Abstract
Podocytes are specialized cells that contribute critically to the normal structure and function of the glomerular filtration barrier. Their depletion plays an important role in the pathogenesis of glomerulosclerosis. Here, we report generation of a genetic model of conditional podocyte ablation and regeneration in zebrafish using a bacterial nitroreductase strategy to convert a prodrug, Metronidazole, into a cytotoxic metabolite. A transgenic zebrafish line was generated that expresses a green fluorescence protein (GFP) and the nitroreductase fusion protein under the control of the podocin promoter Tg(podocin:nitroreductase-GFP). Treatment of these transgenic zebrafish with Metronidazole results in podocyte apoptosis, a loss of nephrin and podocin expression, foot process effacement, and a leaky glomerular filtration barrier. Following Metronidazole washout, proliferating cells were detected in the glomeruli of recovering transgenic fish with a restoration of nitroreductase-GFP fluorescence, nephrin and podocin expression, a reestablishment of normal foot process architecture and glomerular barrier function. Thus, our studies show that zebrafish podocytes are capable of regenerating following depletion and establish the Tg(podocin:NTR-GFP) fish as a new model to study podocyte injury and repair.
Keywords: Podocyte, nitroreductase, zebrafish, ablation, kidney regeneration
Introduction
The kidney is a vital organ that performs a number of essential functions including blood filtration and clearance of endogenous waste products. Podocytes are specialized epithelial cells that contribute critically to the kidney’s “filtration apparatus”. Podocyte dysfunction and/or damage has been associated with both acute and chronic glomerular diseases including focal segmental glomerulosclerosis, diabetic nephropathy and HIV nephropathy (1, 2, 3, 4–6). Podocyte depletion leads to glomerulosclerosis in murine models and recent studies suggest that short-lived localized insults can trigger a cascade of secondary damage that causes more global injury (7–9). Understanding how podocytes respond to injury and whether they are capable of regeneration will provide valuable information for the development of new therapies that seek to replace damaged or lost podocytes (10).
The Zebrafish is a widely used vertebrate model organism for the study of developmental mechanisms and disease pathologies for many organs. It combines many advantages including genetic tractability of both forward and reverse genetics, accessibility to observation and manipulation during organogenesis, and a great capability for regeneration after injury(11–18). Studies from multiple groups have established zebrafish as a useful model system to study kidney development and function(19–22). Despite the structural simplicity of the zebrafish pronephros, consisting of a single glomerulus in connection with two pronephric tubules, it possesses a glomerular filtration apparatus with a similar complexity to that of the mammalian kidney(21, 23). More recently, it has been utilized as an alternative in vivo model for studying kidney injury and regeneration (16, 24–26).
The zebrafish kidney has a remarkable ability to regenerate after injury, and kidney stem/progenitor cells have been identified in adults(16, 26). Similar to a recent report (27), we have independently established two transgenic zebrafish lines where green fluorescence protein (GFP) and a fusion protein of GFP and the bacterial nitroreductase (NTR) are expressed in podocytes under the control of the podocin promoter. In the Tg(podocin:GFP) line, the podocytes are fluorescently tagged allowing them to be visualized, isolated, and tracked in vivo whereas the Tg(podocin:NTR-GFP) line utilizes bacterial NTR to convert the nontoxic pro-drug metronidazole (Mtz) into a cytotoxic, DNA cross-linking agent that induces cell death(28, 29). Here, we report that specific podocyte ablation and glomerular dysfunction occurs in Tg(podocin:NTR-GFP) embryos after treatment with Mtz. Interestingly, following Mtz washout there is a recovery of glomerular filtration barrier function that is associated with podocyte proliferation in the glomerulus and a restoration of normal podocyte foot-process architecture. These findings suggest that zebrafish podocytes are capable of regeneration following depletion and establish the Tg(podocin:NTR-GFP) line as a useful model to identify new therapeutic targets involved in the response of podocytes to injury.
