Abstract
Given the early onset of neuropsychiatric disease and the potential response to immunosuppressive therapy, neuropsychiatric disease is considered a primary disease manifestation in SLE. However, the pathogenesis is not fully understood and optimal treatment has yet to be determined. TWEAK is a TNF family ligand that mediates pleotropic effects through its receptor Fn14, including the stimulation of inflammatory cytokines by astrocytes, endothelial cells, and other non-hematopeotic cell types, and induction of neuronal death. Furthermore, TWEAK-inducible mediators are implicated in neuropsychiatric lupus. Thus, we hypothesized that the TWEAK/Fn14 pathway may be involved in the pathogenesis of neuropsychiatric SLE. We generated MRL-lpr/lpr (MRL/lpr) mice deficient for Fn14, the sole known signaling receptor for TWEAK. Neuropsychiatric disease was compared in age- and gender-matched MRL/lpr Fn14 wild type (WT) and knockout (KO) mice, using a comprehensive battery of neurobehavioral tests. We found that MRL/lpr Fn14WT mice displayed profound depression-like behavior as seen by increased immobility in a forced swim test and loss of preference for sweetened fluids, which were significantly ameliorated in Fn14KO mice. Similarly, MRL/lpr Fn14WT mice had impaired cognition, and this was significantly improved in Fn14KO mice. To determine the mechanism by which Fn14 deficiency ameliorates neuropsychiatric disease, we assessed the serum levels of autoantibodies and local expression of cytokines in the cortex and hippocampus of lupus mice. No significant differences were found in the serum levels of antibodies to nuclear antigens, or autoantibodies specifically associated with neuropsychiatric disease, between MRL/lpr Fn14WT and KO mice. However, MRL/lpr Fn14KO mice had significantly decreased brain expression of RANTES, C3, and other proinflammatory mediators. Furthermore, MRL/lpr Fn14KO mice displayed improved blood brain barrier integrity. In conclusion, several central manifestations of neuropsychiatric lupus, including depression-like behavior and altered cognition, are normalized in MRL/lpr mice lacking Fn14. Our results are the first to indicate a role for the TWEAK/Fn14 pathway in the pathogenesis of neuropsychiatric lupus, and suggest this ligand-receptor pair as a potential therapeutic target for a common and dangerous disease manifestation.
Keywords: Systemic lupus erythematosus (SLE), neuropsychiatric lupus, TWEAK, Fn14
1. Introduction
The neuropsychiatric manifestations that appear in lupus patients are quite diverse, and may include seizures, depression, anxiety, cognitive disorders, and psychosis [1]. Although the exact prevalence of neuropsychiatric lupus is not known, an estimated >50% of lupus patients display one or more neuropsychiatric manifestations, making it one of the more common expressions of disease [2].
While patients with lupus can have multiple factors that may provoke neuropsychiatric signs and symptoms, depression and cognitive impairment are found even in patients with newly diagnosed disease [3]. In murine models as well, neuropsychiatric disease can appear early, is one of the prominent clinical manifestations, and is primarily driven by the autoimmune process [2]. Significantly, immunosuppressive treatment reverses some of the associated abnormalities in animal models [4].
The mechanisms involved in neuropsychiatric SLE (NPSLE) have yet to be fully clarified. This is particularly important in light of the non-curative nature of therapies in current use and their non-targeted mechanism of action. Multiple pathways have been linked to the pathogenesis of NPSLE, such as autoantibodies, cytokines, complement, vasculitis, and thrombosis, leading to neuroinflammation, neurodegeneration, and cell death [5, 6].
Members of the TNF superfamily play an important role in the pathogenesis of SLE [7]. TWEAK is a secreted member of the TNF-ligand superfamily with pleotropic effects on multiple cell types, including enhancement of the inflammatory milieu and context dependent effects on cell survival and apoptosis. In the central nervous system (CNS) the TWEAK receptor Fn14 is expressed by astrocytes, microglia, neurons, and microvascular endothelial cells [8-10]. TWEAK induces typical inflammatory responses in cultured glial cells [9], and can mediate poly ADP ribose polymerase [PARP] activation and neuronal apoptosis [11]. Of note, the TWEAK/Fn14 pathway was shown to be important in animal models of multiple sclerosis [8], as well as during cerebral ischemia [11].
In preliminary studies we found that patients with NPSLE demonstrate high CSF TWEAK levels [12]. Given the expression of TWEAK and Fn14 in the CNS, this pathway may be directly involved in the pathogenesis of NPSLE by promoting the local secretion of inflammatory cytokines. Indeed, cytokines are among the putative mediators of NPSLE, as they reliably induce depression [13] and cognitive dysfunction [14] in humans and in animal models. Moreover, through its promotion of blood brain barrier [BBB] dysfunction (at least during ischemia) [11], TWEAK can also result in access to the CNS of systemic factors, including autoantibodies, cytokines, or other proinflammatory mediators. Therefore, we hypothesized that TWEAK is involved in the pathogenesis of NPSLE.
Although several lupus prone mouse strains develop neuropsychiatric manifestations, MRL-lpr/lpr (MRL/lpr) mice have several advantages including early onset and rapid progression of disease, and neurobehavioral deficits with many similarities to human patients [2]. To elucidate the role for TWEAK signaling in the pathogenesis of murine NPSLE, we evaluated MRL/lpr mice lacking the TWEAK receptor Fn14 (MRL/lpr Fn14KO) in comparison with MRL/lpr Fn14WT mice.
