Abstract
Endogenous retroviruses are implicated in murine lupus nephritis. They provide a source of nephritogenic retroviral gp70-anti-gp70 immune complexes through the production of serum gp70 protein and anti-gp70 autoantibodies as a result of the activation of TLR7. The Sgp (serum gp70 production) loci identified in lupus-prone mice play distinct roles for the expression of different classes of endogenous retroviruses, as Sgp3 regulates the transcription of xenotropic, polytropic and modified polytropic (mPT) viruses, and Sgp4 the transcription of only xenotropic viruses. In the present study, we extended these analyses to a third locus, Sgp5, using BALB/c mice congenic for the NZW-derived Sgp5 allele and also explored the possible interaction of Sgp3 and Sgp4 loci to promote the expression of endogenous retroviruses and serum gp70. The analysis of Sgp5 BALB/c congenic mice demonstrated that the Sgp5 locus enhanced the expression of xenotropic and mPT viruses, thereby upregulating the production of serum gp70. These data indicate a distinct action of the Sgp5 locus on the expression of endogenous retroviruses, as compared with two other Sgp loci. Moreover, comparative analysis of C57BL/6 double congenic mice for Sgp3 and Sgp4 loci with single congenic mice revealed that Sgp3 and Sgp4 acted synergistically to elevate the transcription of the potentially replicationcompetent Xmv18 provirus and the production of serum gp70. This indicates that the combined effect of three different Sgp loci markedly enhance the expression of endogenous retroviruses and their gene product, serum gp70, thereby contributing to the formation of nephritogenic gp70-anti-gp70 immune complexes in murine lupus.
Keywords: Systemic lupus erythematosus, Endogenous retrovirus, gp70
1. Introduction
Lupus-prone (NZB×NZW)F1, MRL and BXSB mice have relatively large concentrations of the retroviral envelope glycoprotein gp70 in their sera, which is secreted by hepatocytes [1]. The pathogenic role of serum retroviral gp70 has been underlined by the demonstration of high levels of circulating gp70-antigp70 immune complexes (gp70 IC) which correlate remarkably with the development of lupus nephritis [2–7]. The induction of anti-gp70 autoimmune responses in lupus-prone mice is dependent on TLR7 [8, 9], which specifically recognizes single-stranded RNA, and is promoted by the Sgp3 locus [10, 11]. This may proceed through the activation of a TLR7 signaling cascade as a result of an enhanced production of endogenous retroviral virions carrying single-stranded RNA. Thus, the Sgp loci play a dual role in the formation of nephritogenic gp70 IC by promoting the development of anti-gp70 autoantibodies as well as the expression of serum gp70.
Serum concentrations of gp70 are highly variable among different strains of mice [2, 12–14]. Genetic studies involving lupus-prone NZB, NZW and BXSB and non-autoimmune C57BL strains revealed that serum levels of gp70 are controlled by a major Sgp3 (serum gp70 production 3) locus on mid chromosome 13 and a minor Sgp4 locus on distal chromosome 4 [7, 11, 15–19]. In addition to these two loci, the genetic analysis involving BALB/c mice revealed a remarkably strong linkage of serum gp70 levels to a distinct locus on proximal chromosome 12 of both NZB and NZW mice [19]. Since no gene name was given to this locus, we propose to designate it Sgp5.
Endogenous retroviruses are classified by the host range dictated by the envelope gp70 protein as follows: ecotropic, xenotropic and polytropic retroviruses [20]. Based on nucleotide sequences in a variable region of their env genes, the xenotropic viruses have been divided into four subgroups, Xeno-I, Xeno-II, Xeno-III and Xeno-IV [21, 22], and the polytropic viruses into two subgroups, polytropic (PT) and modified PT (mPT) [23]. Analysis of the abundance of different retroviral gp70 RNAs in livers of C57BL/6 (B6) Sgp congenic mice demonstrated that the Sgp3 locus enhanced levels of xenotropic, PT and mPT gp70 RNAs, while the effect of the Sgp4 locus was restricted to xenotropic viruses [8, 22]. Moreover, clonal analysis of xenotropic and mPT viral transcripts revealed that each locus regulates the expression of distinct subpopulations of xenotropic proviruses [24] and that Sgp3 promoted the transcription of a select group of mPT proviruses, including potentially replication-competent viruses [25].
The demonstration of differential roles of Sgp3 and Sgp4 for the transcription of separate sets of endogenous retroviruses prompted us to define the contribution of the third Sgp locus, Sgp5, to the expression of endogenous retroviruses using BALB/c mice congenic for the NZW-derived Sgp5 allele (BALB.Sgp5). In addition, we generated B6 mice congenic for both Sgp3 and Sgp4 loci derived from NZB mice (B6.Sgp3/4) in order to determine a possible synergistic effect between the loci to promote the expression of endogenous retroviruses and their gene product, serum gp70. Results obtained from the present study revealed that the Sgp5 locus acts differently from two other Sgp loci in terms of the specificity to three different classes of endogenous retroviruses and that Sgp3 and Sgp4 loci act synergistically to enhance serum levels of gp70 through selective upregulated expression of the Xmv18 provirus.
2. Materials and methods
2.1 Mice
BALB.Sgp5 congenic mice bearing the NZW-Sgp5 allele on chromosome 12 were generated by backcrossing an NZW-derived interval encompassing markers D12Mit291 (8.1 cM from the centromere) and D12Mit4 (35.5 cM) onto the BALB/c background using marker-assisted selection, as described previously [19]. The generation of B6.Sgp3 congenic mice carrying an NZB interval flanked by markers D13Mit283 (32.8 cM) and D13Mit254 (41.0 cM) and B6.Sgp4 congenic mice carrying an NZB interval flanked by markers D4Mit11 (57.4 cM) and D4Mit33 (81.4 cM) was described previously [22, 26]. B6.Sgp3/4 mice double congenic for Sgp3 and Sgp4 loci were obtained by intercrossing B6.Sgp3 and B6.Sgp4 mice. NZW mice were purchased from the Jackson Laboratory, Bar Harbor. All studies presented were carried out in 2–3 mo-old male mice. Animal studies described in the present study have been approved by the Ethics Committee for Animal Experimentation of the University of Geneva (authorization number: 1005/3701/1).
2.2. Serological assay
Serum levels of retroviral gp70 were determined by ELISA as described previously [27]. Results are expressed as µg/ml of gp70 by referring to a standard curve obtained from a serum pool of NZB mice.