Results and Discussion
Expression of GFP and GFP-NTR under the control of podocyte specific podocin promoter
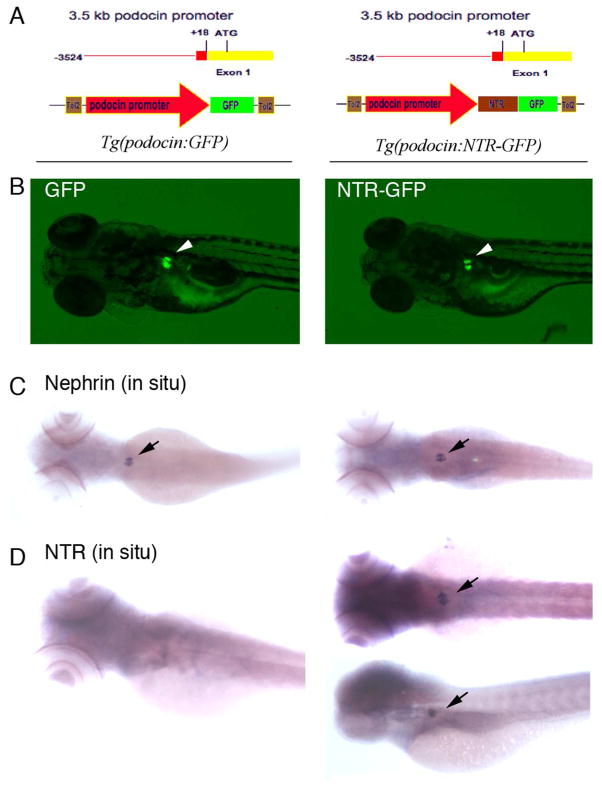
We isolated a 3.5-kb DNA fragment located upstream of the podocin gene that has previously been found to contain the mouse podocin promoter (30, 31). We subsequently ligated GFP and GFP-NTR under the control of this promoter in the Tol2 transposon vector and injected zebrafish embryos with these constructs (32)(Fig. 1A). By out-crossing with wild type fish, we identified 4 independent founders for both transgenic fish lines, Tg(podocin:GFP) and Tg(podocin:NTR-GFP) respectively. Embryos from each of the founders displayed identical expression patterns in which GFP was expressed exclusively in the region of the pronephric glomerulus from 60 hours post-fertilization (hpf) by fluorescence microscopy (Fig. 1B). The founders with the strongest GFP expression were used to collect embryos for study and line maintenance. Consistent with these lines expressing GFP in podocytes, we found that the GFP signal localized to the site of nephrin expression (Fig. 1C), and they both colocalized with the site of NTR expression in Tg(podocin:NTR-GFP) laval fish (Fig. 1D).
Fig. 1. GFP and NTR-GFP expression under the 3.5-kb podocin promoter.
A. Constructs used to generate the podocin driven GFP (left panel) and NTR-GFP (right panel) transgenic lines are shown respectively. B. GFP expression in glomeruli from transgenic fish Tg(podocin:GFP)(left panel, arrow) and Tg(podocin:NTR-GFP)(right panel, arrow). C. GFP expression (panels in B) overlaps with nephrin expression in glomeruli from Tg(podocin:GFP) (left panel, arrow) and Tg(podocin:NTR-GFP) (right panel, arrow) embryos. D. NTR is expressed in glomeruli from Tg(podocin:NTR-GFP)(right panel, arrows), but absent in glomeruli from Tg(podocin:GFP) embryos (left panel).
Conditional ablation of podocytes results in a loss of podocyte marker expression and the slit diaphragm, and defective glomerular barrier function
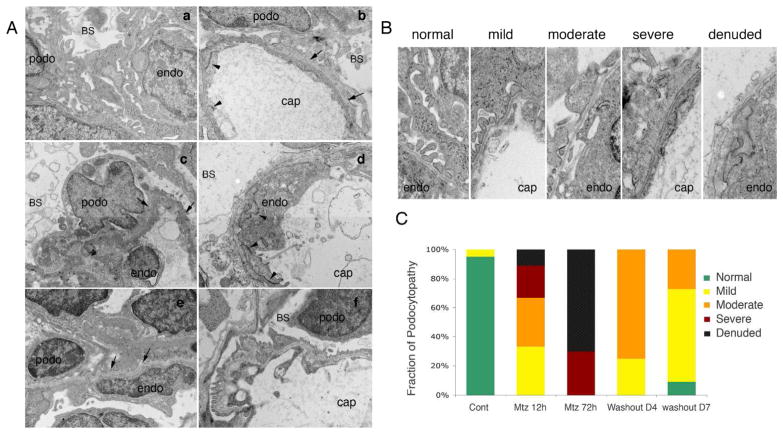
We next determined the conditions under which Mtz will induce conditional ablation of podocytes. Wild type, Tg(podocin:GFP) and Tg(podocin:NTR-GFP) larval fish at 70 hpf were incubated with Mtz for 12–48 hours at concentrations ranging from 1–20 mM. Exposure to Mtz for 12 hours resulted in pericardial edema in Tg(podocin:NTR-GFP) larval fish, consistent with renal failure (Fig. 2A). The extent of pericardia edema was more pronounced with increasing Mtz concentration or prolonged exposure even when low (2 mM) concentration of Mtz was used (data not shown). Concomitant with the presence of pericardial edema, the intensity of the GFP signal in the glomerulus of Mtz treated Tg(podocin:NTR-GFP) larval fish was significantly reduced in a dose dependent fashion (Fig 2B). A robust effect was found when Tg(podocin:NTR-GFP) embryos were exposed to Mtz at 4 or 10 mM for 12 hours with ~95% (n=41/43) of the animals showing a dramatic reduction or loss of GFP fluorescence in the glomerulus (Fig. 2B and 2C, b). No effects on GFP signal or the appearance of pericardial edema was observed in Mtz treated Tg(podocin:GFP) embryos for 12 or 48 hr (Fig. 2A left panel, and 2C, a). When Mtz concentrations >20 mM were used we observed non-specific toxicity, characterized by necrosis of the larva without significant pericardial edema in all groups (Tg(podocin:NTR-GFP), Tg(podocin:GFP) and wild type fish; data not shown). Whole mount in situ hybridization showed that the loss of GFP fluorescence induced by Mtz was concomitant with loss of the expression of nephrin (Fig. 2C, d and Fig. 4A, j and k) and podocin in the glomerulus (Fig. 4A, f and g). Despite significant edema and reduced expression of GFP/nephrin/podocin induced by Mtz in Tg(podocin:NTR-GFP) animals, we did not detected any abnormalities or change of gene expression in Mtz treated Tg(podocin:GFP) and wild type larval fish, or in Tg(podocin:NTR-GFP) larval fish without Mtz treatment. Ultrastructural examination of the glomerulus from Mtz treated Tg(podocin:NTR-GFP) larval fish by electron microscopy (EM) revealed the presence of podocyte foot process effacement (Fig. 3A, b). A more severe disruption in foot process architecture and areas of podocyte denudation was detected in animals following exposure to Mtz for 72 hours (Fig. 3A, d). Consistent with this, quantitation of the podocytopathy by classifying the areas of injury into mild, moderate, severe, and denuded (Fig. 3B), according to established methods (25), confirmed that 72 hrs of Mtz treatment caused greater injury than 12 hrs of Mtz treatment (Fig. 3C). Interestingly, despite severe damage of podocytes in some of the Mtz treated fish, the morphology of the glomerular basement membrane and the endothelium remained well preserved (Fig. 3A, d), indicating that the NTR mediated cell damage is confined to podocytes.