2. Materials and methods
2.1. Mice
Female MRL/lpr Fn14KO mice (backcross generation #9) and WT littermates were bred at Biogen Idec and transferred to the Albert Einstein College of Medicine at 8-16 weeks of age. Control MRL/MpJ mice (MRL/+) were purchased from The Jackson Laboratory (Bar Harbor, Maine). The housing conditions were controlled, with a temperature of 21–23 °C and a 12:12 hour light:dark cycle. All animal study protocols were approved by the institutional animal care and use committee.
2.2. Assessment of disease
Serum IgG autoantibodies against DNA, chromatin, and the NMDA receptor at 16.5 and 22 weeks of age were measured by ELISA, as previously described [15]. Antibodies against ribosomal P (P0, P1, and P2) were measured using a commercially available kit from Aesku (Wendelsheim, Germany).
Measurement of serum IgG antibodies to cardiolipin was performed as previously described [15], with modifications [16]. In brief, cardiolipin at a concentration of 75 μg/ml in ethanol was adsorbed to Immulon II microtiter plates, and allowed to dry overnight at 4°C. Plates were then blocked with 5% fetal calf serum/3% bovine serum albumin (BSA) in PBS for 90 minutes at room temperature, followed by incubation with the serum samples diluted 1:250 in PBS/1% BSA for 2 hours at 37°C. The fetal calf serum in the blocking solution served as the source of beta-2-Glycoprotein I in this assay [16]. Following extensive washing, incubation with labeled secondary antibody and substrate then continued as above [15].
For analysis of anti-dsDNA antibodies in the CSF, plates were coated and blocked in 2% BSA for 1 hour as described [15]. CSF samples at a dilution of 1:20 in PBS/2% BSA were incubated on the plates for 4 hours at 37°C. Plates were then developed with biotinylated goat anti-mouse IgG (Southern Biotechnology, Birmingham, AL) at a 1:500 dilution for 2 hours, followed by streptavidin-alkaline phosphatase diluted 1:500 in PBS/2% BSA and substrate.
2.3. Behavioral assessment
A series of tests previously described in detail [17, 18] were employed to test the affective behavior, social behavior, and cognition of female MRL/+, MRL/lpr Fn14WT, and MRL/lpr Fn14KO mice at 10-40 weeks of age. Included in the panel were robust and reliable tests, which have been previously used in this and other labs to assess neuropsychiatric manifestations in murine lupus [17-20]. These tests were used to evaluate the impact of the Fn14 knockout on well established and reproducible behavioral abnormalities in MRL/lpr mice such as depressive-like behavior and cognitive dysfunction [2], and to assess potential unintended negative consequences of the Fn14KO. These tests included forced swim, open field testing, object placement and object recognition tests, balance beam crossing, and social preference. In addition, saccharin preference and olfactory tests were performed as described [21, 22].
For all the behavioral tests, mice were transferred to the test room and equilibrated for 30 minutes prior to the tests under low incandescent light. Except for the balance beam, all tests were digitally recorded by Viewer tracking software (Biobserve, Bonn, Germany).
2.4. Real-time PCR
RNA isolated from the cerebral cortex and hippocampus of 19 week old mice from a second independent cohort, and from half of a brain (cut in the sagittal plane) of a third mouse cohort at 40 weeks old, was used in real-time PCR studies to confirm the gene expression patterns. Real-time PCR was carried out in triplicate for TWEAK, Fn14, and selected genes. Values for the tested genes were normalized to GAPDH.
2.5. Immunohistochemistry
Five μm thick formalin fixed paraffin-embedded brain sections from mice perfused at sacrifice were incubated with rabbit anti-mouse RANTES (R&D Systems, Minneapolis, MN), or rabbit anti-mouse Fn14 primary antibody (Epitomics, Burlingame, CA) followed by the DakoCytomation Envision + Dual Link System-HRP kit (Dako, Carpinteria, CA), using the manufacturer’s instructions. Immunoreactivity was visualized with a 3,3-diaminobenzidine chromogen solution, and the slides then counterstained with Mayer’s hematoxylin and mounted.
2.6. Albumin quotient and IgG index
The permeability of the blood brain barrier was assessed by calculating the albumin quotient as follows: Albumin quotient = cerebrospinal fluid (CSF) albumin/serum albumin. Intrathecal IgG synthesis was measured by calculating the IgG index as follows: IgG index = (CSF IgG/serum IgG)/albumin quotient [23]. Albumin was quantitated using an albumin ELISA kit from Bethyl Laboratories (Montogomery, TX).
2.7. Statistics
JMP (SAS: Cary, NC) was used for the statistical analysis. An unpaired dunnet’s post hoc t test (two tailed) was used to compare the differences between two groups after significant main effects in an ANOVA. Correlation and linear regression analysis were also performed. For all the statistics, significance was defined as p<0.05.