2.3. Quantitative real-time PCR
RNA from livers was purified with TRIzol reagent (Invitrogen AG, Basel, Switzerland) and treated with DNase I (Amersham Biosciences Corp., Piscataway, NJ). The abundance of xenotropic, mPT and PT gp70 RNAs (genomic RNA and mRNA) was quantified by real-time PCR, as described [8, 22, 26]. Levels of G protein-coupled receptor kinase 5 (Grk5) mRNA were quantified as described [24]. PCR was performed using the iCycler iQ Real-Time PCR Detection System (Bio-Rad, Philadelphia, PA) and iQ SYBR green Supermix (Bio-Rad). Results were quantified using a standard curve generated with serial dilutions of a reference cDNA preparation from NZB livers and normalized using TATA-binding protein (TBP) mRNA.
2.4. RT-PCR
For the amplification of subgroups of xenotropic gp70 cDNA, a common Xeno277F forward primer and reverse primers on the env genes specific for four different subgroups of xenotropic viruses were used as described [22]. Since the reverse primer designed for the amplification of Xeno-III gp70 cDNA is also able to amplify Xeno-II gp70 cDNA, the RT-PCR products obtained with this set of primers contain both Xeno-II and Xeno-III amplicons. The presence of three different species of mPT env RNAs, intact wild-type (WT) and two deletion mutants (D1 and D2), was detected by RT-PCR as described [8]. The abundance of three different species of mPT env RNAs was semi-quantified with 5-fold serially diluted cDNA templates. As a control, levels of Gapdh cDNA were determined in a parallel assay. PCR products were visualized by staining with ethidium bromide after electrophoresis on 2% agarose gels.
2.5. Genomic PCR
The presence of four different subgroups of xenotropic proviruses in the genome was determined by PCR on genomic DNA, as described [22]. The presence of the Xmv15, Mpmv4, and Mpmv11 proviruses in mice was verified by genomic PCR analysis with the following combinations of primers for 5’- and 3’-flanking sequences and those for the U3 sequence of the long terminal repeat (LTR): 5’-Xmv15 flanking forward primer (5’-GAAAGCTATACTGCCCTTCC- 3’), 3’-Xmv15 flanking reverse primer (5’-TCTCTGCCTCCATTGTTCTC-3’); 5’- Mpmv4 flanking forward primer (5’-CAGAGGTCCTGAGTTCAATTCC-3’), 3’- Mpmv4 flanking reverse primer (5’-AGACATGGGCAAAGCATCTG-3’); 5’- Mpmv11 flanking forward primer (5’-CTATGCTTAGCAGGCAGGTATC-3’) and 3’-Mpmv11 flanking reverse primer (5’-AAGGTTGTCTGGCTTGAAGG-3’); U3 forward (5’-AAGCTAGCTGCAGTAACGCCATTTTGC-3’) and reverse (5’- AGCTTGCTAAGCCTTATGGTGG-3’) primers.
2.6. RT-PCR cloning
Xenotropic and mPT viral cDNA spanning the env gene and the U3 region of LTR was amplified with Xeno1098F or mPT858F forward primer and Uniltr-4R reverse primer to determine the U3 sequence of the LTR, as described [8]. The amplified fragments were purified and ligated into the pBluescript-SK+ plasmid. The origin of Xmv and Mpmv proviruses expressed in livers was determined through the nucleotide sequence analysis of the U3 region [24, 25].
2.7. Statistical analysis
Unpaired comparison for serum levels of gp70 and levels of different retroviral gp70 RNAs and Grk5 mRNA in livers was analyzed by Student's t test. Probability values <5% were considered significant.
3. Results
3.1. Enhanced hepatic expression of xenotropic and mPT gp70 RNAs in BALB/c congenic mice bearing the NZW-Sgp5 allele
BALB.Sgp5 congenic mice bearing the NZW-Sgp5 allele displayed a 6.5- fold higher concentration of gp70 in sera than that observed with BALB/c mice (P < 0.0001; Table 1). However, their gp70 levels were still more than 10 times lower than those of NZW mice (P < 0.0001). Moreover, it is noted that serum levels of gp70 in BALB/c and BALB.Sgp5 mice were substantially lower than those of B6 and B6.Sgp3 mice, respectively (P < 0.0001; Table 2).
Table 1.
Levels of serum gp70 and hepatic retroviral gp70 RNAs in BALB/c, BALB.Sgp5 and NZW mice.
| gp70 RNAb | ||||
|---|---|---|---|---|
| Mice | Serum gp70a | Xeno | PT | mPT |
| BALB/c | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| BALB.Sgp5 | 5.2 ± 0.2 | 24.6 ± 5.5 | 1.0 ± 0.1 | 102.4 ± 2.6 |
| NZW | 65.5 ± 3.8 | 457.8 ± 56.0 | 5.1 ± 0.3 | 118.7 ± 13.2 |
Serum levels of gp70 (µg/ml; mean ± SEM of 10–12 male mice at 2–3 months of age).
Levels of each gp70 RNA (mean ± SEM of 5 male mice at 2–3 months of age) were quantified using a standard curve generated with serial dilutions of a reference cDNA preparation and normalized using TBP mRNA. Results are expressed as fold increases of each transcript relative to BALB/c mice.
Table 2.
Levels of serum gp70 and hepatic retroviral gp70 RNAs in B6 Sgp congenic mice.
| gp70 RNAb | ||||
|---|---|---|---|---|
| Mice | Serum gp70a | Xeno | PT | mPT |
| B6 | 3.6 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| B6.Sgp3 | 19.7 ± 1.3 | 10.4 ± 1.9 | 4.3 ± 0.5 | 14.9 ± 1.8 |
| B6.Sgp4 | 7.8 ± 1.1 | 4.5 ± 0.7 | 1.5 ± 0.4 | 1.0 ± 0.1 |
| B6.Sgp3/4 | 47.1 ± 3.9 | 25.4 ± 3.5 | 4.2 ± 0.5 | 17.4 ± 2.8 |
| NZB | 78.5 ± 5.7 | 75.3 ± 4.1 | 6.1 ± 0.4 | 30.7 ± 2.7 |
Serum levels of gp70 (µg/ml; mean ± SEM of 10–12 male mice at 2–3 months of age).
Levels of each gp70 RNA (mean ± SEM of 5–6 male mice at 2–3 months of age) were quantified using a standard curve generated with serial dilutions of a reference cDNA preparation and normalized using TBP mRNA. Results are expressed as fold increases of each transcript relative to B6 mice.
