Abstract
Objective
To study changes in lipid profiles at 24 weeks among early rheumatoid arthritis (RA) patients participating in the Treatment of Early Rheumatoid Arthritis (TEAR) Trial randomized to initiate methotrexate plus etanercept (MTX+ETA), triple therapy (TT) [MTX plus sulfasalazine plus hydroxychloroquine] or aggressively-titrated MTX monotherapy.
Methods
The TEAR biorepository study had 459 participating patients. Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured in serum plasma at 0 and 24 weeks.
Results
At 24 weeks, there were statistically significant mean increases in cholesterol levels in the MTX + ETA, TT, and MTX monotherapy arms, the observed increases were 31.4, 28.7 and 30 mg/dL in LDL-C; 19.3, 22.3 and 20.6 mg/dL in HDL-C and 56.8, 53 and 57.3 mg/dL values in TC (p < 0.001 all compared to baseline). There was a statistically significant decrease in TC/HDL-C ratio at 24 weeks in all 3 treatment groups from baseline. There was no difference in any lipid changes between the 3 treatment arms. After multivariable adjustment, change in C-reactive protein was associated with change in LDL-C (p=0.03), HDL-C (p=0.09), and TC (p=0.01), but disease activity score in 28-joints was not. Baseline glucocorticoid use was associated with changes in HDL-C (p=0.03) and TC (p=0.02).
Conclusion
Levels of TC, LDL-C, and HDL-C increased equivalently shortly after initiation of MTX + ETA, TT and MTX monotherapy among early RA patients with active disease participating in a clinical trial. The clinical relevance of short term changes in traditional lipids on cardiovascular outcomes remains to be determined.
Keywords: rheumatoid arthritis, etanercept, methotrexate, cholesterol, lipoprotein, cardiovascular
INTRODUCTION
Rheumatoid arthritis (RA) significantly increases the risk for coronary heart disease (CHD) and survival is reduced approximately 5–10 years compared to patients without RA (1). Traditional risk factors such as dyslipidemia are associated with CHD in the general population, but the role of lipids in CHD in RA patients is not well established. Inflammation has been considered to be a major contributor to the development of CHD in RA through multiple mechanisms including endothelial damage (2). RA therapies which decrease inflammation such as anti-tumor necrosis factor (TNF) agents and methotrexate (MTX) (3–6) have been proposed to decrease CHD risk in RA, but this relationship is complex. RA patients prior to treatment tend to have lower levels of total cholesterol (TC), triglycerides (TG) and low density lipoprotein cholesterol (LDL-C) compared to non-RA patients (7–9) and even before the onset of clinical manifestations of RA (10). The dual observations of lower lipid levels but higher rates of cardiac events (1) in RA vs. non-RA patients have suggested to some that lipid levels and their associated clinical interpretation of impact on cardiovascular disease (CVD) risk might be different in RA patients compared to the general population (11) However, this so-called ‘paradoxical’ effect remains yet to be proved. Moreover, additional work (12) has suggested that the relative contribution of lipids and conventional risk factors to cardiac events may be smaller in patients with RA compared to controls.
TNF and other pro-inflammatory cytokines play an important role in elevation of triglycerides and very low density lipoprotein (VLDL). TNF is a major cytokine that drives the inflammation seen in RA, and its effect on lipid metabolism, inflammation and associated atherosclerosis may be an important factor related to mortality from CHD in patients with RA. There is evidence to support that HDL-C is protective against CHD in the general population through multiple anti-atherogenic properties, including its cellular cholesterol efflux capacity, and its anti-oxidative and anti-inflammatory activities (13, 14), which can be compromised in metabolic diseases associated with accelerated atherosclerosis. In RA and other inflammatory states, however, the protein cargo in HDL particles is shifted from an anti-atherogenic and anti-inflammatory profile to a pro-atherogenic and pro-inflammatory one (14–16, 18). Thus, patients with chronic inflammation may have ‘dysfunctional’ or proinflammatory HDL-C even while having normal HDL levels. Changes in the size and composition of LDL-C (15) and HDL-C lipid particles have also been observed (16).
Whether RA therapies have risk modifying activity with respect to lipid metabolism and CHD death has not been widely studied. The COBRA trial showed that both in established, but especially in early RA, effective non-biologic RA treatment was associated with increase in lipid (e.g. HDL) levels and a more favorable (lower) atherogenic index (TC/HDL-C ratio) (17). Other data that examined the association between changes in lipids associated with biologic therapies has been summarized in a systematic literature review (18) and found that lipids seemed to increase after initiating anti-TNF therapy. Recently, data from a clinical trial on golimumab showed larger increase in TC, LDL-C and HDL-C in the golimumab group compared to the MTX monotherapy group (19). However, most of the data available was from small, observational and uncontrolled studies. The Treatment of Early Rheumatoid Arthritis (TEAR) Trial (20) provided the opportunity to examine the effect of MTX monotherapy, in a head-to-head comparison to two combination therapy regimens, on lipid levels in patients with untreated early RA.