3. Results
3.1. Fn14 is upregulated in MRL/lpr versus MRL/+ mice
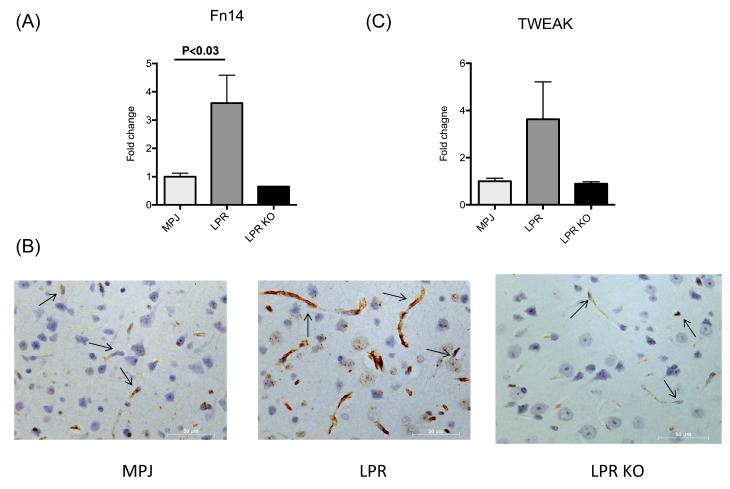
To assess the relevance of the TWEAK/Fn14 pathway in the pathogenesis of NPSLE, we analyzed the expression of TWEAK and Fn14 in 14 week old female MRL/lpr Fn14WT and MRL/+ mice. We found a significant increase in Fn14 gene expression in the cortex of MRL/lpr Fn14WT mice as compared to MRL/+ mice (Figure 1A). By immunohistochemistry, Fn14 expression was detected primarily on endothelial cells, and present both in cortex (Figure 1B) and hippocampus (data not shown). A similar trend was found with increased TWEAK gene expression in the cortex of MRL/lpr mice (Figure 1C).
Figure 1. Fn14 expression is upregulated in brains of MRL/lpr mice.
Fn14 (A, B) and TWEAK (C) expression were measured in cortical tissue of 14 week old female MRL/+ (“MPJ”) (n=4), MRL/lpr Fn14WT (“LPR”) (n=4), and MRL/lpr Fn14KO (“LPR KO”) (n=3) by real-time PCR (A,C) and Fn14 immunohistochemistry (B). For the PCR, fold changes were calculated in reference to the control MRL/+ mice, whose mean value was set at 1. In (B), representative images of Fn14 staining in frontal cortical tissue from MRL/+, MRL/lpr Fn14WT, and MRL/lpr Fn14KO (“LPR KO”) mice are shown. The arrows in the panels point to endothelial cells. Sections were examined using a Zeiss AxioObserver at 40X magnification.
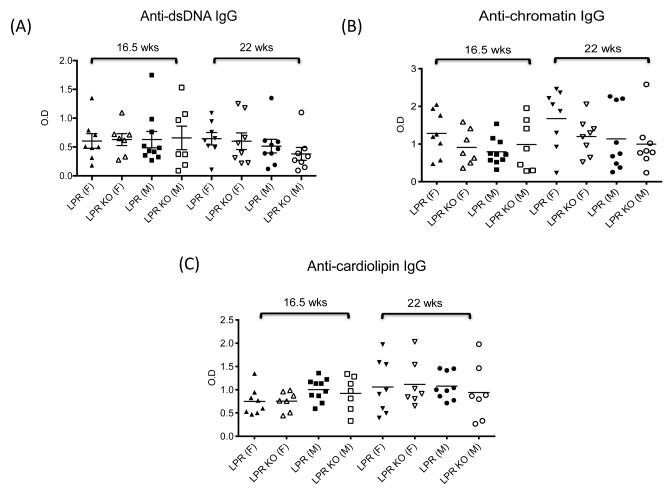
To assess possible effects on disease progression, we studied serum autoantibody titers. We found no significant differences in the titer of IgG anti-double stranded (ds) DNA and anti-chromatin antibodies in MRL/lpr Fn14WT compared to KO mice at weeks 16.5 and 22 (Figure 2A,B), around the time the first set of neurobehavioral assessments was performed. Similarly, no significant differences were observed in the titer of anti-single stranded (ss) DNA (IgM and IgG), and IgM anti-dsDNA and anti-chromatin antibodies (data not shown). Moreover, significant proteinuria had yet to develop in any of the test cohorts when the mice were first evaluated at 10-20 weeks of age (data not shown).
Figure 2. Titers of serum autoantibodies are similar in MRL/lpr Fn14WT and KO mice.
Titers of serum IgG anti-dsDNA (A), anti-chromatin (B), anti-cardiolipin (C), anti-ribosomal P (D), and anti-NMDA receptor (E) antibodies in MRL/lpr Fn14WT and KO mice at 16.5 and 22 weeks of age were determined by ELISA, as described in the Materials and methods.
Autoantibodies with particular antigenic specificities, including anti-cardiolipin, anti-ribosomal P, and anti-NMDA receptor antibodies, have been linked to the pathogenesis of NPSLE [24]. However, we found no significant differences in IgG anti-cardiolipin, anti-ribosomal P, and anti-NMDA receptor antibodies between MRL/lpr Fn14WT and KO mice at 16.5 and 22 weeks of age (Figure 2C-E).