Fig. 2. Metronidazole induces podocyte specific ablation.
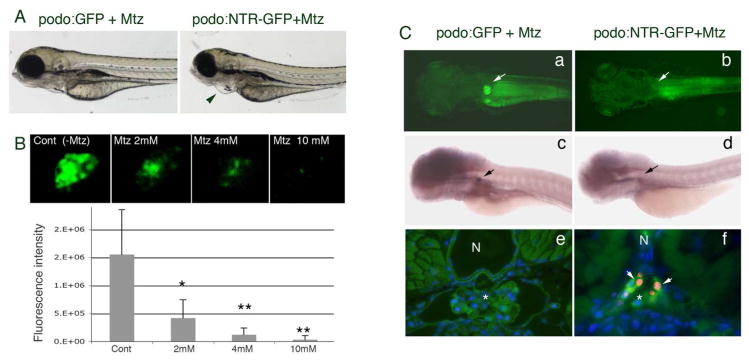
A. Mtz induced pericardial edema is seen in Tg(podocin:NTR-GFP) fish (right panel, indicated by the arrowhead) but not in Tg(podocin:GFP) fish (left panel). B. Treatment with Mtz results in attenuation and eventually loss of the GFP signal in the glomerulus of Tg(podocin:NTR-GFP) animals in a dose dependent manner (live images of glomeruli are shown in the upper panels and quantitation of fluorescence is shown in the lower graph). * indicates p<0.05, ** indicates p<0.01. C. A loss of GFP fluorescence and nephrin transcripts is seen in the glomeruli of Tg(podocin:NTR-GFP) fish (b and d respectively), but not in Tg(podocin:GFP)(a and c respectively) after treatment with Mtz for 12 hours. Arrows indicate the glomerulus. TUNEL-positive apoptotic cells (red) that co-label with the anti-panCrb antibody (green) are detected in the glomerulus of Mtz treated Tg(podocin:NTR-GFP) animals (f, arrows), but not in Mtz treated Tg(podocin:GFP) fish (e). N, notochord. * indicates the glomerulus.
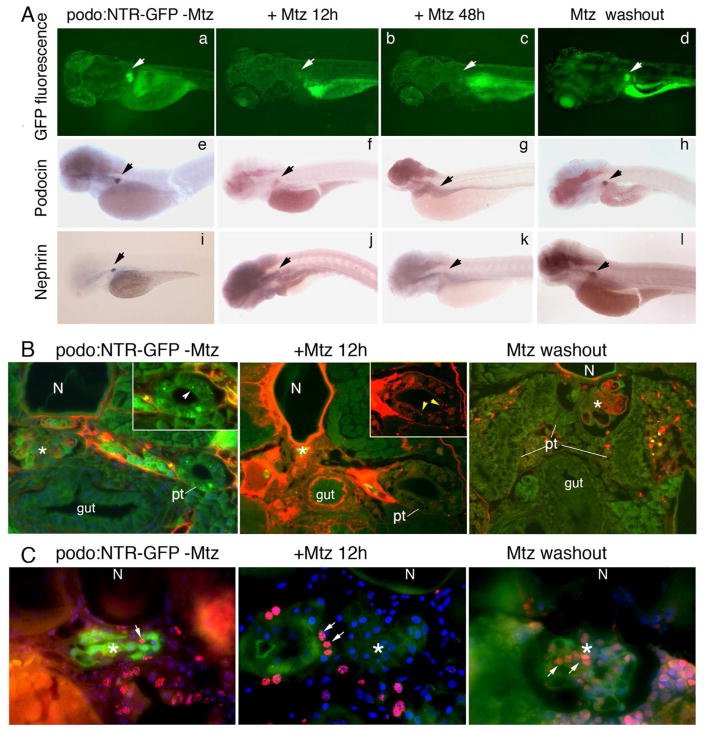
Fig. 4. Dynamic changes in gene expression, glomerular function, and cell proliferation during Mtz induced podocyte injury and recovery after Mtz washout.