Using data from this large, randomized, investigator initiated clinical trial of early RA patients, we investigated whether treatment with 3 different regimens (MTX + sulfasalazine [SSZ] + hydroxycloroquine [HCQ], also known as “triple therapy” (TT), MTX + ETA and MTX monotherapy) affected lipid levels similarly at the end of 24 weeks among participants in the TEAR Trial (20). We also evaluated factors associated with changes in lipids, with a particular focus on RA disease activity and inflammation.
METHODS
Study cohort and treatments
This study utilized data and serum samples from the TEAR Trial (20). All patients fulfilled the American College of Rheumatology (ACR) 1987 revised criteria for RA. The TEAR Trial was a 2-year investigator initiated, randomized, 4-arm, placebo-controlled trial of 755 disease-modifying anti-rheumatic drugs (DMARDs)-naïve early RA patients. Participants in this study were randomized to 4 different treatment groups: two arms included aggressively-titrated to 20 mg/week of MTX monotherapy with “step-up” to MTX + etanercept (ETA) 50 mg weekly or to triple therapy at 6 months for patients who did not achieve low disease activity in 28 joints (DAS28(ESR) < 3.2 at 6 months); MTX+ETA initiated at baseline; and triple therapy (TT) [MTX (titrated to 20 mg/week) + SSZ at 500 mg twice a day, and if tolerated, escalated to 1000 mg twice a day + HCQ 200 mg twice a day] initiated at baseline. Details of the TEAR Trial have been reported previously (20). In this analysis, we examined only the first 24 weeks, so two of the treatment arms (the two step-up arms) were considered as a single MTX-monotherapy group.
Measurement of laboratory and clinical data
Total Cholesterol, TG, LDL-C and HDL-C levels were measured in study participants at 0 and 24 weeks. As samples were measured non-fasting, LDL was calculated according to the Friedewald formula as follows: LDL-C = total cholesterol − HDL-cholesterol − TG/5 (21). Samples were assayed separately in two laboratories [University of Alabama at Birmingham (UAB) and University of California at Los Angeles (UCLA)]. Results were similar, and the results from the UCLA lab were used for this analysis. Samples with TG levels > 400 mg/dL were excluded. Serum cholesterol, HDL-C and triglycerides were measured by a Beckman-Coulter (previously Olympus Corporation) AU400 automated chemistry analyzer using Beckman-Coulter reagents (Brea, CA, USA). The analyzer was maintained and operated according to the manufacturers User’s Guide. The coefficients of variation for TC and HDL-C were 2.5% and 1% within run, and 3.5% and 2.9% between run (day-to-day) (22). C-reactive protein (CRP) was analyzed by Clinical and Epidemiological Research Laboratory at Children’s Hospital in Boston using a high-sensitivity immunoturbidimetric assay on a Hitachi 917 autoanalyzer (Roche Diagnostics, Indianapolis, IN), with the use of reagents and calibrators from Denka Seiken (Tokyo, Japan).
All other clinical data (e.g. tender and swollen joint counts, patient and physical global, comorbidities, concomitant medications) were obtained from the TEAR trial data at baseline and week 24.
Statistical analyses
The distribution of variables was tested for normality. Data were presented as mean ± SD (changes from baseline), or percentages, and p-values < 0.05 were considered significant. Changes in LDL-C, HDL-C and TC, and the TC/HDL-C ratio were determined from baseline to 24 weeks for each treatment group. The data for each treatment arm were also analyzed vis a vis changes in DAS28(ESR) and CRP from baseline to week 24. Lipid changes also were stratified based on DAS28(ESR) score < 3.2 or >3.2) at 24 weeks for each treatment arm to determine if there was any significant change in lipid profile based on the category of disease activity. The proportions of patients that shifted their LDL-C level to higher or lower categories at 24 weeks were evaluated. A bivariate and multivariate analysis using linear regression was used to determine the changes in LDL-C, HDL-C and TC at 24 weeks after controlling for age, body mass index (BMI), diabetes (DM), CVD [defined as any of the following: history of acute myocardial infarction, cerebrovascular accident, unstable angina, angioplasty or stent placement, congestive heart failure and coronary artery bypass graft (CABG)], respiratory disease (e.g. chronic obstructive pulmonary disease, asthma), LDL-C at baseline, DAS28(ESR) change between baseline and week 24, CRP and statin and steroid use at baseline or any time within the first 24 weeks.
RESULTS
Patient characteristics
A total of 459 patients (61% of the population from the main TEAR Trial population) with valid data and available serum samples were included in the analysis (Table 1). Characteristics of the participants electing to participate in the bio-repository study of TEAR were similar to those in the main TEAR Trial (data not shown). Patients in the TEAR Trial were essentially DMARDs naïve, including biologics. Approximately 40% of the participants in each treatment group were using prednisone at the time of enrollment. Participants had a very short RA disease duration (mean 3.8 ± 1.1 months), 80% were Caucasian, and had a mean DAS28(ESR) at baseline of 5.8 ± 1.1. There were no significant baseline differences between treatment groups.
