histology in the meniscectomized guinea pig model of osteoarthritis. Osteoarthritis Cartilage. 2003;11:412–23. doi: 10.1016/s1063-4584(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 37.Botter SM, van Osch GJ, Clockaerts S, Waarsing JH, Weinans H, van Leeuwen JP. Osteoarthritis induction leads to early and temporal subchondral plate porosity in the tibial plateau of mice: an in vivo microfocal computed tomography study. Arthritis Rheum. 2011;63:2690–9. doi: 10.1002/art.30307. [DOI] [PubMed] [Google Scholar]
- 38.Intema F, Sniekers YH, Weinans H, Vianen ME, Yocum SA, Zuurmond AM, et al. Similarities and discrepancies in subchondral bone structure in two differently induced canine models of osteoarthritis. J Bone Miner Res. 2010;25:1650–7. doi: 10.1002/jbmr.39. [DOI] [PubMed] [Google Scholar]
- 39.Li G, DeFrate LE, Park SE, Gill TJ, Rubash HE. In vivo articular cartilage contact kinematics of the knee: an investigation using dual-orthogonal fluoroscopy and magnetic resonance image-based computer models. Am J Sports Med. 2005;33:102–7. doi: 10.1177/0363546504265577. [DOI] [PubMed] [Google Scholar]
- 40.Moro-oka TA, Hamai S, Miura H, Shimoto T, Higaki H, Fregly BJ, et al. Dynamic activity dependence of in vivo normal knee kinematics. J Orthop Res. 2008;26:428–34. doi: 10.1002/jor.20488. [DOI] [PubMed] [Google Scholar]
- 41.Dennis DA, Komistek RD, Hoff WA, Gabriel SM. In vivo knee kinematics derived using an inverse perspective technique. Clin Orthop Relat Res. 1996:107–17. doi: 10.1097/00003086-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13:632–41. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Langenskiold A, Michelsson JE, Videman T. Osteoarthritis of the knee in the rabbit produced by immobilization. Attempts to achieve a reproducible model for studies on pathogenesis and therapy. Acta Orthop Scand. 1979;50:1–14. doi: 10.3109/17453677909024083. [DOI] [PubMed] [Google Scholar]
- 44.Smith RL, Thomas KD, Schurman DJ, Carter DR, Wong M, van der Meulen MC. Rabbit knee immobilization: bone remodeling precedes cartilage degradation. J Orthop Res. 1992;10:88–95. doi: 10.1002/jor.1100100111. [DOI] [PubMed] [Google Scholar]
- 45.Takakura Y, Tanaka Y, Sugimoto K, Akiyama K, Tamai S. Long-term results of arthrodesis for osteoarthritis of the ankle. Clin Orthop Relat Res. 1999:178–85. doi: 10.1097/00003086-199904000-00023. [DOI] [PubMed] [Google Scholar]
- 46.Vanwanseele B, Eckstein F, Knecht H, Spaepen A, Stussi E. Longitudinal analysis of cartilage atrophy in the knees of patients with spinal cord injury. Arthritis Rheum. 2003;48:3377–81. doi: 10.1002/art.11367. [DOI] [PubMed] [Google Scholar]
- 47.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 48.van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994;71:279–90. [PubMed] [Google Scholar]
- 49.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Bone mass is preserved and cancellous architecture altered due to cyclic loading of the mouse tibia after orchidectomy. J Bone Miner Res. 2008;23:663–71. doi: 10.1359/JBMR.080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.