3.2. Fn14 deficiency ameliorates depression-like behavior and improves cognition in MRL/lpr mice
To comprehensively evaluate the effect of Fn14 deficiency on neuropsychiatric disease, we employed a battery of tests commonly used in the evaluation of murine behavior and in assessment of spontaneous and induced mouse lupus models [5, 17]. MRL/lpr mice have been extensively studied and have been previously shown to exhibit emotional abnormalities and impaired cognition [19, 25]; therefore, we assessed these specific features in MRL/lpr Fn14WT and KO mice. Since lupus manifestations appear earlier and are generally more severe in female MRL/lpr mice, female and male mice were analyzed separately.
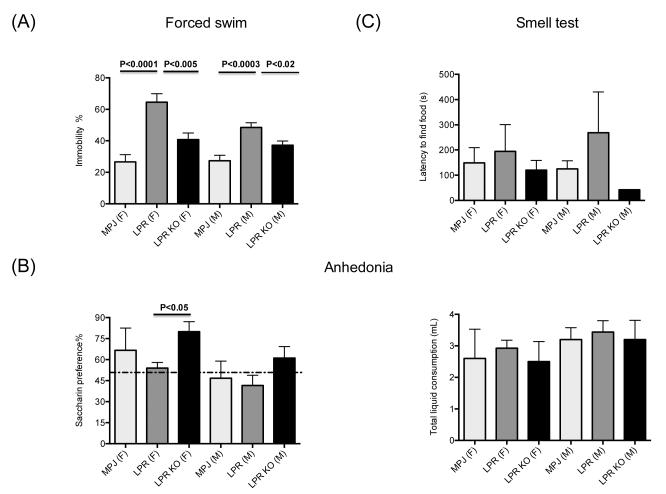
Depression is an early and common manifestation of neuropsychiatric disease in human lupus. Previously, we demonstrated that MRL/lpr mice display depressive-like behavior at an early age, before other systemic manifestations [17]. To investigate whether the TWEAK/Fn14 pathway plays a role in depressive-like behavior, we compared the performance of 10-20 week old MRL/lpr Fn14WT and KO mice in the forced swim test. As we described previously [17], we found that both female and male MRL/lpr mice had increased immobility compared to MRL/+ controls (Figure 3A) as indicated by a main effect of genotype in a 2-way ANOVA (Fgenotype 2,54=29.4, p<0.0001: Fsex 1,54 =3.3, p<0.07: Fsex X genotype 2,54 =1.8, p<0.17) and subsequent pairwise comparisons (p<0.02). Notably, we found that this depression-like behavior was significantly ameliorated in MRL/lpr Fn14KO as compared to sex and aged matched MRL/lpr Fn14WT mice (p<0.02) (Figure 3A).
Figure 3. Depression like behavior is ameliorated in MRL/lpr Fn14KO mice.
(A) Forced swim test was performed on 10-20 week old MRL/+ (“MPJ”)(10 females (F), 10 males (M)), MRL/lpr Fn14WT (“LPR”) (10F/14M) and MRL/lpr Fn14KO (“LPR KO”)(8F/13M) mice. (B) Lack of preference for sweetened liquids (anhedonia) was measured by comparing the amount of fluid intake in individual 10-20 week old MRL/+ (“MPJ”) (5F/5M), MRL/lpr Fn14WT (“LPR”) (8F/9M), and MRL/lpr Fn14KO (“LPR KO”) (6F/6M) mice given a choice of drinking from two identical water bottles, one containing artificially sweetened water (left panel). Normal mice are expected to have >50% preference for sweetened fluid (above the dotted line). The right panel shows the total amount of fluid (sweetened and unsweetened) consumed by the mice in each group in this experiment. (C) Olfactory competency was assessed in 10-20 week old MRL/+ (“MPJ”)(5F/5M), MRL/lpr Fn14WT (“LPR”) (5F/2M) and MRL/lpr Fn14KO (“LPR KO”) (4F/1M) mice by measuring the time to uncover buried food. Subsequent figures utilize the same designations for each genotype, with the (F) and (M) label after the genotype in the figure legend denoting female and male mice, respectively.
Anhedonia, which is a lack of response to pleasure or reward, is a hallmark diagnostic symptom of depression. Anhedonia is assessed in rodents as loss of the typical preference to drink sweetened fluids, and correlates with a depression-like phenotype in the forced swim test. To corroborate the depression-like behavior observed in the forced swim test, we next performed a saccharin preference test of anhedonia in MRL/lpr Fn14WT and KO mice at 10-20 weeks of age. As shown in Figure 3B (left panel), female MRL/lpr Fn14KO exhibit significantly improved saccharin preference compared to female MRL/lpr Fn14WT mice (p<0.05) (2-way ANOVA: Fgenotype 2,33=3.7, p<0.04: Fsex 1,33 =5.5, p<0.025: Fsex X genotype 2,33 =0.11, p<0.88). Total liquid consumption was not different between genotypes (Figure 3B, right panel). Similar apparent amelioration of anhedonia was present in the male MRL/lpr Fn14KO as compared to the MRL/lpr Fn14WT (Figure 3B), although this did not reach statistical significance.