Table 1.
Baseline Characteristics of Patients Randomized to MTX + Etanercept, Triple Therapy, or MTX Monotherapy
| MTX + ETA N=155 | Triple Therapy N=78 | MTX Monotherapy N=226 | |
|---|---|---|---|
| Demographics and RA related variables | |||
| Age (years) | 50.8 ± 13.2 | 47.6 ± 12.4 | 49.0 ± 12.2 |
| BMI | 29.0 ± 6.9 | 29.6 ± 8.6 | 30.2 ± 7.0 |
| Female | 73.6% | 76.9% | 70.8% |
| RF+ | 88.4% | 92.3% | 87.6% |
| Smoking (current) | 34.8% | 30.8% | 33.6% |
| Disease Duration (months) | 3.5 ± 6.6 | 5.1 ± 8.5 | 3.6 ± 6.3 |
| DAS28(ESR) | 5.7 ± 1.1 | 5.8 ± 1.1 | 5.8 ± 1.1 |
| CRP (mg/dL) | 1.6 ± 2.7 | 1.5 ± 2.4 | 1.6 ± 2.0 |
| Race | |||
| African American | 10.3% | 7.7% | 10.2% |
| Caucasian | 80.0% | 76.9% | 82.7% |
| Other | 9.7% | 15.4% | 7.1% |
| Medications | |||
| Any oral glucocorticoid use | 43.9% | 47.4% | 42.0% |
| Statins | 15.5% | 10.3% | 11.5% |
| Comorbidities | |||
| Diabetes | 79.4% | 87.2% | 81.4% |
| Cardiovascular disease* | 29.7% | 24.4% | 27.4% |
| Respiratory disease** | 23.2% | 16.7% | 16.4% |
| Lipid levels at baseline | |||
| Total Cholesterol, mg/dL | 189.3 ± 44.7 | 183.3 ± 44.9 | 189.4 ± 48.2 |
| LDL-C, mg/dL | 107.0 ± 35.1 | 96.1 ± 31.2 | 107.1 ± 38.0 |
| HDL-C, mg/dL | 56.2 ± 14.7 | 57.9 ± 19.8 | 54.4 ± 14.8 |
| Ratio between Total Cholesterol and HDL-C | 3.5 ± 0.9 | 3.3 ± 0.8 | 3.6 ± 0.9 |
Data shown as mean ± SD, or n (%); HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; CRP = C-reactive protein; DAS28 = disease activi9ty score in 28 joints; ESR = erythrocyte sedimentation rate. MTX = methotrexate; ETA = etanercept.
includes history of myocardial infarction, heart failure, unstable angina, angioplasty, stent placement, coronary bypass graft angioplasty and stroke.
includes asthma, chronic obstructive pulmonary disease and other chronic pulmonary disorders.
Changes in lipid levels
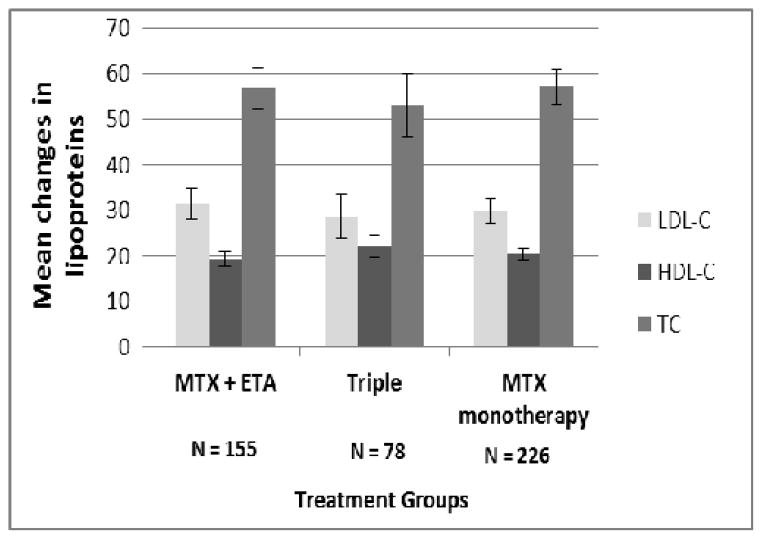
At 24 weeks, there were statistically significant mean increases in TC (56.8, 53.0, and 57.3 mg/dL), HDL-C (19.3, 22.3 and 20.6 mg/dL) and LDL-C (31.4, 28.7 and 30.0 mg/dL) for the MTX + ETA, TT and MTX monotherapy groups respectively (Figure 1) compared to baseline (p < 0.001 for all), but there were no significant baseline differences between treatment arms. Despite the increase observed in TC, LDL-C and HDL-C, there was a statistically significant mean decrease compared to baseline in the TC/HDL-C ratio (23) at 24 weeks of −0.1 for MTX + ETA (p = 0.012 compared to baseline) and of −0.3 and −0.2 for the TT and MTX monotherapy groups respectively (p < 0.001 for each compared to baseline). When the ETA and TT groups were compared to MTX monotherapy, there was no statistically significant difference between the groups in the change in TC/HDL-C ratio. Changes in lipids were not statistically significant within each treatment group comparing those with DAS28(ESR) <3.2 and those with DAS28(ESR) >3.2 at 24 weeks (data not shown).