A. GFP fluorescence is reduced and then absent after Mtz treatment for 12 and 48 hours, respectively (b–c), but reappears at 7 days after Mtz wash out (d). Arrows indicate the glomerulus. Whole mount in situ hybridization for podocin (f–h) and nephrin (j–l) transcripts reveals the concomitant loss and re-appearance of expression corresponding with the change in GFP fluorescence in the glomerulus of Mtz-treated Tg(podocin:NTR-GFP) animals. No change in GFP expression (a), or podocin (e) and nephrin (i) expression is seen in the glomerulus of Tg(podocin:NTR-GFP) animals without Mtz treatment. B. Assessment of glomerular filtration function using the rhodamine-conjugated albumin filtration assay. 5–6 hours after retro-orbital injection of rhodamine-albumin, rhodamine-albumin containing vesicles are detected inside pronephric tubule cells of Mtz treated Tg(Podocin:NTR-GFP) fish (middle panel, inset shows a higher magnified view of the tubule with arrowheads indicating red rhodamine-albumin containing vesicles inside the proximal tubular cells). Rhodamine-positive vesicles are not seen in untreated Tg(Podocin:NTR-GFP) control fish, although the uptake of freely filtered 10 kDa FITC-dextran is detected in these animals (left panel, arrowhead in inset indicates green FITC-dextran containing vesicles). No rhodamine-positive vesicles are seen in the pronephric tubule cells in recovered Tg(Podocin:NTR-GFP) animals at 7 days post Mtz washout (right panel). C. Detection of proliferating cells by BrdU incorporation. A small number of BrdU-positive cells are seen in the glomerulus of untreated Tg(podocin:NTR-GFP) laval fish (left panel, arrow indicates the red-colored BrdU signal in the nucleus. Green fluorescence marks NTR-GFP expressing podocytes). In Tg(Podocin:NTR-GFP) fish larvae treated with Mtz for 12 hours, despite the presence of BrdU labeling in neighboring cells of the glomerulus and pronephric tubules (arrows), almost no BrdU incorporation is detected in the glomerulus and the GFP fluorescence of podocytes is significant reduced (middle panel). Greatly increased BrdU staining is detected in the glomerulus of Tg(podocin:NTR-GFP) fish at 7 days post Mtz washout (right panel) (arrows). Overall GFP fluorescence is also increased in the glomerulus of these recovered animals. Some of the BrdU labeled cells are apparently also expressing GFP. N, notochord. Pt, pronephric tubule. * indicates glomerulus*.
Fig. 3. Ultrastructural examination of podocytes in Mtz treated Tg(podocin:GFP) and Tg(podocin:NTR-GFP) fish larvae.
A. Electron microscopy examination of Mtz treated Tg(podocin:GFP) larval fish shows normal podocyte morphology, intact foot processes and normal appearing glomerular basement membrane (a). Examination of Tg(podocin:NTR-GFP) fish treated for 12 hours with Mtz reveals the presence of foot process enfacement (b and c, indicated by arrows). Chromatin condensation and early nuclear fragmentation in podocytes is clearly seen at 12 hours post exposure to Mtz indicating podocyte apoptosis (c). A complete loss of foot process and significant podocyte destruction are observed in fish treated with Mtz for 72 hours (d), however, the morphology of neighboring endothelial cells appear grossly normal with intact intercellular junctional structures and glomerular basement membrane (b and d, arrowheads indicate intercellular junctional structures). 4 days after Mtz washout, foot process-like structures appear attached to the glomerular basement membrane in the glomerulus (arrows in e). 7 days after washout, near complete recovery of foot processes and slit diaphragms are found in the glomerulus (f). B. The change in podocyte ultrastructure in response to Mtz treatment and subsequent recovery after Mtz washout is categorized into mild, moderate, severe and denuded injuries based on established methods (25). C. Quantitation of damage of podocytes after Mtz treatment and podocyte recovery 4 day and 7 days after Mtz washout. Podo, podocyte. Endo, endothelial cell. BS, Bowman’s space. Cap, capillary space.
NTR is known to induce cell death by converting Mtz into a DNA cross-linking agent (33). In line with this, chromatin condensation, a hallmark of the onset of apoptosis, was clearly detected by EM in some of the podocytes in Mtz treated Tg(Podocin:NTR-GFP) larval fish (Fig. 3A, c). We further investigated Mtz induced podocyte apoptosis using the TUNEL assay (Fig. 2C, e and f). While no apoptotic cells were detected in the glomeruli of Mtz treated wild type (data not shown) and Tg(Podocin:GFP) (Fig. 2C, e) controls (n=30 respectively), we observed strong apoptotic signals in the glomeruli of Mtz-treated Tg(Podocin:NTR-GFP) animals (n=30) (Fig. 2C, f). These apoptotic cells co-stained with the anti-panCrb antibody, which recognizes the Crumbs protein Crb2b on podocytes (34), confirming that the dying cells were podocytes (Fig. 2C, f). Taken together, these results indicate that apoptosis of podocytes is responsible for the loss of GFP fluorescence and nephrin/podocin expression in the glomeruli of Mtz-treated Tg(Podocin:NTR-GFP) animals. However, we cannot rule-out that NTR-induced injury downregulates podocin and nephrin expression independently of podocyte cell death or that podocyte cell death causes secondary damage to surrounding cells such as the endothelium, pronephric tubules, and mesangial cells. Interplays between podocyte, endothelial cells and mesangial cell has been suggested to be critical for the survival and/or development of the complex architecture and function of the glomerulus (35–38).