When the levels of different retroviral gp70 RNAs were quantified, we observed that BALB.Sgp5 congenic mice displayed 25- and 102-fold increases in xenotropic and mPT gp70 RNAs (P < 0.005 and P < 0.0001), respectively, while the presence of the NZW-Sgp5 allele failed to upregulate the expression of PT gp70 RNA in BALB/c mice (Table 1). Notably, the levels of mPT RNA were comparable to those of NZW mice, while the proportion of xenotropic gp70 RNA in BALB.Sgp5 mice accounted for only 5% of those found in NZW mice.
3.2. Expression of restricted and distinct subpopulations of xenotropic proviruses in BALB/c and BALB.Sgp5 mice
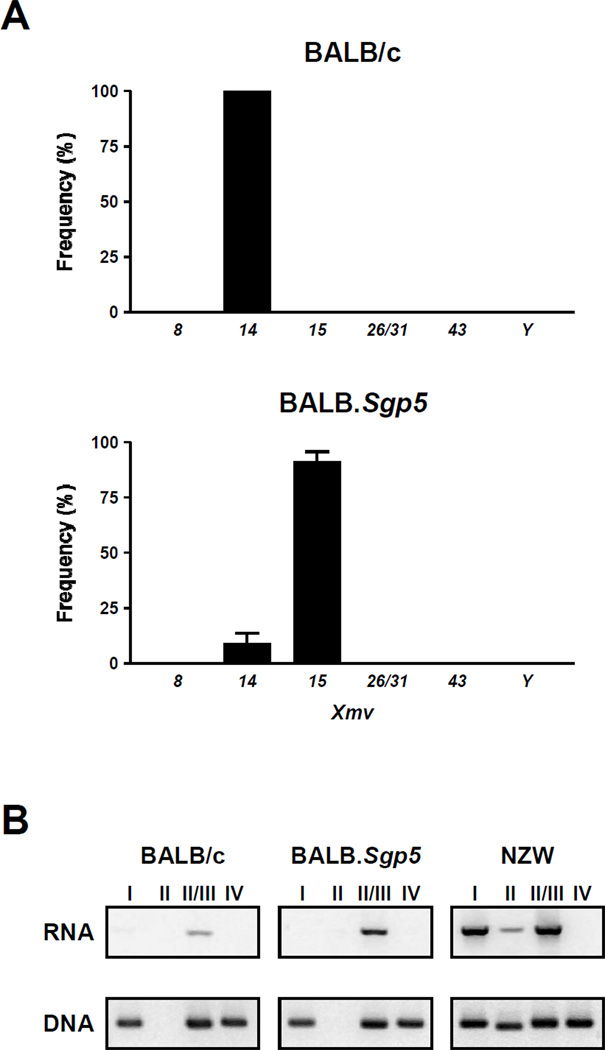
Restriction fragment length polymorphism analysis indicated that BALB/c mice carry 7 xenotropic proviral loci [28]. Five of them (Xmv8, Xmv14, Xmv15, Xmv43 and XmvY) are shared with B6 mice, while the Xmv10 provirus constitutively expressed in B6 mice [24] is absent in BALB/c mice (Table S1). Clonal analysis of xenotropic viral transcripts in livers revealed the expression of a single xenotropic proviral sequence, identical to that of Xmv14, in BALB/c mice (Fig. 1A). However, because of the lack of the information of nucleotide sequences of Xmv proviruses (Xmv26 and Xmv31) present in the BALB/c but not B6 genome, we could not formally exclude that the sequences of these proviruses are indistinguishable from Xmv14. In contrast, the presence of two distinct xenotropic viral transcripts was observed in BALB.Sgp5 congenic mice. The U3 sequence of the predominant xenotropic transcript with a frequency (mean ± SEM) of 80.5 ± 7.5% was identical to that of Xmv15, and the second corresponded to that of Xmv14 expressed in BALB/c mice (Fig. 1A). Both Xmv14 and Xmv15 belong to the Xeno-III subgroup [24] among four subgroups of xenotropic viruses, which differ by nucleotide sequences in a variable region of their env genes [21, 22]. The analysis of RT-PCR confirmed that only Xeno-III gp70 transcripts were detectably transcribed in BALB/c and BALB.Sgp5 congenic mice, despite the presence of Xeno-I, Xeno-III and Xeno-IV proviral sequences in the BALB/c genome (Fig. 1B).
Fig. 1.
Frequencies of different Xmv transcripts and the expression patterns of four different subgroups of xenotropic viruses in livers from BALB/c and BALB.Sgp5 mice.
(A) A region spanning the xenotropic env gene and the U3 region of xenotropic viral cDNA derived from livers was amplified, cloned and sequenced to identify the origin of Xmv proviruses expressed in livers of BALB/c and BALB.Sgp5 mice. ~20 clones from each of 3–4 individual mice were analyzed, and results are expressed as mean percentages (+ SEM) of each Xmv provirus expressed in livers.
(B) The presence of Xeno-I, Xeno-II, Xeno-III and Xeno-IV gp70 RNAs in livers of BALB/c, BALB.Sgp5 and NZW mice was determined by RT-PCR with a common forward primer (Xeno277F) and reverse primers specific for the four different subgroups of xenotropic viruses. Since the reverse primer designed for the amplification of Xeno-III gp70 also amplifies Xeno-II gp70, the results obtained with this primer are indicated as Xeno-II/III. Genomic DNA from different strains of mice was analyzed for the presence of Xeno-I, Xeno-II, Xeno- III and Xeno-IV gp70 sequences by PCR with the same sets of primers used for the detection of gp70 RNAs.
Our clonal analysis could not exclude the possibility that a xenotropic virus carrying the U3 sequence identical to Xmv15 is present in the NZW-Sgp5 interval and co-transferred when BALB.Sgp5 congenic mice were generated. However, this possibility was excluded, since none of the 11 xenotropic viral transcripts identified in NZW mice, designated Xmv-Nzw1-11 (Fig. S1), carried the U3 sequence of Xmv15. The absence of Xmv15 in NZW mice was corroborated by genomic PCR analyses (data not shown).