Figure 1.
Changes in mean LDL-C, HDL-C and TC from baseline to 24 weeksamong patients randomized to MTX + ETA, Triple Therapy or MTX monotherapy. All p values compared results at 24 weeks to baseline were p < 0.0001. There were no significant differences between the 3 treatment arms for any of the 3 different lipoproteins. HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; TC = Total cholesterol; MTX = methotrexate; ETA = etanercept; Triple = methotrexate + hydroxychloroquine + Sulfasalazine.
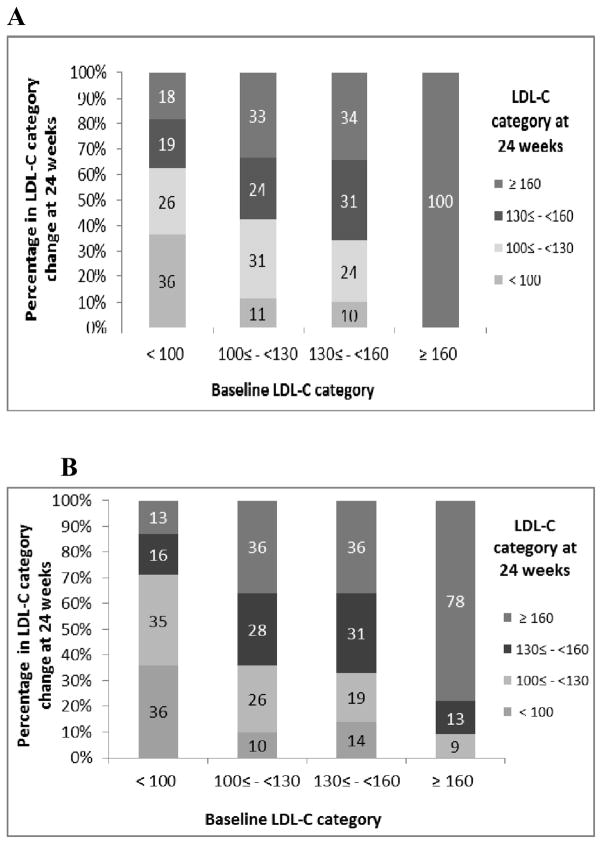
Comparing MTX + ETA to MTX monotherapy patients, there were similar proportions of patients who shifted their LDL-C at week 24 into a higher category (Figure 2a and 2b). Most patients shifted to a higher LDL-C category. Results were similar for the TT treatment arm.
Figure 2.
Shift in LDL-C category at 24 weeks compared to baseline among Methotrexate + etanercept patients and methotrexate monotherapy patients. A. Methotrexate and etanercept. B. Methotrexate monotherapy. LDL-C = low density lipoprotein cholesterol
CRP and BMI were significantly and negatively associated with the LDL-C and TC at 24 weeks after multivariable adjustment (Table 2). Changes in DAS28(ESR) were associated with LDL-C and TC in bivariate analyses; however, after controlling for multiple factors using multivariable adjustment, these associations no longer remained significant. Prednisone use was positively associated with the changes in HDL-C and TC. As indicated by the R2 values, the factors examined in the MV analysis explained 12 – 49% of the variability in the change in LDL-C, HDL-C, TC, and TC/HDL-C ratio.
Table 2.