Albuminuria is considered to be a hallmark of glomerulopathy and has been widely used as an important clinical marker for diagnosis, monitoring disease progression and remission in patients with chronic kidney diseases (39, 40). To functionally assess the effects of podocyte depletion on the glomerular filtration barrier of Mtz-treated Tg(Podocin:NTR-GFP) animals, we injected rhodamine-conjugated albumin into the circulation of larval fish. If the glomerular filtration barrier is compromised, the albumin tracer will pass into the pronephros and be taken up by proximal tubule cells. 5–6 hours after injection, rhodamine-albumin containing vesicles were detected inside the cells of the pronephric tubules in Mtz treated Tg(Podocin:NTR-GFP) larval fish (Fig. 4B, middle), but not in Tg(Podocin:NTR-GFP) larval fish without Mtz treatment (Fig. 4B, left panel) or in Mtz treated Tg(Podocin:GFP) fish (data not shown).
Structural and functional recovery of podocytes after NTR/Mtz induced podocyte ablation
Although still controversial in mammals, recent studies have shown that the zebrafish kidney contains renal stem/progenitor cells and regenerates after acute kidney injury(16, 26). To investigate the regenerative capacity of podocytes after NTR/Mtz-mediated ablation, we performed Mtz washout experiments. After treatment with 4 mM Mtz for 12 hours, Mtz was washed away and the fish were observed periodically under light and fluorescence microscope to assess their pericardial edema and glomerular GFP fluorescence. Four days post Mtz washout, weak but visible GFP signal re-appeared in the glomeruli of Tg(podocin:NTR-GFP) animals. By 7 days post Mtz washout, the glomerular GFP fluorescence had become more intense and both nephrin and podocin transcripts could be re-detected (Fig. 4A, d, h, l). In addition, the pericardial edema had resolved (data not shown).
To determine whether the re-appearance of GFP fluorescence and expression of podocin and nephrin also results in a corresponding recovery in the structure of the previously ablated glomerulus, we examined podocyte ultrastructure by EM. A re-establishment of slit diaphragms was found in Mtz-treated Tg(podocin:NTR-GFP) animals starting at 4 days post Mtz washout although the majority of podocytes displayed a ‘moderate’ degree of effacement at this stage (Fig. 3A, e, and 3C). At 7 days post Mtz washout, well-formed foot processes linked by slit diaphragms were found with the majority of podocytes showing only a ‘mild’ degree of effacement (Fig. 3A, f and 3C). A recovery in the glomerular barrier function was examined by the rhodamine conjugated-bovine albumin filtration. In Tg(podocin:NTR-GFP) animals 7 days post Mtz washout, no rhodamine-albumin-positive vesicles were detected in the proximal tubules (Fig. 4B, right panel) consistent with a functional recovery of the NTR/Mtz damaged glomerulus.
To understand whether podocyte proliferation contributes to the recovery of the NTR/Mtz-damaged glomerulus, cell proliferation was examined by 5-bromo-2-deoxyuridine (BrdU) labeling. BrdU was injected into circulation and two to four hours later, larval fish were fixed and BrdU incorporation detected by immunofluorescence staining using anti-BrdU antibody. A low level of BrdU incorporation was seen in glomeruli from Tg(podocin:NTR-GFP) larval fish without Mtz treatment (Fig. 4C, left panel). Despite the presence of BrdU labeling in neighboring cells of the glomerulus and pronephric tubules, almost no BrdU incorporation was detected in the glomeruli in Tg(Podocin:NTR-GFP) larval fish treated with Mtz for 12 hours. The GFP fluorescence signal was also significantly reduced in the glomerulus of these animals (Fig. 4C, middle panel). However, greatly increased BrdU labeling was observed in Tg(podocin:NTR-GFP) animals 7 days post Mtz washout. The majority of these BrdU labeled cells also expressed GFP, consistent with being podocytes and implicating podocyte proliferation in the glomerular recovery from NTR-induced injury.
In summary, we have shown here the utility of the Mtz/NTR system to cause glomerular damage by using the podocin promoter to specifically restrict the apoptotic-inducing effects of NTR to podocytes. This system is temporally inducible, highly efficient, and the duration of the ablation can be exquisitely controlled by washout experiments. Using this genetic tool, we demonstrated that podocyte depletion leads to effacement and a loss of slit diaphragms and glomerular barrier function. Remarkably, about a week following Mtz washout, the glomerulus is able to recover functionality, restoring foot process architecture and filtration integrity. This regeneration is associated with podocyte proliferation, suggesting that zebrafish podocytes can re-enter the cell cycle and replenish cells lost to injury. Alternatively, new podocytes may be derived from a resident stem/progenitor cell population, such as the putative CD133+CD24+CD106+ stem cells identified in the Bowman’s capsule of human glomeruli (41). By combining this tool with forward genetic or chemical screens it should now be possible to identify new genes and novel compounds that accelerate podocyte recovery from injury
CONCISE METHODS
Zebrafish culture
Wild type and transgenic zebrafish (Danio rerio) embryos, larvae and adult fish were raised and maintained under standard laboratory conditions(42).