Acquired anosmia (loss of smell) is associated with an increased risk of depression; moreover, it has been recently reported that patients with lupus have a defective sense of smell [26]. Since lupus autoantibodies can bind to olfactory pathways [20], we explored whether the depressive-like behavior in MRL/lpr mice was associated with anosmia. However, there were no significant differences in the latency to find buried food between any of the MRL/+, MRL/lpr Fn14WT, and MRL/lpr Fn14KO mouse groups at 10-20 weeks of age (Figure 3C).
Mice have an innate tendency to interact with another mouse in preference to an inanimate object. Social withdrawal can be one of the symptoms of depression, and reduced social behavior can be ameliorated by anxiolytics and antidepressants. No significant decrease in social preference was observed in either male or female MRL/lpr Fn14WT mice compared to the MRL/+ controls (Fgenotype 1,52=4.8, p<0.01: Fsex 1,52 =1.4, p<0.23: Fsex X genotype 2,52 =0.70, p<0.49) (Figure 4A). Lack of social withdrawal in MRL/lpr mice despite the depression-like behavior evident in the other behavioral domains of despair could be considered consistent with the absence of anxiety-like behavior we reported previously in MRL/lpr mice [17]. Interestingly, male MRL/lpr Fn14KO displayed significantly higher social preference than MRL/lpr Fn14WT mice (p<0.04) (Figure 4A).
Figure 4. Cognitive deficits in the object placement test in MRL/lpr mice are reversed by Fn14 deficiency.
In social preference (A), the % of the time 10-20 week old MRL/+ (10F/10M), MRL/lpr Fn14WT (10F/14M) and MRL/lpr Fn14KO (8F/13M) mice spend interacting with another mouse relative to an inanimate object is measured. In the object placement test (B), the relative preference of MRL/+, MRL/lpr Fn14WT and KO mice to explore a familiar object in a novel location was measured. At 10-20 weeks of age, the number of mice in the MRL/+, MRL/lpr Fn14WT and MRL/lpr Fn14KO groups were 5F/5M, 3F/7M, and 2F/6M, respectively. In mice 21-40 weeks of age, the number of mice in the MRL/+, MRL/lpr Fn14WT, and MRL/lpr Fn14KO groups were 9F/10M, 7F/7M, and 5F/6M, respectively. Only for the 10-20 week old mice in the object placement test were the MRL/+ mice tested at a different time than the MRL/lpr mice, for technical reasons. Otherwise, the MRL/+, MRL/lpr Fn14WT, and MRL/lpr Fn14KO mice groups had their neurobehavioral assessments done concurrently. In object recognition (C), the relative preference of MRL/lpr Fn14WT and KO mice to explore a novel object was measured. At 10-20 weeks of age, the number of mice in the MRL/+, MRL/lpr Fn14WT and MRL/lpr Fn14KO groups was 5F/5M, 8F/9M, and 6F/6M, respectively. In mice 21-40 weeks of age, the number of mice in the MRL/+, MRL/lpr Fn14WT, and MRL/lpr Fn14KO groups were 10F/10M, 7F/7M, and 4F/7M, respectively. The dotted line in panels A-C denotes normal (>50% preference).
One important parameter defining neuropsychiatric disease in lupus mice is impaired cognition. We compared the performance of MRL/lpr Fn14WT and KO mice in object placement and object recognition tests which assess visuospatial and recognition memory, respectively. These assays utilize the rodent’s innate preference to explore novel objects and infer that they remember being exposed to other objects previously. Thus, a preference score of ≤50% represents a cognitive deficit as the animals spend equal time exploring the novel (or displaced) object versus a familiar one. Young (10-20 week old) MRL/lpr Fn14WT mice perform at chance levels in the object placement test, indicating deficits in spatial memory (Figure 4B). The roughly 50% preference score of both female and male MRL/lpr Fn14WT is lower than the normal preference score of MRL/+ mice. At 10-20 weeks of age, female and male MRL/lpr Fn14KO mice have significantly improved preference scores in the object placement test, signifying better spatial memory than the MRL/lpr Fn14WT mice (Figure 4B; p<0.05). A similar result is seen at 21-40 weeks (2-way ANOVA: Fgenotype 2,41=4.1, p<0.02: Fsex 1,41 =0.2, p<0.64: Fsex X genotype 2,41=0.21, p<0.80), where male MRL/lpr Fn14KO mice have significantly (p<0.04) improved spatial memory compared to MRL/lpr Fn14WT (Figure 4B). Despite the deficits in visuospatial memory, object recognition memory was generally intact in 10-20 and 21-40 week old MRL/lpr Fn14WT mice (Figure 4C). However, young male MRL/lpr Fn14KO have significantly (p<0.04) improved recognition memory compared to MRL/lpr Fn14WT mice (3 way ANOVA: Fsex X genotype X age 2,51 =3.8, p<0.03: F genotype 2,51 =2.9 , p<0.06) (Figure 4C, left panel). Therefore, cognitive behavior was improved in MRL/lpr Fn14KO mice, albeit in a sex and task dependent manner.