Results from the bivariate and multivariate analysis used to determine the changes in LDL-C, HDL-C and TC at 24 weeks. Multivariable adjusted factors associated with change in LDL-C, HDL-C, TC-C and TC/HDL ratio at week 24*
| LDL-C | HDL-C | TC | TC/HDL-C Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Bivariate(std) | Multivariable-Adjusted(std) | Bivariate(std) | Multivariable-Adjusted(std) | Bivariate(std) | Multivariable-Adjusted(std) | Bivariate(std) | Multivariable-Adjusted(std) | |
| Age (by 10 years) | 0.019 (0.016) (p=0.22) | 0.023 (0.017) (p=0.18) | 0.005 (0.007) (p=0.50) | 0.015 (0.008) (p=0.05) | 0.033 (0.022) (p=0.13) | 0.051 (0.023) (p=0.03) | 0.034 (0.028) (p=0.24) | 0.002 (0.023) (p=0.95) |
| Gender (F) | −4.38 (4.40) (p=0.32) | 0.54(4.41) (p=0.90) | 1.41(2.07) (p=0.50) | 8.55 (2.08) (p<.0001) | −5.63(6.17) (p=0.36) | 5.93(6.01) (p=0.32) | −0.43 (0.08) (p<.0001) | −0.21 (0.06) (p=0.001) |
| CRP change between week 24 and baseline (mg/dL) | −0.026 (0.009) (p=0.003) | −0.019(0.009) (p=0.03) | −0.009(0.004) (p=0.03) | −0.007(0.004) (p=0.09) | −0.043(0.012) (p=0.0004) | −0.03 (0.012) (p=0.01) | −0.018 (0.016) (p=0.26) | −0.013 (0.012) (p=0.28) |
| DAS 28(ESR) change between week 24 and baseline | −2.65(1.32) (p=0.04) | −1.38(1.34) (p=0.31) | −1.05(0.62) (p=0.09) | −0.26(0.61) (p=0.67) | −4.21(1.84) (p=0.02) | −1.82(1.83) (p=0.32) | −0.02 (0.02) (p=0.38) | 0.0029 (0.0187) (p=0.88) |
| BMI | 0.46 (0.27) (p=0.09) | 0.76 (0.27) (p=0.005) | 0.13 (0.13) (p=0.29) | −0.04 (0.12) (p=0.72) | 0.79 (0.38) (p=0.04) | 1.14 (0.36) (p=0.002) | 0.24 (0.005) (p<.0001) | 0.009 (0.005) (p=0.01) |
| Lipid value at baseline | −0.31 (0.05) (p<.0001) | −0.34 (0.06) (p<0.0001) | −0.48 (0.05) (p<.0001) | −0.56 (0.06) (p<.0001) | −0.41 (0.06) (p<.0001) | −0.45 (0.06) (p<.0001) | 0.62 (0.03) (p<.0001) | 0.58 (0.03) (p<.0001) |
| Using Steroids at baseline | 4.34 (3.94) (p=0.27) | 5.06 (3.87) (p=0.19) | −0.27 (1.86) (p=0.88) | 3.92 (1.80) (p=0.03) | 7.90 (5.52) (p=0.15) | 12.02 (5.26) (p=0.02) | 7.90 (5.52) (p=0.15) | 0.04 (0.05) (p=0.41) |
| R-square from the model | 0.12 | 0.19 | 0.18 | 0.49 | ||||
Controlled for diabetes, cardiovascular disease, respiratory disease and statins; these were not independently significant); TC = total cholesterol; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; CRP = C-reactive protein; BMI = body mass index.
Data shown are B coefficients (standard errors) with associated p values
DISCUSSION
Among early RA patients participating in the TEAR Trial with biospecimens available, we found a robust increase in TC, LDL-C and HDL-C after treatment for 6 months with MTX + ETA, MTX + sulfasalazine + hydroxychloroquine combination, or MTX monotherapy with similar magnitude of change observed for each of these lipoproteins between the 3 TEAR treatment arms. Despite the increase in TC, LDL-C and HDL-C, the TC/HDL-C ratio (i.e. atherogenic index) decreased slightly in all treatment arms. Results from the Framingham Study have shown that the TC/HDL-C ratio was a better predictor of CHD risk than was LDL or TC (24). Importantly, our study also demonstrated that the magnitude of the changes in lipids were similar with aggressive titration of methotrexate monotherapy, as governed by the TEAR protocol, compared to treatment with triple therapy or MTX + ETA.
This study is among the few reports of lipid changes related to blinded, randomized use of single and combination RA medications among patients with untreated, very early RA and active disease. Moreover, lipid changes were able to be directly compared between both biologic and non-biologic RA treatment regimens (e.g. triple therapy, MTX monotherapy, and MTX + ETA) in the same study from a randomized clinical trial. This design provides for a more direct comparison of therapies in a homogeneous group of patients under controlled conditions.
We observed that changes in LDL-C and TC correlated negatively with CRP changes even after multivariate adjustment for potentially confounding factors. The association with HDL-C was of borderline significance (p=0.09) but trended in the same direction. Although there was a negative association between changes in DAS28(ESR) after 6 months of therapy and changes in LDL-C and TC, this observation did not persist after multivariate adjustment for CRP and other factors. Similar to our study, Seriolo et al (25) demonstrated in a small prospective study of 34 women that changes in lipids did not correlate with disease activity level after 16 weeks of treatment, although it did after 24 weeks. On the other hand, and similar to our study, 4 months of etanercept treatment for patients enrolled in the Etanercept Treatment in Rheumatoid Arthritis (ETRA) study (26) was associated with elevations in TC, and LDL-C, although the magnitude of changes was much lower in the ETRA study. In that same study, the changes in TC were also stratified by changes in CRP, ESR and DAS28(ESR) over time, and a negative association between these clinical and laboratory response variables and TC and HDL-C levels was found. Although our results are generally consistent with ETRA, key differences between the design of TEAR and ETRA may account for some dissimilarity in the magnitude of some associations observed in the two studies. ETRA was an observational cohort study of patients with established RA and median disease duration of 8 years with high disease activity (median DAS28(ESR) of 5.21); 31% were previously treated with anti-TNF medication, and concomitant treatment with MTX or prednisone occurred for 76% and 29% of patients, respectively. Our results are also compatible with the COBRA trial which found increases in lipid levels but decreases in the atherogenic index after starting aggressive non-biologic DMARDs treatment (17). We also found that glucocorticoid use (at baseline, allowed to be continued but not provided as part of the TEAR treatment protocol) was independently associated with changes in HDL-C and TC. This is consistent with prior observations that glucocorticoids increases lipid levels (27) and the COBRA trial that found greater increases in lipids in the treatment arm that included glucocorticoid use (17).