Chemicals, reagents and antibodies
Rhodamine B isothiocyanate conjugated bovine albumin, FITC-dextran 10 kDa, 5-bromo-2-deoxyuridine (BrdU), Metronidazole (Mtz) were purchased from Sigma-Aldrich (St. Louis MO). The terminal deoxynucleotidyl transferase–mediated deoxyuridinetriphosphate nick end-labeling (TUNEL)-In Situ Cell Death Detection Kit, TMR red was from Roche (catalog no. 12156792910). Mouse anti –BrdU antibody was purchased from Invitrogen. The rabbit anti-panCrb antibody was a kind gift from Dr. J. Malicki (34, 43). Fluorescence conjugated secondary antibodies were obtained from Jackson laboratory.
Generation of Podocin driven GFP and GFP-Nitroreductase constructs and zebrafish lines
A 3540 bp DNA fragment from zebrafish genomic DNA corresponding to the 5′ end of the zebrafish podocin gene was cloned by PCR amplification using primers (forward) 5′-TACGCTTGAGCAACTAAATGAATGGC-3; (reverse) 5′-GTGAAGTGTCCTCTGGTGTTTGG-3′. The PCR fragment was subsequently cloned into a pGEM-T Easy vector (pGEM-PodP). To generate the constructs for Tg(podocin:NTR-GFP) and Tg(podocin:GFP) transgenic fish, we use the pTol2-Slc2a15b-NTR/GFP and pTol2-Slc2a15b-GFP constructs (Dr. Davidson, NZ) as templates to replace the Slc2a15b promoter with podocin promoter fragment. After obtaining pTol2-podocin:GFP and pTol2-podocin:NTR-GFP constructs, they were co-injected with Tol2 transposase RNA(32) into two-cells stage zebrafish embryos for genomic integration and generating stable transgenic lines. Adult carriers of Tg(podocin:GFP) and Tg(podocin:NTR-GFP) were identified by screening their progeny for GFP fluorescence. Adult fish expressing transgene were out-crossed to wild type fish to obtain the germ-line transgenics.
NTR/Metronidazole mediated podocyte ablation
Zebrafish embryos were collected from timed pair mating of F1 Tg(podocin:GFP), Tg(podocin:GFP-NTR) and wild-type fish about 60–70 hpf (with visible GFP fluorescence in the glomerulus) and dechorionated. Mtz was freshly prepared in 0.1% ETOH and added to fish water. The larval fish were treated with various concentrations of Mtz for 12, 24, 48 and 72 hours in the dark. For recovery experiment, the Mtz-containing medium was replaced with 3–4 changes of fresh embryo medium, and embryos/ larvae were returned to 28°C, and monitored every 6–12 hours. Minimal 15 embryos/larvae were in each group under each treatment condition. Each experiment was repeated for at least three times. The control experiment was set up as following: wild-type +ETOH (control 1); Tg(Podocin:NTR-GFP) + ETOH (control 2); wild-type +Mtz (control 3) and Tg(podocin:GFP) +Mtz (control 4) and Tg(podocin:NTR-GFP) +Mtz (experiment). The morphology of the fish and the intensity of the fluorescence signal in the glomerulus were monitored by stereomicroscope and fluorescence microscope respectively.
Whole mount in situ hybridization
Whole mount in situ hybridization was performed as previously reported (44) with the modification of longer proteinase K treatment of 30 minutes for 4 dpf embryos and 1 hour of treatment for fish at or beyond 7 dpf.
Transmission electron microscopy
Zebrafish larvae were first fixed in 4% paraformaldehyde/PBS at 4°C overnight, then transferred to 2.0% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4 (Electron Microscopy Sciences, Hatfield, PA) overnight at 4°C, then rinsed in cacodylate buffer and post-fixed in 1.0% osmium tetroxide in cacodylate buffer for one hour at room temperature, followed by dehydration through a graded series of ethanol to 100%. They were then infiltrated with Epon resin (Ted Pella, Redding, CA) in a 1:1 solution of Epon: 100% ethanol overnight on a rotator. The following day, they were embedded in fresh Epon at 60°C overnight. Thin sections were cut on a Leica EM UC7 ultramicrotome, collected onto formvar-coated grids and stained with uranyl acetate and lead citrate. All grids were examined in a JEOL JEM 1011 transmission electron microscope at 80 kV. Images were collected using an AMT digital imaging system (Advanced Microscopy Techniques, Danvers, MA).
TUNEL cell death assay
The terminal deoxynucleotidyl transferase–mediated deoxyuridinetriphosphate nick end-labeling (TUNEL) assay was performed using the In Situ Cell Death Detection Kit, TMR red (Roche catalog no. 12156792910) and follow manufacturer’s instruction with modification. After staining, fish embryos were embedded in 2% agarose block, dehydrated and embedded in JB-4 resin (Polysciences). After polymerization, the resin block was cut into 5 μM-thick section, mounted and viewed under fluorescence microscopy.