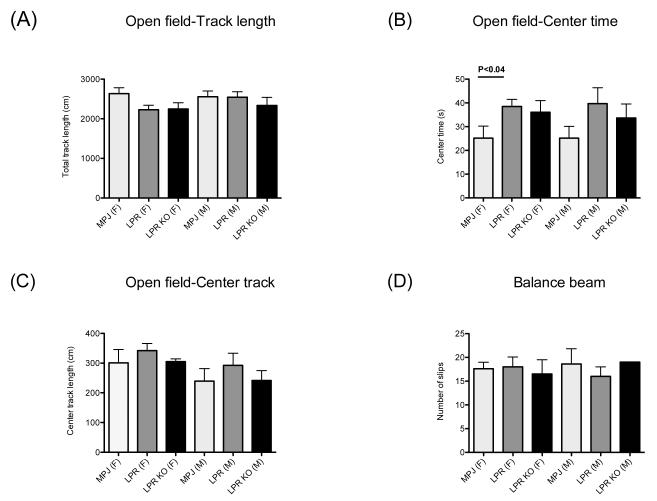
3.3. Loss of Fn14 does not alter general locomotor activity or motor coordination
To verify that the increased immobility observed in the swim test was not a function of sickness related behavior or neuromuscular weakness, we next assessed whether Fn14 deficiency altered locomotor activity (track length, Figure 5A). In addition, anxiety-like behavior was assessed in the open field by tracking the time a mouse spends in the center zone (center time), and the distance it covers while exploring the center (center track length) (Figure 5B,C). Anxiety-like behavior in this test manifests as decreased center entries and less time spent in the center, also known as thigmotaxis, i.e. the tendency to remain close to the walls. As seen in Figure 5, there was essentially no effect of Fn14 deficiency on activity or anxiety-like behavior, although there was a general effect of genotype on duration spent in the center zone (Fgenotype 2,54=3.7, p<0.03) evident as a significant increase in center time of MRL/lpr Fn14WT females compared to MRL/+ controls (p<0.04).
Figure 5. General locomotor activity and motor coordination are preserved and do not differ between MRL/lpr Fn14WT and KO mice.
(A-C) General locomotor activity was assessed in individual 10-20 week old MRL/+ (10F/9M), MRL/lpr Fn14WT (10F/12M), and MRL/lpr Fn14KO (8F/10M) mice for 6 minutes in an 39 × ***39 cm open field, with the center 15 × ***15 cm defined as the center zone. Total (A) and center (C) track length and the time spent in the center zone (B) were analyzed by Viewer software. (D) Motor coordination was assessed by crossing a 100 cm long, 1.5 cm diameter balance beam, with the number of slips defined as at least one of the paws passing below the midline of the beam.
Motor coordination was assessed in the balance beam as the number of slips while crossing the beam (Figure 5D). Consistent with the lack of obvious arthritis or neuromuscular weakness, there were no difference in motor performance on the balance beam among MRL/lpr Fn14WT, MRL/lpr Fn14KO, and MRL/+ mice.
3.4. MRL/lpr Fn14KO mice display diminished expression of proinflammatory mediators in the cortex and hippocampus relative to Fn14WT mice
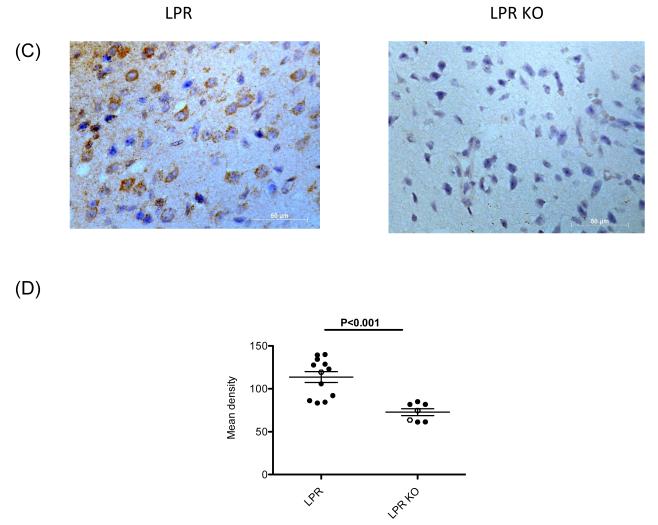
TWEAK can stimulate astrocytes, brain microvascular endothelial cells, macrophages, and perhaps microglia to secrete cytokines and other inflammatory mediators that can modulate neurobehavioral phenotypes [10, 11]. To assess whether Fn14 deficiency alters the brain cytokine microenvironment, we compared cytokine expression in the cortex and hippocampus of female MRL/lpr Fn14WT and KO mice at 19 weeks of age by real-time PCR. The focus was particularly on genes previously shown to be closely associated with NPSLE. We found that in the cortex of female MRL/lpr Fn14KO mice there was attenuated expression of several proinflammatory genes as compared to MRL/lpr Fn14WT mice, including RANTES, C3 (p<0.003), and CXCL11 (p<0.04) (Figure 6A). A similar tendency towards decreased RANTES expression was seen in the cortex of age matched male MRL/lpr Fn14KO mice (Figure 6A). When female and male mice were considered together, the difference in RANTES became significant (p<0.03) (not shown). Cortical brain tissue expression of CCL3 (p<0.05) and MCP-1 (p<0.01), but not TNF, was also significantly decreased in MRL/lpr Fn14KO mice (data not shown). Furthermore, RANTES and C3 were significantly decreased in MRL/lpr Fn14KO mice in total brain tissue examined from 40 week old mice (Figure 6B). To determine whether the altered RANTES gene expression levels are reflected at the protein level as well, we performed immunohistochemical staining. As shown in Figure 6C-D, there was a significant decrease in brain RANTES staining, predominantly in neurons, in Fn14 deficient mice.