3.3. Expression of restricted and distinct subpopulations of mPT proviruses in BALB/c and BALB.Sgp5 mice
Many strains of mice expressed low levels of the intact WT form of mPT env transcript and/or two different env deletion mutants (D1 and D2) derived from Mpmv10 and Mpmv13, respectively [8]. In contrast, lupus-prone mice predominantly expressed the WT mPT env RNA at the near exclusion of the defective transcripts. This specific expression pattern was also observed in B6 mice bearing the Sgp3 locus derived from lupus-prone mice [8]. Therefore, we assessed the relative expression of the three different species of mPT env transcripts in BALB/c and BALB.Sgp5 mice. As shown in Fig. 2A, only D1 and D2 transcripts were detectable in BALB/c mice, while BALB.Sgp5 mice predominantly expressed the WT mPT env RNA, as in the case of lupus-prone NZW mice. The analysis of semi-quantitative RT-PCR specific for the three different mPT env sequences confirmed the absence of the WT mPT env transcript in BALB/c mice and its marked elevation in BALB.Sgp5 mice at levels comparable to NZW mice (Fig. 2B). Furthermore, no appreciable increases in D1 and D2 mPT env RNAs in BALB.Sgp5 mice were observed. Notably, the presence of multiple copies of mPT proviruses bearing the intact env gene in BALB/c mice was confirmed by genomic PCR (Fig. 2C).
Fig. 2.
RT-PCR and genomic PCR analysis of WT, D1 and D2 mPT env RNAs and proviruses in BALB/c, BALB.Sgp5 and NZW mice.
(A) The presence of three different species of mPT env RNAs in livers was determined by RT-PCR. Representative results of three individual animals are shown. As a control (Ctl), a mixture of three different plasmids containing WT, D1 and D2 clones was included. Note the predominant expression of WT env transcripts in BALB.Sgp5 and NZW mice. Lane 1: BALB/c; lane 2: BALB.Sgp5; lane 3: NZW.
(B) Semi-quantitative RT-PCR analysis for WT, D1 and D2 mPT env RNAs with reverse primers specific for the three different mPT env genes and a common forward mPT-specific primer was carried out with 5-fold serially diluted cDNAs from BALB/c, BALB.Sgp5 and NZW mice. As a control, the abundance of Gapdh mRNA was assessed in parallel. Representative results of three individual mice analyzed are shown. Note the absence of WT and D2 env transcripts in BALB/c and NZW mice, respectively.
(C) Genomic DNA from different strains of mice was analyzed for the presence of WT, D1 and D2 env sequences by PCR. Genomic DNA of B6 mice was used as a control. Note the absence of Mpmv13 (D2) provirus in NZW mice. Lane 1: BALB/c; lane 2: BALB.Sgp5; lane 3: NZW.
It was reported that BALB/c mice carry 8 mPT proviral loci [29]. While four of them (Mpmv3, Mpmv4, Mpmv10 and Mpmv13) are shared with B6 mice, the Mpmv6 provirus expressed in B6 mice [25] is absent in the BALB/c genome (Table S2). Clonal analysis of mPT viral transcripts in livers confirmed the expression of only two Mpmv10 (D1) and Mpmv13 (D2) deletion mutants in BALB/c mice, in which Mpmv13 (D2) was predominantly transcribed at a frequency of 89.8 ± 4.9% (mean ± SEM; Fig. 3). In contrast, three different mPT viral transcripts were identified in BALB.Sgp5 congenic mice (Fig. 3). The most abundant transcript (89.6 ± 4.1%) carried the U3 sequence identical to that of Mpmv4, which is potentially replication competent since it appears to carry the intact gag, pol and env sequences [25]. Two other less frequent transcripts were derived from Mpmv13 (D2) (4.1 ± 2.0%) and an unidentified Mpmv provirus (6.4 ± 3.6%), which is one of the 4 Mpmv proviruses (Mpmv18, Mpmv19, Mpmv28 and Mpmv30) present in the BALB/c but not B6 genome. The possibility that an mPT virus carrying the U3 sequence identical to Mpmv4 was co-transferred from NZW mice into BALB.Sgp5 congenic mice was unlikely, because none of the 6 mPT viral transcripts identified in NZW mice, designated Mpmv-Nzw1-6 (Fig. S1), carried the sequence identical to that of Mpmv4 expressed in BALB.Sgp5 mice. Notably, the absence of Mpmv4 in the NZW genome was confirmed by genomic PCR analysis (data not shown).
Fig. 3.
Frequencies of different Mpmv transcripts in livers from BALB/c and BALB.Sgp5 mice.
A region spanning the mPT env gene and the U3 region of mPT viral cDNA derived from livers was amplified, cloned and sequenced to identify the origin of Mpmv proviruses expressed in BALB/c and BALB.Sgp5 mice. ~20 clones from each of 3–4 individual mice were analyzed, and results are expressed as mean percentages (+ SEM) of each Mpmv provirus expressed in livers.
3.4. Synergistic effect of the Sgp3 and Sgp4 loci on the production of serum gp70 and the expression of xenotropic gp70 RNA in B6 mice
Our previous studies of B6 congenic mice indicated that Sgp3 locus elevated levels of xenotropic, PT and mPT gp70 RNAs, while the Sgp4 locus elevated only xenotropic viruses [8, 22]. In those studies it was noted that levels of serum gp70 and hepatic xenotropic viral gp70 RNA in NZB and NZW mice were still far higher than the additive levels present in Sgp3 and Sgp4 B6 congenic mice [22], suggesting a synergistic effect of the two loci. To investigate this possibility, B6.Sgp3/4 double congenic mice were generated to examine the production of serum gp70. Serum gp70 levels in B6.Sgp3/4 mice were much higher than those of B6 (13.1-fold) or those of B6.Sgp3 (2.4-fold) and B6.Sgp4 (6.0-fold) mice (P < 0.0001 in each comparison; Table 2). Notably, mean increases of serum gp70 concentrations (43.5 µg/ml) in double congenic mice as compared with B6 mice exceeded the combined increases (20.3 µg/ml) obtained by the two single congenic mice, indicating that the two loci acted in a synergistic manner.
When the levels of different retroviral gp70 RNAs in livers were quantified, Sgp3/4 double congenic mice displayed highly significant increases in levels of xenotropic gp70 RNAs, as compared with Sgp single congenic mice (B6.Sgp3, P < 0.005 and B6.Sgp4, P < 0.0005; Table 2). Again, the extent of increases in xenotropic gp70 RNA in double congenic mice were approximately 2-fold larger than that of the combined effect of the two single congenic mice. In contrast, the abundance of PT and mPT RNAs in double congenic mice was comparable to that of B6.Sgp3 congenic mice (Table 2), further confirming that the Sgp4 locus only upregulated the expression of xenotropic gp70 RNA [8, 22].