Glomerular filtration assay
Zebrafish larvae aged 3.5–4 dpf were anaesthetized with Tricaine prior to injections. Rhodamine B isothiocyanate conjugated bovine albumin (Sigma) was diluted in PBS to make a final concentration of 1 mg/ml. FITC-dextran 10 kDa was diluted to 10mg/ml in PBS. Approximately 23 nl of rhodamine-albumin or FITC-dextran solution was injected into retro-orbital vasculature in each animal. Six hours after injection, animals were harvested and fixed in 4% paraformaldehyde/PBS, and processed for embedding in JB-4 resin as mentioned previously. Finally the block was sectioned and viewed directly under fluorescence microscopy. In addition to rhodamine conjugated-albumin, FITC-dextran of 10 kDa (10 mg/ml, 23 nl total) was also injected into each animal as an internal control. In some of the recovery experiments, 10 kDa FITC-dextran was avoided because of the interference of FITC dextran signal with the re-appearance of GFP signal in recovered podocytes. 10 kDa FITC dextran is freely filtered by the glomerulus into the tubules system and be uptake by tubular cells. However, the rhodamine labeled albumin will not be filtered under normal circumstance by the glomerulus and therefore will not appear in the tubular system/tubular cells unless there is a leakiness/destruction of the glomerular filtration barrier. This assay has frequently been used as a readout of the permeability/barrier function of the glomerulus in animals (45).
Cell proliferation assay- BrdU incorporation
5-bromo-2-deoxyuridine (BrdU) (Sigma) was diluted in PBS to make a final concentration of 100 uM. Approximate 23 nl of the BrdU solution was injected into each animal. Two hours after BrdU injection, animals were harvested and fixed in 4% paraformaldehyde/PBS and processed for JB4 embedding. After section, tissues slices were stained with anti-BrdU antibody following manufacturer’s instruction (Invitrogen).
Acknowledgments
We thank Dr. Dennis Brown for assistance with electron microscopy analysis. Dr. Yawei Kong for assistance with generating the constructs and transgenic zebrafish. Drs. J. Malicki, Lem (tufts University, Boston), Tepass and Silva-Gagliardi (University of Toronto, Canada) for providing anti-crumbs antibody (panCrb). Dr. Iain Drummond for assistance with image analysis of zebrafish and providing constructs for probe generation. We thank Renee Ethier and David Machon in the zebrafish facility at Massachusetts General Hospital for their dedicated support. Dr. Patricia K. Donahoe for providing support for image acquiring and analysis. Dr. A.J Davidson is supported by the Rutherford Foundation, Marsden Fund, and the Auckland Medical Research Foundation. H. J. Lu is supported by an NIH KO8 grant DK075940, NIH RO3 DK084295 and a Gottschalk research grant from the American Society of Nephrology (ASN). The zebrafish facility is supported by the Center for Regenerative Medicine (MGH). The Program in Membrane Biology receives additional support from the Boston Area Diabetes and Endocrinology Research Center (NIH DK-57521) and from the Center for the Study of Inflammatory Bowel Disease (NIH DK-43351).
Footnotes
COMPETING FINANCIAL INTERESTS
None
References
- 1.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 2.Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol. 2007;22:2011–2022. doi: 10.1007/s00467-007-0524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno JA, Sanchez-Nino MD, Sanz AB, et al. A slit in podocyte death. Curr Med Chem. 2008;15:1645–1654. doi: 10.2174/092986708784911542. [DOI] [PubMed] [Google Scholar]
- 4.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 5.Hishiki T, Shirato I, Takahashi Y, et al. Podocyte injury predicts prognosis in patients with iga nephropathy using a small amount of renal biopsy tissue. Kidney Blood Press Res. 2001;24:99–104. doi: 10.1159/000054214. [DOI] [PubMed] [Google Scholar]
- 6.Lemley KV, Lafayette RA, Safai M, et al. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–1485. doi: 10.1046/j.1523-1755.2002.00269.x. [DOI] [PubMed] [Google Scholar]
- 7.D’Agati VD. Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts) Kidney Int. 2008;73:399–406. doi: 10.1038/sj.ki.5002655. [DOI] [PubMed] [Google Scholar]
- 8.Matsusaka T, Xin J, Niwa S, et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16:1013–1023. doi: 10.1681/ASN.2004080720. [DOI] [PubMed] [Google Scholar]
- 9.Macary G, Rossert J, Bruneval P, et al. Transgenic mice expressing nitroreductase gene under the control of the podocin promoter: a new murine model of inductible glomerular injury. Virchows Arch. 2009 doi: 10.1007/s00428-009-0840-9. [DOI] [PubMed] [Google Scholar]
- 10.Ortmann J, Amann K, Brandes RP, et al. Role of podocytes for reversal of glomerulosclerosis and proteinuria in the aging kidney after endothelin inhibition. Hypertension. 2004;44:974–981. doi: 10.1161/01.HYP.0000149249.09147.b4. [DOI] [PubMed] [Google Scholar]
- 11.Fishman MC, Stainier DY. Cardiovascular development. Prospects for a genetic approach. Circ Res. 1994;74:757–763. doi: 10.1161/01.res.74.5.757. [DOI] [PubMed] [Google Scholar]
- 12.Ingham PW. Zebrafish genetics and its implications for understanding vertebrate development. Hum Mol Genet. 1997;6:1755–1760. doi: 10.1093/hmg/6.10.1755. [DOI] [PubMed] [Google Scholar]