Figure 6. Fn14 deficiency decreases expression of RANTES, C3 and CXCL11 in the brain of MRL/lpr mice.
(A) The gene expression of RANTES, C3 and CXCL11 in the cortex of 19 week old, female and male MRL/lpr Fn14WT and Fn14KO mice was measured by real-time PCR, and normalized to GAPDH. For the PCR, fold changes were calculated in reference to the MRL/lpr Fn14KO mice, whose mean value was set at 1. (B) The gene expression of RANTES, C3 and CXCL11 in the whole brain of 40 week old, female and male MRL/lpr Fn14WT and Fn14KO mice was measured by real-time PCR, and normalized to GAPDH. For the PCR, fold changes were calculated in reference to the MRL/lpr Fn14KO mice, whose mean value was set at 1. (C) Representative images of the immunohistochemical staining for RANTES in the frontal cortex of female MRL/lpr Fn14WT and Fn14KO mice at 40 weeks of age. Sections were examined using a Zeiss AxioObserver at 40X magnification. (D) RANTES staining quantitation was performed by Image J software. Staining intensity in 10 randomly selected areas was quantitated in each section, and the average of the density for each section was then calculated. Female mice, full dots; Male mice, open dots.
3.5. BBB integrity is preserved in MRL/lpr Fn14KO mice
In order for circulating mediators to contribute to the pathogenesis of NPSLE these would need to cross the BBB, which under normal conditions significantly restricts their diffusion from the serum. Prior studies indicate that TWEAK/Fn14 interactions may play a role in maintaining barrier integrity [11]. Furthermore, it has been shown that aged (19-30 weeks old) MRL/lpr mice have increased CSF concentrations of IgG and albumin, as compared to age-matched MRL/+ or young (4 week old) MRL/lpr mice [23]. To investigate whether altered BBB permeability may contribute to the pathogenesis of neuropsychiatric disease and whether it may be dependent on the TWEAK/Fn14 pathway, we calculated the albumin quotient as an indicator of BBB permeability. Nineteen week old MRL/lpr Fn14WT mice (before the onset of kidney disease) had significantly increased albumin quotients as compared to MRL/+ mice (Figure 7A). Notably, in comparison to MRL/lpr Fn14WT mice, the albumin quotient was significantly decreased in MRL/lpr Fn14KO mice, indicating better preservation of BBB integrity relative to MRL/lpr Fn14WT mice.
Figure 7. MRL/lpr Fn14KO mice display improved BBB permeability.
The permeability of the blood brain barrier in individual 10-20 week old female MRL/lpr Fn14KO (n=6) and MRL/lpr Fn14WT (n=6) mice was assessed by calculating the albumin quotient, defined as cerebrospinal fluid (CSF) albumin/serum albumin. Intrathecal IgG synthesis was studied by calculating the IgG index, defined as IgG index = (CSF IgG/serum IgG)/albumin quotient). (A) Albumin quotient. (B) IgG ratio (CSF IgG/serum IgG). (C) IgG index. (D) Anti-dsDNA antibody titers in CSF obtained from 15-23 week old female MRL/lpr Fn14KO and WT mice were measured as described in the Materials and methods. Age and gender matched MRL/+ mice served as controls. Fold change was calculated relative to the OD values in MRL/+ control mice, which were normalized to a value of 1.
Increased IgG concentrations in the CSF may reflect either increased BBB permeability, heightened local intrathecal secretion, or both. We found an increased IgG ratio (CSF IgG/serum IgG) in MRL/lpr Fn14WT mice (Figure 7B), indicating increased CSF IgG concentrations; however, since the IgG index (CSF IgG/serum IgG)/(CSF albumin/serum albumin) was not significantly different between MRL/lpr Fn14WT, MRL/lpr Fn14KO, and MRL/+ mice (Figure 7C), the increased IgG ratio in MRL/lpr Fn14WT mice was likely due to compromised BBB integrity.
3.6. MRL/lpr Fn14KO mice display lower autoantibody concentrations in the CSF relative to Fn14WT mice
While serum levels of potentially neuropathic autoantibodies such as antibodies to dsDNA were not different between MRL/lpr Fn14KO and WT mice (Figure 2), changes in the integrity of the BBB may affect the concentrations of antibodies that actually reach the brain. To address this possibility, we measured the levels of anti-dsDNA antibodies in the CSF of MRL/lpr Fn14WT and MRL/lpr Fn14KO mice. We found that MRL/lpr Fn14WT mice had markedly increased CSF anti-dsDNA titers, as compared to MRL/+ control mice (Figure 7D). While levels of CSF anti-dsDNA antibodies were still elevated in MRL/lpr Fn14KO mice, these were significantly less than those found in MRL/lpr Fn14WT mice (Figure 7D).