3.5. Enhanced transcription of the Xmv18 provirus in B6.Sgp3/4 mice
Among 14 xenotropic proviruses present in the B6 genome, only two proviruses (Xmv10 and Xmv14) were actively transcribed in B6 mice [24]. In contrast, Sgp3 induced the transcription of three additional xenotropic viruses (Xmv15, Xmv17 and Xmv18), while Sgp4 induced the expression of a different xenotropic virus (Xmv13) (Fig. 4). Notably, Xmv13 and Xmv14 proviruses are deleted in B6.Sgp3/4 B6 mice upon the introduction of the NZB-derived Sgp3 and Sgp4 intervals into B6 mice because of their presence within the respective Sgp intervals of B6 mice, but absence in NZB mice (Table S1). Therefore, markedly increased levels of xenotropic gp70 RNA in double congenic mice suggested that Sgp3 and Sgp4 acted synergistically to elevate the expression of all or some of the Xmv proviruses (Xmv10, Xmv15, Xmv17 and Xmv18).
Fig. 4.
Frequencies of different Xmv transcripts in livers from B6.Sgp3, B6.Sgp4 and B6.Sgp3/4 congenic mice.
A region spanning the xenotropic env gene and the U3 region of xenotropic viral cDNA derived from livers was amplified, cloned and sequenced to identify the origin of Xmv proviruses expressed in different B6 Sgp congenic mice. ~20 clones from each of 4–5 individual mice were analyzed, and results are expressed as mean percentages (+ SEM) of each Xmv provirus expressed in livers. The results of B6.Sgp3 and B6.Sgp4 mice were derived from the previous study [24]. Potentially replication-competent viruses are indicated by asterisks.
A clonal analysis of xenotropic viral transcripts revealed the expression of four xenotropic proviruses (Xmv10, Xmv15, Xmv17 and Xmv18) in livers of B6.Sgp3/4 congenic mice, as expected from the expression profiles of Sgp3 and Sgp4 single congenic mice and the deletion of Xmv13 and Xmv14 in double congenic mice (Fig. 4). However, the frequency of expression of the xenotropic proviruses in the B6.Sgp3/4 mice was markedly different from the frequencies observed in the single congenic mice. Most notably, double congenic mice expressed Xmv18 at the highest frequency of 53.3 ± 4.6% (mean ± SEM), while the expression of Xmv18 was rather modest in B6.Sgp3 (18.5 ± 3.4%; P < 0.0001) or undetectable in B6.Sgp4 mice. It should be stressed that Xmv18 is potentially replication competent since it appears to carry the intact gag, pol and env gene sequences [24]. It is also noteworthy that the frequencies of Xmv10 and Xmv15 were differentially affected in the double congenic mice compared to the single congenic mice. Xmv10, the most frequently expressed xenotropic virus in B6.Sgp3 (62.5 ± 2.7%) and B6.Sgp4 (59.6 ± 12.8%), exhibited a lower frequency in the B6.Sgp3/4 mice (21.7 ± 4.1%; P < 0.0001 and P < 0.05, respectively). In contrast, the frequency of Xmv15 was not decreased but instead appeared to be elevated in the double congenic mice (B6.Sgp3/4: 15.7 ± 6.8% and B6.Sgp3: 3.1 ± 1.3%), although the difference was not statistically significant.
Xmv10 belongs to the Xeno-I subgroup, while Xmv18 and Xmv15 belong to the Xeno-III subgroup [24]. Indeed, levels of Xeno-I gp70 RNA of double congenic mice were comparable to those of Sgp3 and Sgp4 mice (Table 3). In contrast, 7- and more than 100-fold increases in Xeno-II/III gp70 RNA in B6.Sgp3/4 mice were noted as compared with Sgp3 and Sgp4 mice (P < 0.0001). These increases were likely to be due to a selectively enhanced transcription of the Xeno-III virus, since the abundance of Xeno-II gp70 RNA in B6.Sgp3/4 congenic mice was nearly identical to that of B6.Sgp3 congenic mice, and Xeno- II gp70 RNA was hardly detectable in B6.Sgp4 mice. These data thus confirmed increased frequencies of Xmv18 and Xmv15 (Xeno-III) transcripts and a decrease in the frequency of Xmv10 (Xeno-I) transcript in B6.Sgp3/4 congenic mice, as compared with Sgp single congenic mice.
Table 3.
Levels of xenotropic gp70 RNAs in livers of B6 Sgp congenic mice.
| gp70 RNAa | |||
|---|---|---|---|
| Mice | Xeno-I | Xeno-II | Xeno-II/III |
| B6 | 0.15 ± 0.02 | <0.01 | 0.20 ± 0.04 |
| B6.Sgp3 | 1.00 ± 0.13 | 1.00 ± 0.27 | 1.00 ± 0.11 |
| B6.Sgp4 | 1.27 ± 0.11 | <0.01 | 0.04 ± 0.01 |
| B6.Sgp3/4 | 1.32 ± 0.09 | 1.03 ± 0.15 | 6.83 ± 0.24 |
Levels of each gp70 RNA (mean ± SEM of 5–7 male mice at 2–3 months of age) were quantified using a standard curve generated with serial dilutions of a reference cDNA preparation and normalized using TBP mRNA. Results are expressed as fold increases of each transcript relative to B6.Sgp3 mice, since Xeno II and Xeno II/III gp70 RNAs were very weak or hardly detectable in WT B6 and B6.Sgp4 mice.
High-level transcription of Xmv18 can be due to a unique integration site of this provirus, as it is integrated in the opposite transcription direction within the 2nd intron of the Grk5 gene encoding G protein-coupled receptor kinase 5 [24]. However, the abundance of Grk5 mRNA was not significantly elevated in livers of B6.Sgp3/4 mice (mean ± SEM: 1.6 ± 0.4), as compared with B6 and Sgp single congenic mice (B6, 1.0 ± 0.3; B6.Sgp3, 1.3 ± 0.3; B6.Sgp4, 1.4 ± 0.3). These results thus argued against the possibility that the enhanced expression of Xmv18 in B6.Sgp3/4 mice was a result of co-regulated transcription of the host gene, in which Xmv18 is integrated.
4. Discussion
The envelope glycoprotein, gp70, of endogenous retroviruses secreted by hepatocytes represents one of the major nephritogenic autoantigens implicated in murine SLE. Its expression is controlled by the major and minor regulatory loci, Sgp3 and Sgp4, respectively, in lupus-prone mice [11, 16, 18]. The Sgp3 locus influences the transcription of xenotropic, PT and mPT viruses, while the Sgp4 locus the transcription of only xenotropic viral sequences [8, 22]. To further define the genetic mechanism responsible for the expression of endogenous retroviruses and their gene product, serum gp70, we extended our analysis to the third Sgp5 locus linked to serum levels of gp70 [19] and also examined a possible interaction between Sgp3 and Sgp4 loci to promote the expression of endogenous retroviruses. Our results showed that the Sgp5 locus regulated in trans the expression of xenotropic and mPT viruses, which contrasts with the specificity of Sgp3 for three different classes of retroviruses and Sgp4 for only one class. Moreover, our results demonstrated that Sgp3 and Sgp4 loci do act synergistically to markedly elevate the transcription of a potentially replication-competent xenotropic virus, Xmv18, the expression of which was induced by the presence of the Sgp3, but not Sgp4 locus alone.