Another important factor to consider in terms of disease duration and its effect on lipids relate to multiple observations suggesting that RA itself may impact lipid levels. Myasoedova and colleagues (10) observed that patients who developed RA had lower levels of TC and LDL-C in the preclinical state than matched controls who did not develop RA. In a large observational study of commercially insured patients, lipid levels were found to significantly differ between RA and OA patients even after controlling for multiple confounding factors (28). Consistent with these findings, it is possible that the elevation in lipids shortly after treatment in our study could reflect a ‘normalization’ of lipid levels back to their pre-disease values.
RA therapies other than those examined in our study such as the novel IL-6 receptor monoclonal antibody tocilizumab (29–33), have been shown to be associated with elevation in serum lipid levels. In clinical trials, tocilizumab with MTX caused a significant mean increase of LDL and HDL of 20 mg/dL and 5 mg/dL, respectively at 24 weeks (34). This evidence, coupled with our data showing that both non-biologic and etanercept treatment increased lipids, suggests that the increase in lipids observed in patients with RA after initiation of treatment may not be wholly due to a direct effect of any particular RA medication or drug class on lipids but due to a decrease in inflammation and/or response to treatment.
A synthesis of the lipid changes in patients with RA following anti-TNF treatment was published in a systematic review (18). Although some studies demonstrated isolated increases in triglycerides, most studies reported modest increases in lipid levels. The data from these observational studies might be difficult to interpret since some of the studies included patients with rheumatic diseases other than RA; some studies had small sample sizes, and the proportion of RA patients with high disease activity was low (18). All of this might explain the modest discrepancies in magnitude of the difference in the results between most of these studies compared to the findings in TEAR. Nevertheless, the majority of the studies summarized in that review suggested that there was an overall increase in lipids in patients with RA that were treated with anti-TNF agents. In a more recent systematic review of lipid profiles changes with anti-TNF medications use in RA patients (35), a significant increase in TC, HDL-C and triglycerides was observed (mean increase of 10.4 mg/dL for HDL and TC and 10.8 for triglycerides), while LDL-C and TC/HDL-C ratio remained unchanged.
The clinical importance of dyslipidemia with respect to CHD events or death in RA is unclear. Even though there is observational data that suggests that there is no significant difference between RA and non-RA subjects in the risk imparted by hyperlipidemia (12), it could be possible that non-traditional CHD risk factors, or other lipid parameters besides those found in cholesterol profiles, explain a greater proportion of CHD risk than in non-RA patients (36). For example, it has been proposed that the non-fasting apoB/apoA-I ratio was superior to any of the cholesterol ratios for estimation of the risk of acute myocardial infarction (37). Some have suggested that the elevation in lipids after RA therapy might be offset by a reduction of more atherogenic molecules (26) such as apoB and apoA-I. These were not able to be measured in this analysis, nor were other molecules suggested to have a significant predictive value in CHD outcomes in RA (e.g. apoC-III, oxidized LDL). As with most clinical trials, there were few ischemic events in TEAR trial participants, and the changes in lipids observed could not be correlated to clinical events such as myocardial infarction or stroke. Additionally, comorbidities (e.g. prior CHD, lung disease) measured in TEAR were characterized with limited precision. As another potential limitation of this analysis, lipid samples in TEAR were collected non-fasting. However, a recent study showed that fasting time had little association with lipids (e.g. variation in LDL of only up to 10%) and suggested that fasting for routine lipid levels may be largely unnecessary (38). Whether colleting lipid levels in fasting conditions is optimal or not remains controversial, but some data suggests that non-fasting lipid assessment may be more useful to optimize CVD risk stratification (39).
Conclusions
The implications of elevation in lipids in terms of CHD outcomes and as a result of aggressive DMARDs and anti-TNF therapy require further investigation. Nevertheless, changes in lipids associated with initiation of effective RA treatments (including MTX monotherapy) appear to be less related to the specific type of RA therapy and more related to a decrease in inflammation. This blinded clinical trial may provide a higher level of evidence to support this assertion for early RA patients with high levels of disease activity. Physicians should be cognizant of optimizing cardiovascular risk factors such as lipoproteins in RA patients, particularly in relation to the initiation of RA medications. Further work to understand longer-term changes in lipid levels, to evaluate novel CV biomarkers, and to better characterize important aspects of lipid metabolism (e.g. particle size, oxidation), and the clinical significance of these factors, is warranted.