- 13.Houdebine LM, Chourrout D. Transgenesis in fish. Experientia. 1991;47:891–897. doi: 10.1007/BF01929879. [DOI] [PubMed] [Google Scholar]
- 14.Zon LI. Zebrafish: a new model for human disease. Genome Res. 1999;9:99–100. [PubMed] [Google Scholar]
- 15.Dong J, Stuart GW. Transgene manipulation in zebrafish by using recombinases. Methods Cell Biol. 2004;77:363–379. doi: 10.1016/s0091-679x(04)77020-x. [DOI] [PubMed] [Google Scholar]
- 16.Diep CQ, Ma D, Deo RC, et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 470:95–100. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heasman J. Morpholino oligos: making sense of antisense? Dev Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- 18.Thisse B, Heyer V, Lux A, et al. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol. 2004;77:505–519. doi: 10.1016/s0091-679x(04)77027-2. [DOI] [PubMed] [Google Scholar]
- 19.Drummond IA, Davidson AJ. Zebrafish kidney development. Methods Cell Biol. 100:233–260. doi: 10.1016/B978-0-12-384892-5.00009-8. [DOI] [PubMed] [Google Scholar]
- 20.Drummond I. Making a zebrafish kidney: a tale of two tubes. Trends Cell Biol. 2003;13:357–365. doi: 10.1016/s0962-8924(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 21.Drummond IA, Majumdar A, Hentschel H, et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- 22.Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- 23.Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol. 2005;16:299–304. doi: 10.1681/ASN.2004090754. [DOI] [PubMed] [Google Scholar]
- 24.Hentschel DM, Park KM, Cilenti L, et al. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 2005;288:F923–929. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- 25.Hentschel DM, Mengel M, Boehme L, et al. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol. 2007;293:F1746–1750. doi: 10.1152/ajprenal.00009.2007. [DOI] [PubMed] [Google Scholar]
- 26.Zhou W, Boucher RC, Bollig F, et al. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol. 299:F1040–1047. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol. 23:1039–1047. doi: 10.1681/ASN.2011080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3:948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pisharath H, Rhee JM, Swanson MA, et al. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shigehara T, Zaragoza C, Kitiyakara C, et al. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol. 2003;14:1998–2003. doi: 10.1681/ASN.V1481998. [DOI] [PubMed] [Google Scholar]
- 31.He B, Ebarasi L, Hultenby K, et al. Podocin-green fluorescence protein allows visualization and functional analysis of podocytes. J Am Soc Nephrol. 22:1019–1023. doi: 10.1681/ASN.2010121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawakami K. Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 2004;77:201–222. doi: 10.1016/s0091-679x(04)77011-9. [DOI] [PubMed] [Google Scholar]
- 33.Felmer RN, Clark JA. The gene suicide system Ntr/CB1954 causes ablation of differentiated 3T3L1 adipocytes by apoptosis. Biol Res. 2004;37:449–460. doi: 10.4067/s0716-97602004000300009. [DOI] [PubMed] [Google Scholar]
- 34.Ebarasi L, He L, Hultenby K, et al. A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol. 2009;334:1–9. doi: 10.1016/j.ydbio.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Rossini M, Yang HC, et al. Effects of podocyte injury on glomerular development. Pediatr Res. 2007;62:417–421. doi: 10.1203/PDR.0b013e31813cbed1. [DOI] [PubMed] [Google Scholar]
- 36.Fogo AB, Kon V. The glomerulus--a view from the inside--the endothelial cell. Int J Biochem Cell Biol. 42:1388–1397. doi: 10.1016/j.biocel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 37.St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60:1037–1046. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee HS, Song CY. Differential role of mesangial cells and podocytes in TGF-beta-induced mesangial matrix synthesis in chronic glomerular disease. Histol Histopathol. 2009;24:901–908. doi: 10.14670/HH-24.901. [DOI] [PubMed] [Google Scholar]
- 39.Cravedi P, Ruggenenti P, Remuzzi G. Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol. 8:301–306. doi: 10.1038/nrneph.2012.42. [DOI] [PubMed] [Google Scholar]
- 40.Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the U.S. Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 156:570–581. doi: 10.7326/0003-4819-156-8-201204170-00004. [DOI] [PubMed] [Google Scholar]
- 41.Angelotti ML, Ronconi E, Ballerini L, et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 30:1714–1725. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 42.Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) 3 1995. [Google Scholar]
- 43.Pellikka M, Tanentzapf G, Pinto M, et al. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- 44.Thisse CaT B. High resolution whole-mount in situ hybridization. Eugene: Univesrsity of Oregon Press; 1998. [Google Scholar]
- 45.Pugliese G, Ricci C, Iacobini C, et al. Glomerular barrier dysfunction in glomerulosclerosis- resistant Milan rats with experimental diabetes: the role of renal haemodynamics. J Pathol. 2007;213:210–218. doi: 10.1002/path.2226. [DOI] [PubMed] [Google Scholar]