Among 7 Xmv and 8 Mpmv proviruses present in the BALB/c genome, a xenotropic (Xmv14) and two mPT (Mpmv10 and Mpmv13) viruses were selectively expressed in BALB/c mice. Our previous studies showed that B6 mice actively transcribed two xenotropic (Xmv10 and Xmv14) and three mPT (Mpmv6, Mpmv10 and Mpmv13) viruses [24, 25]. These differences reflect the absence of Xmv10 and Mpmv6 proviruses in the BALB/c genome and are also consistent with the finding that levels of xenotropic and mPT gp70 RNAs in livers as well as serum levels of gp70 in BALB/c mice were much lower than those in B6 mice [8].
It is noteworthy that increases in serum levels of gp70 in BALB.Sgp5 mice were modest, as compared with those observed in B6.Sgp3 congenic mice, despite the fact that Sgp5 was identified as the major quantitative trait locus in genetic crosses involving NZW and BALB/c mice [19]. This quantitative difference in the effects of the two loci is likely explained by the findings that Sgp5 induced the transcription of only one xenotropic and two mPT viruses in BALB/c mice, while Sgp3 is able to induce the expression of multiple xenotropic and mPT viruses in B6 mice [24, 25]. This difference is in part due to the absence in BALB/c mice of several proviruses (Xmv10, Xmv17, Xmv18 and Mpmv11) which were transcribed at a high frequency in B6.Sgp3 mice. Thus, levels of serum gp70 in various murine strains can be determined by the inheritance of not only multiple regulatory genes but also selective subsets of endogenous retroviruses responsive to different regulatory genes.
We have previously shown that the Xmv13 and Xmv14 proviruses are respectively located in the Sgp3 and Sgp4 intervals of B6 mice, but deleted in B6.Sgp3/4 double congenic mice because of their absence in NZB mice [24]. Despite the absence of these two proviruses which were actively transcribed in Sgp4 and Sgp3 congenic mice, respectively, we observed that the serum levels of gp70 and hepatic xenotropic gp70 RNA in double congenic mice were more than two times larger than the added values of two single Sgp congenic mice. These data indicate that Sgp3 and Sgp4 act synergistically to promote the transcription of xenotropic viruses and hence the production of serum gp70. Notably, clonal analysis of xenotropic viral transcripts in livers of B6.Sgp3/4 double congenic mice revealed an unexpected expression pattern of Xmv proviruses, in which the frequency of Xmv18 transcript was the highest. This was contrasted with the fact that Xmv10 was most frequently transcribed in both Sgp3 and Sgp4 single congenic mice and that Xmv18 transcripts were not detectably induced in B6.Sgp4 mice. This suggests that the Sgp4 locus harbors a gene which does not directly induce the transcription of Xmv18, but promotes the transcription by influencing a gene(s) controlling the expression of Xmv18 present in the Sgp3 locus. This suggests an additional level of complexity of the genetic control of the expression of endogenous retroviruses.
Analyses of BALB.Sgp5 congenic mice revealed marked increases in mPT gp70 RNA at a level comparable to that of NZW mice, but only moderate increases in xenotropic gp70 RNA, the level of which was still ~20 times less than that of NZW mice. Notably, serum levels of gp70 in BALB.Sgp5 mice were far lower than those in NZW mice, supporting the idea that xenotropic viral gp70 represents the major source of serum gp70 [30–32]. This is in agreement with the demonstration that marked elevation in serum levels of gp70 in B6.Sgp3/4 double congenic mice as compared with single congenic mice was associated with selective increases in xenotropic gp70 RNA.
It has previously been shown that the expression patterns of three different species (WT and 2 deletion mutants) of mPT env RNAs in livers were highly variable among various strains of mice [8]. Part of this variation is due to the absence of the Mpmv13 (D2) provirus in several strains, such as NZW. In addition, the present study revealed that the absence of the WT mPT env RNA in BALB/c mice is likely to be due to the lack of Mpmv6 carrying the intact env gene, which is constitutively expressed in B6 mice, independently of the presence of the Sgp3 locus [25]. Moreover, like Sgp3, Sgp5 induced the predominant and abundant expression of the WT mPT proviruses [8]. Thus, the presence of both Sgp3 and Sgp5 loci has an impact on the expression of mPT viruses in lupus-prone mice, thereby promoting the formation of nephritogenic anti-gp70 autoantibodies.
Both Sgp3 and Sgp5 loci have been shown to contribute to the development of anti-gp70 autoimmune responses [10, 11, 19], which is dependent on TLR7 signaling [8, 9]. Since it is unlikely that virion-free serum gp70 would trigger TLR7, endogenous retroviral virions carrying single-stranded RNA may promote the formation of autoantibodies against serum gp70 through the activation of TLR7. Our previous and present analyses raised the possibility that the Sgp3 and Sgp5 loci are able to induce the generation of replication-competent mPT viruses [25], which are able to utilize the xenotropic and polytropic retrovirus receptor (XPR1) for infectious entry [33]. Thus, the highly enhanced expression of replication-competent mPT viruses in lupus-prone mice by the presence of multiple Sgp loci could facilitate their internalization and ability to gain access to TLR7 in plasmacytoid dendritic cells. Activation of these cells could aggravate the autoimmune process, as they secrete robust amounts of IFNα, a cytokine prominently involved in the pathogenesis of SLE [34, 35]. Notably, it has been shown that the spontaneous formation of anti-DNA autoantibodies was linked to the Sgp3 and Sgp5 loci [10, 11, 18, 36]. Moreover, the present analysis suggested that Sgp3 and Sgp4 loci act synergistically to markedly enhance the production of a potentially replicationcompetent Xmv18 virus in B6 mice. Although the polymorphic form of XPR1 expressed in laboratory strains of mice does not confer susceptibility to xenotropic viruses [33], non-infectious xenotropic viruses can be internalized through gp70-specific BCR and gain access to TLR7. This may lead to the activation of gp70-specific B cells, thereby contributing to the development of lupus nephritis.