Acknowledgments
Funding: Amgen provided funding for the TEAR Trial but was not responsible for data collection or analysis; UAB and co-authors, are solely responsible for all data collection, management, and statistical analysis. This ancillary study was funded by the Arthritis Foundation through a grant to JRC. The TEAR bio-repository was funded by NIH R01 AR052658 (Dr. Bridges). Dr. Curtis receives support from the NIH (AR053351) and the Agency for Healthcare Research and Quality (R01HS018517) Dr. Charles-Schoeman receives support from the NHLBI (5K23HL094834) and NIAMS (R21 AR 057913-01A1) and grants and consulting compensation from Pfizer and Bristol Myers Squibb. Dr. Bathon receives support from the NIH (2R01 AR050026-07) and from the American College of Rheumatology. Dr. Moreland receives funding from the Margaret J. Miller Endowed Professor of Arthritis Research chair.
Footnotes
Competing Interests/Disclosures:
Dr. Curtis reports receipt of grant and consulting compensation from Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, Abbott, and UCB. Dr. Cofield reports receipt of compensation from Teva Neuroscience and the American Shoulder and Elbow Society (2008–2011; for consultancy work) and from Centocor Ortho Biotech Services L.L.C. (2008–2011; for DSMB activities). None of the compensation reported by these investigators was related to the work in this manuscript. Other authors reported no potential conflicts.
References
- 1.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65(12):1608–12. doi: 10.1136/ard.2005.050377. Epub 2006/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tousoulis D, Antoniades C, Bosinakou E, Kotsopoulou M, Tsoufis C, Marinou K, et al. Differences in inflammatory and thrombotic markers between unstable angina and acute myocardial infarction. Int J Cardiol. 2007;115(2):203–7. doi: 10.1016/j.ijcard.2006.03.011. Epub 2006/06/22. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F, Michaud K. The risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis: a cohort and nested case-control analysis. Arthritis Rheum. 2008;58(9):2612–21. doi: 10.1002/art.23811. Epub 2008/09/02. [DOI] [PubMed] [Google Scholar]
- 4.Atzeni F, Turiel M, Caporali R, Cavagna L, Tomasoni L, Sitia S, et al. The effect of pharmacological therapy on the cardiovascular system of patients with systemic rheumatic diseases. Autoimmun Rev. 2010 doi: 10.1016/j.autrev.2010.07.018. Epub 2010/08/04. [DOI] [PubMed] [Google Scholar]
- 5.Carmona L, Descalzo MA, Perez-Pampin E, Ruiz-Montesinos D, Erra A, Cobo T, et al. All-cause and cause-specific mortality in rheumatoid arthritis are not greater than expected when treated with tumour necrosis factor antagonists. Ann Rheum Dis. 2007;66(7):880–5. doi: 10.1136/ard.2006.067660. Epub 2007/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–7. doi: 10.1002/art.24092. Epub 2008/11/28. [DOI] [PubMed] [Google Scholar]
- 7.Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: an explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford) 2011;50(2):324–9. doi: 10.1093/rheumatology/keq295. Epub 2010/10/05. [DOI] [PubMed] [Google Scholar]
- 8.del Rincon I, Freeman GL, Haas RW, O’Leary DH, Escalante A. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52(11):3413–23. doi: 10.1002/art.21397. Epub 2005/10/29. [DOI] [PubMed] [Google Scholar]
- 9.Lazarevic MB, Vitic J, Mladenovic V, Myones BL, Skosey JL, Swedler WI. Dyslipoproteinemia in the course of active rheumatoid arthritis. Semin Arthritis Rheum. 1992;22(3):172–8. doi: 10.1016/0049-0172(92)90017-8. Epub 1992/12/01. [DOI] [PubMed] [Google Scholar]
- 10.Myasoedova E, Crowson CS, Kremers HM, Fitz-Gibbon PD, Therneau TM, Gabriel SE. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1310–4. doi: 10.1136/ard.2009.122374. Epub 2009/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Therneau TM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70(3):482–7. doi: 10.1136/ard.2010.135871. Epub 2011/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez A, Maradit Kremers H, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67(1):64–9. doi: 10.1136/ard.2006.059980. Epub 2007/05/23. [DOI] [PubMed] [Google Scholar]
- 13.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58(3):342–74. doi: 10.1124/pr.58.3.1. Epub 2006/09/14. [DOI] [PubMed] [Google Scholar]
- 14.Feingold KR, Hardardottir I, Grunfeld C. Beneficial effects of cytokine induced hyperlipidemia. Z Ernahrungswiss. 1998;37(Suppl 1):66–74. Epub 1998/04/29. [PubMed] [Google Scholar]
- 15.Rizzo M, Spinas GA, Cesur M, Ozbalkan Z, Rini GB, Berneis K. Atherogenic lipoprotein phenotype and LDL size and subclasses in drug-naive patients with early rheumatoid arthritis. Atherosclerosis. 2009;207(2):502–6. doi: 10.1016/j.atherosclerosis.2009.07.015. Epub 2009/08/01. [DOI] [PubMed] [Google Scholar]