A cluster of 21 KRAB (Krüppel-associated box)-ZFPs (zinc finger protein) [37, 38] has been identified among 30 genes mapped to the Sgp3 locus, which has been narrowed down to an interval between 64.5 and 70.0 Mb of chromosome 13. Although the target genes regulated by most of these Zfp genes are still unknown [37, 38], it has been shown that the expression of endogenous retroviruses was down-modulated by several ZFPs [39–41] and that ZFP809 suppressed the transcription of a retroviral gene through recognition of a point mutation in the tRNA primer-binding site [40]. Thus, distinct sets of endogenous retroviruses could be the target of different KRAB-ZFPs. It is of interest to note that 3 and 2 Zfp genes are also mapped to the Sgp4 and Sgp5 loci, respectively. Analysis of polymorphism and expression levels of these different Zfp genes might help identify the candidate genes for Sgp.
In conclusion, the present analysis revealed that the Sgp5 locus acts differently from two other Sgp loci in terms of the specificity to three different classes of retroviruses and that Sgp3 and Sgp4 cooperate in a synergistic manner to enhance the transcription of xenotropic viruses and serum gp70. Thus, a concerted action of these three loci could lead to a markedly increased expression of endogenous retroviruses and their gene product, serum gp70, thereby participating in the development of murine lupus nephritis. Further studies on the actions and interactions of these loci will help further define the extent to which endogenous retroviruses contribute to the development of murine SLE. The eventual identification of the Sgp genes will enable us to address the relevance of their human counterparts, thus providing a clue for the potential role of endogenous retroviruses in human SLE.
Supplementary Material
Highlights.
Retroviral gp70 is one of the major nephritogenic antigens in murine lupus nephritis.
Endogenous xenotropic retrovirus is the major source of serum retroviral gp70.
Its expression is controlled by Sgp3, Sgp4 and Sgp5 loci in lupus-prone mice.
Each locus independently regulates three distinct classes of endogenous retroviruses.
Sgp3 and Sgp4 act synergistically to enhance the expression of xenotropic viruses.
Acknowledgments
This work was supported by the Swiss National Foundation for Scientific Research (grant 310030_127644). L.H.E. was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank Mr. Guy Brighouse and Ms Montserrat Alvarez for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hara I, Izui S, Dixon FJ. Murine serum glycoprotein gp70 behaves as an acute phase reactant. J Exp Med. 1982;155:345–357. doi: 10.1084/jem.155.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izui S, McConahey PJ, Theofilopoulos AN, Dixon FJ. Association of circulating retroviral gp70-anti-gp70 immune complexes with murine systemic lupus erythematosus. J Exp Med. 1979;149:1099–1116. doi: 10.1084/jem.149.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izui S, McConahey PJ, Clark JP, Hang LM, Hara I, Dixon FJ. Retroviral gp70 immune complexes in NZB x NZW F2 mice with murine lupus nephritis. J Exp Med. 1981;154:517–528. doi: 10.1084/jem.154.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maruyama N, Furukawa F, Nakai Y, Sasaki Y, Ohta K, Ozaki S, et al. Genetic studies of autoimmunity in New Zealand mice. IV. Contribution of NZB and NZW genes to the spontaneous occurrence of retroviral gp70 immune complexes in (NZB x NZW)F1 hybrid and the correlation to renal disease. J Immunol. 1983;130:740–746. [PubMed] [Google Scholar]
- 5.Izui S, Kelley VE, McConahey PJ, Dixon FJ. Selective suppression of retroviral gp70-anti-gp70 immune complex formation by prostaglandin E1 in murine systemic lupus erythematosus. J Exp Med. 1980;152:1645–1658. doi: 10.1084/jem.152.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vyse TJ, Drake CG, Rozzo SJ, Roper E, Izui S, Kotzin BL. Genetic linkage of IgG autoantibody production in relation to lupus nephritis in New Zealand hybrid mice. J Clin Invest. 1996;98:1762–1772. doi: 10.1172/JCI118975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haywood MEK, Vyse TJ, McDermott A, Thompson EM, Ida A, Walport MJ, et al. Autoantigen glycoprotein 70 expression is regulated by a single locus, which acts as a checkpoint for pathogenic anti-glycoprotein 70 autoantibody production and hence for the corresponding development of severe nephritis, in lupus-prone BXSB mice. J Immunol. 2001;167:1728–1733. doi: 10.4049/jimmunol.167.3.1728. [DOI] [PubMed] [Google Scholar]
- 8.Yoshinobu K, Baudino L, Morito N, Dunand-Sauthier I, Morley BJ, Evans LH, et al. Selective up-regulation of intact, but not defective env RNAs of endogenous modified polytropic retrovirus by the Sgp3 locus of lupusprone mice. J Immunol. 2009;182:8094–8103. doi: 10.4049/jimmunol.0900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santiago-Raber ML, Dunand-Sauthier I, Wu T, Li QZ, Uematsu S, Akira S, et al. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J Autoimmun. 2010;34:339–348. doi: 10.1016/j.jaut.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Obata Y, Tanaka T, Stockert E, Good RA. Autoimmune and lymphoproliferative disease in (B6-GIX+ × 129)F1 mice: relation to naturally occurring antibodies against murine leukemia virus-related cell surface antigens. Proc Natl Acad Sci USA. 1979;76:5289–5293. doi: 10.1073/pnas.76.10.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rankin J, Boyle JJ, Rose SJ, Gabriel L, Lewis M, Thiruudaian V, et al. The Bxs6 locus of BXSB mice is sufficient for high-level expression of gp70 and the production of gp70 immune complexes. J Immunol. 2007;178:4395–43401. doi: 10.4049/jimmunol.178.7.4395. [DOI] [PubMed] [Google Scholar]
- 12.Yoshiki T, Mellors RC, Strand M, August JT. The viral envelope glycoprotein of murine leukemia virus and the pathogenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med. 1974;140:1011–1027. doi: 10.1084/jem.140.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strand M, August JT. Oncornavirus envelope glycoprotein in serum of mice. Virology. 1976;75:130–144. doi: 10.1016/0042-6822(76)90012-x. [DOI] [PubMed] [Google Scholar]
- 15.Tucker RM, Vyse TJ, Rozzo S, Roark CL, Izui S, Kotzin BL. Genetic control of gp70 autoantigen production and its influence on immune complex levels and nephritis in murine lupus. J Immunol. 2000;165:1665–1672. doi: 10.4049/jimmunol.165.3.1665. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi S, Fossati-Jimack L, Moll T, Amano H, Amano E, Ida A, et al. Differential role of three major NZB-derived loci linked with Yaa -induced murine lupus nephritis. J Immunol. 2005;174:1111–1117. doi: 10.4049/jimmunol.174.2.1111. [DOI] [PubMed] [Google Scholar]
- 17.Santiago ML, Mary C, Parzy D, Jacquet C, Montagutelli X, Parkhouse RME, et al. Linkage of a major quantitative trait locus to Yaa gene-induced lupuslike nephritis in (NZW × C57BL/6)F1 mice. Eur J Immunol. 1998;28:4257–4267. doi: 10.1002/(SICI)1521-4141(199812)28:12<4257::AID-IMMU4257>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Laporte C, Ballester B, Mary C, Izui S, Reininger L. The Sgp3 locus on mouse chromosome 13 regulates nephritogenic gp70 autoantigen and predisposes to autoimmunity. J Immunol. 2003;171:3872–3877. doi: 10.4049/jimmunol.171.7.3872. [DOI] [PubMed] [Google Scholar]
- 19.Rigby RJ, Rozzo SJ, Gill H, Fernandez-Hart T, Morley BJ, Izui S, et al. A novel locus regulates both retroviral glycoprotein 70 and anti-glycoprotein 70 and antibody production in New Zealand mice when crossed with BALB/c. J Immunol. 2004;172:5078–5085. doi: 10.4049/jimmunol.172.8.5078. [DOI] [PubMed] [Google Scholar]
- 20.Stoye JP, Coffin JM. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J Virol. 1987;61:2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamont C, Culp P, Talbott RL, Phillips TR, Trauger RJ, Frankel WN, et al. Characterization of endogenous and recombinant proviral elements of a highly tumorigenic AKR cell line. J Virol. 1991;65:4619–4628. doi: 10.1128/jvi.65.9.4619-4628.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baudino L, Yoshinobu K, Morito N, Kikuchi S, Fossati-Jimack L, Morley BJ, et al. Dissection of genetic mechanisms governing the expression of serum retroviral gp70 implicated in murine lupus nephritis. J Immunol. 2008;181:2846–2854. doi: 10.4049/jimmunol.181.4.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frankel WN, Stoye JP, Taylor BA, Coffin JM. Genetic identification of endogenous polytropic proviruses by using recombinant inbred mice. J Virol. 1989;63:3810–3821. doi: 10.1128/jvi.63.9.3810-3821.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kihara M, Leroy V, Baudino L, Evans LH, Izui S. Sgp3 and Sgp4 control expression of distinct and restricted sets of xenotropic retroviruses encoding serum gp70 implicated in murine lupus nephritis. J Autoimmun. 2011;37:311–318. doi: 10.1016/j.jaut.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leroy V, Kihara M, Baudino L, Brighouse G, Evans LH, Izui S. Sgp3 and TLR7 stimulation differentially alter the expression profile of modified polytropic retroviruses implicated in murine systemic lupus. J Autoimmun. 2012;38:361–368. doi: 10.1016/j.jaut.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baudino L, Changolkar LN, Pehrson JR, Izui S. The Sgp3 locus derived from the 129 strain is responsible for enhanced endogenous retroviral expression in macroH2A1-deficient mice. J Autoimmun. 2010;35:398–403. doi: 10.1016/j.jaut.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merino R, Fossati L, Lacour M, Lemoine R, Higaki M, Izui S. H-2-linked control of the Yaa gene-induced acceleration of lupus-like autoimmune disease in BXSB mice. Eur J Immunol. 1992;22:295–299. doi: 10.1002/eji.1830220202. [DOI] [PubMed] [Google Scholar]
- 28.Frankel WN, Stoye JP, Taylor BA, Coffin JM. Genetic analysis of endogenous xenotropic murine leukemia viruses: association with two common mouse mutations and the viral restriction locus Fv-1. J Virol. 1989;63:1763–1774. doi: 10.1128/jvi.63.4.1763-1774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frankel WN, Stoye JP, Taylor BA, Coffin JM. A linkage map of endogenous murine leukemia proviruses. Genetics. 1990;124:221–236. doi: 10.1093/genetics/124.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elder JH, Jensen FC, Bryant ML, Lerner RA. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differential antigens encoded by a multi-gene family. Nature. 1977;267:23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- 31.Elder JH, Gautsch JW, Jensen FC, Lerner RA, Chused TM, Morse HC, et al. Differential expression of two distinct xenotropic viruses in NZB mice. Clin Immunol Immunopathol. 1980;15:493–501. doi: 10.1016/0090-1229(80)90061-6. [DOI] [PubMed] [Google Scholar]
- 32.Izui S, Elder JH, McConahey PJ, Dixon FJ. Identification of retroviral gp70 and anti-gp70 antibodies involved in circulating immune complexes in NZB × NZW mice. J Exp Med. 1981;153:1151–1160. doi: 10.1084/jem.153.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marin M, Tailor CS, Nouri A, Kozak SL, Kabat D. Polymorphisms of the cell surface receptor control mouse susceptibilities to xenotropic and polytropic leukemia viruses. J Virol. 1999;73:9362–9368. doi: 10.1128/jvi.73.11.9362-9368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 35.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigby RJ, Rozzo SJ, Boyle JJ, Lewis M, Kotzin BL, Vyse TJ. New loci from New Zealand Black and New Zealand White mice on chromosomes 4 and 12 contribute to lupus-like disease in the context of BALB/c. J Immunol. 2004;172:4609–4617. doi: 10.4049/jimmunol.172.7.4609. [DOI] [PubMed] [Google Scholar]
- 37.Krebs CJ, Larkins LK, Price R, Tullis KM, Miller RD, Robins DM. Regulator of sex-limitation (Rsl) encodes a pair of KRAB zinc-finger genes that control sexually dimorphic liver gene expression. Genes Dev. 2003;17:2664–2674. doi: 10.1101/gad.1135703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krebs CJ, Larkins LK, Khan SM, Robins DM. Expansion and diversification of KRAB zinc-finger genes within a cluster including Regulator of sex-limitation 1 and 2. Genomics. 2005;85:752–761. doi: 10.1016/j.ygeno.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Bulliard Y, Wiznerowicz M, Barde I, Trono D. KRAB can repress lentivirus proviral transcription independently of integration site. J Biol Chem. 2006;281:35742–35746. doi: 10.1074/jbc.M602843200. [DOI] [PubMed] [Google Scholar]
- 40.Wolf D, Goff SP. Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature. 2009;458:1201–1204. doi: 10.1038/nature07844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowe HM, Jakobsson J, Mesnard D, Rougemont J, Reynard S, Aktas T, et al. KAP1 controls endogenous retroviruses in embryonic stem cells. Nature. 2010;463:237–240. doi: 10.1038/nature08674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.