- 16.Chung CP, Oeser A, Raggi P, Sokka T, Pincus T, Solus JF, et al. Lipoprotein subclasses determined by nuclear magnetic resonance spectroscopy and coronary atherosclerosis in patients with rheumatoid arthritis. J Rheumatol. 2010;37(8):1633–8. doi: 10.3899/jrheum.090639. Epub 2010/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boers M, Nurmohamed MT, Doelman CJ, Lard LR, Verhoeven AC, Voskuyl AE, et al. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62(9):842–5. doi: 10.1136/ard.62.9.842. Epub 2003/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollono EN, Lopez-Olivo MA, Lopez JA, Suarez-Almazor ME. A systematic review of the effect of TNF-alpha antagonists on lipid profiles in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29(9):947–55. doi: 10.1007/s10067-010-1405-7. Epub 2010/04/13. [DOI] [PubMed] [Google Scholar]
- 19.Kirkham BW, Wasko MC, Hsia EC, Fleischmann RM, Genovese MC, Matteson EL, et al. Effects of golimumab, an anti-tumour necrosis factor-alpha human monoclonal antibody, on lipids and markers of inflammation. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202089. Epub 2013/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreland LW, O’Dell JR, Paulus HE, Curtis JR, Bathon JM, William St Clair E, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early, aggressive rheumatoid arthritis. Arthritis Rheum. 2012 doi: 10.1002/art.34498. Epub 2012/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. Epub 1972/06/01. [PubMed] [Google Scholar]
- 22.Stanbio L. Stanbio Cholesterol LiquiColor. USA: Stanbio; 2004. [Google Scholar]
- 23.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. Epub 2002/12/18. [PubMed] [Google Scholar]
- 24.Kannel WB, Wilson PW. Efficacy of lipid profiles in prediction of coronary disease. American heart journal. 1992;124(3):768–74. doi: 10.1016/0002-8703(92)90288-7. Epub 1992/09/01. [DOI] [PubMed] [Google Scholar]
- 25.Seriolo B, Paolino S, Sulli A, Fasciolo D, Cutolo M. Effects of anti-TNF-alpha treatment on lipid profile in patients with active rheumatoid arthritis. Ann N Y Acad Sci. 2006;1069:414–9. doi: 10.1196/annals.1351.039. Epub 2006/07/21. [DOI] [PubMed] [Google Scholar]
- 26.Jamnitski A, Visman IM, Peters MJ, Dijkmans BA, Voskuyl AE, Nurmohamed MT. Beneficial effect of 1-year etanercept treatment on the lipid profile in responding patients with rheumatoid arthritis: the ETRA study. Ann Rheum Dis. 2010;69(11):1929–33. doi: 10.1136/ard.2009.127597. Epub 2010/05/26. [DOI] [PubMed] [Google Scholar]
- 27.Maxwell SR, Moots RJ, Kendall MJ. Corticosteroids: do they damage the cardiovascular system? Postgraduate medical journal. 1994;70(830):863–70. doi: 10.1136/pgmj.70.830.863. Epub 1994/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis JR, John A, Baser O. Dyslipidemia and changes in lipid profiles associated with rheumatoid arthritis and initiation of anti-TNF therapy. Arthritis Care Res (Hoboken) 2012 doi: 10.1002/acr.21693. Epub 2012/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–97. doi: 10.1016/S0140-6736(08)60453-5. Epub 2008/03/25. [DOI] [PubMed] [Google Scholar]
- 30.Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54(9):2817–29. doi: 10.1002/art.22033. Epub 2006/09/02. [DOI] [PubMed] [Google Scholar]
- 31.Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69(1):88–96. doi: 10.1136/ard.2008.105197. Epub 2009/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67(11):1516–23. doi: 10.1136/ard.2008.092932. Epub 2008/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63(3):609–21. doi: 10.1002/art.30158. Epub 2011/03/02. [DOI] [PubMed] [Google Scholar]
- 34.GENETECH. Prescibing Information. 2010 http://wwwgenecom/gene/products/information/actemra/pdf/pipdf.
- 35.Daien CI, Duny Y, Barnetche T, Daures JP, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis. 2012;71(6):862–8. doi: 10.1136/annrheumdis-2011-201148. Epub 2012/01/24. [DOI] [PubMed] [Google Scholar]
- 36.Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69(11):1920–5. doi: 10.1136/ard.2009.122226. Epub 2010/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy--a review of the evidence. J Intern Med. 2006;259(5):493–519. doi: 10.1111/j.1365-2796.2006.01643.x. Epub 2006/04/25. [DOI] [PubMed] [Google Scholar]
- 38.Sidhu D, Naugler C. Fasting Time and Lipid Levels in a Community-Based Population: A Cross-sectional Study. Archives of internal medicine. 2012:1–4. doi: 10.1001/archinternmed.2012.3708. Epub 2012/11/14. [DOI] [PubMed] [Google Scholar]
- 39.Eberly LE, Stamler J, Neaton JD Multiple Risk Factor Intervention Trial Research G. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Archives of internal medicine. 2003;163(9):1077–83. doi: 10.1001/archinte.163.9.1077. Epub 2003/05/14. [DOI] [PubMed] [Google Scholar]