4. Discussion
Using the MRL/lpr strain as an established animal model to explore NPSLE [17-19, 27], we report for the first time that the TWEAK/Fn14 pathway is important in the pathogenesis of this major organ manifestation. Specifically, we found that Fn14 is upregulated in brains of MRL/lpr mice, and that the severe depression-like behavior observed in MRL/lpr Fn14WT mice is significantly ameliorated in Fn14 deficient mice. Moreover, visospatial and recognition memory performance was improved in MRL/lpr Fn14KO mice. The negative behavioral outcomes exhibited by MRL/lpr Fn14WT compared to KO mice are unlikely to be due to non-specific illness or peripheral organ pathology, since they were exhibited as early as 10 weeks of age, before other disease manifestations. Moreover, despite the immobility in the forced swim test, anhedonia, and deficits in spatial memory, MRL/lpr Fn14WT and KO mice did not differ in general locomotor activity, motor coordination, total liquid consumption and social preference. These data also indicate that loss of Fn14 does not have a negative impact in these behavioral domains. The explanation for the selective effect of Fn14 deficiency on certain neurobehavioral patterns but not on others is not immediately obvious, and will need to be further explored.
The identification of antibodies that bind to brain tissue and that are closely associated with particular neurologic manifestations indicates that autoantibodies may play a role in the pathogenesis of NPSLE [24, 28]. While anti-cardiolipin antibodies are primarily associated with focal NPSLE manifestations, mood disorders and cognitive deficits have been described as well [24, 28, 29]. A number of studies have demonstrated a strong association of anti-ribosomal P antibodies with several NPSLE manifestations, particularly psychosis [24]. More recently, Shoenfeld et al has shown that intracerebroventricular injection of anti-ribosomal P antibodies in mice induces abnormal olfactory function and depression-like behavior [20, 30]. Furthermore, CSF anti-ribosomal P antibodies can induce very high levels of α-interferon (a cytokine with prominent CNS side effects including confusion, malaise, and depression), as well as IP-10, IL-8, and MCP-1 [31]. Finally, following pharmacologic abrogation of the BBB in non-autoimmune mice, anti-dsDNA antibodies cross reactive with the NMDA receptor induce location-specific neuronal cell death, and significant neurologic deficits [5]. Nevertheless, in our study we did not find a significant difference in serum autoantibody titers against cardiolipin, ribosomal P, DNA, and NMDA receptor between MRL/lpr Fn14WT and KO mice, consistent with prior studies showing that the TWEAK/Fn14 pathway does not play a prominent role in the regulation of adaptive immunity [32]. However, autoantibodies could still be involved in the more severe neuropsychiatric phenotype observed in MRL/lpr Fn14WT as compared to Fn14KO mice, due to differential amounts of potentially pathogenic antibodies reaching the brain via increased BBB permeability. Indeed, in support of this explanation we found that MRL/lpr Fn14KO mice had significantly lower titers of anti-dsDNA antibodies in the CSF as compared to MRL/lpr Fn14WT mice, presumably reflective of their preserved barrier integrity. In addition, while very limited mouse CSF sample availability poses significant technical challenges, it will be interesting to investigate whether changes in antibody fine specificity, or differences in titers of pathogenic antibodies that were not assessed, may also have contributed to the severe neurobehavioral abnormalities found in MRL/lpr Fn14WT mice.
There are several additional mechanisms by which TWEAK/Fn14 signaling is likely to be important in NPSLE. Various cytokines are highly expressed in the brain of NPSLE patients, and these are believed to have important roles in the pathogenesis of NPSLE [33]. Lupus patients with CNS manifestations have higher CSF levels of TNF, IL-6, and IL-10 as compared to patients with multiple sclerosis or controls with non-inflammatory neurologic disease [34], while intrathecal levels of IL-6, IL-8, and RANTES, all of which are TWEAK-inducible, were significantly increased in NPLSE patients [35]. Indeed, there is interest in using CSF cytokine profiling for the diagnosis of acute confusional state in lupus patients [36], or to distinguish between NPSLE and non-NPSLE complicated with CNS infection [37].
TWEAK has been shown to induce cytokines across many cell and tissue types [38, 39]. Consistent with this mechanism, our current study showed increased proinflammatory mediator expression in the CNS of MRL/lpr Fn14WT as compared to Fn14KO mice. By inducing proinflammatory cytokines (coming from the circulation, or produced locally), TWEAK signaling may result in negative CNS outcomes and particularly depression and cognitive dysfunction [33] via several mechanisms that are likely to be complementary. These include activation of microglia and astrocytes, and degradation of the integrity of the BBB (discussed separately below). TWEAK-inducible cytokines can also alter neurotransmitter systems such as serotonin thought to be important in depression and cognition [40], by regulating neurotransmitter metabolism [41, 42] and the availability of essential amino acid precursors (such as serotonin) in plasma and in the brain [41]. Finally, TWEAK-induced chemokines may also further propagate inflammation via recruitment of inflammatory cells from the circulation (e.g. chemotaxis of T cells by RANTES). The spectrum of mediators present intrathecally in lupus patients which correlate with CNS disease activity and change with treatment [35, 43], many of which are TWEAK inducible (including IL-6, IL-8, MCP-1, RANTES, IP-10, and MMP-9), support a model in which mediators induced in response to TWEAK signaling contribute to the pathogenesis of NPSLE. Although it will also be important in future studies to exclude histopathological differences contributing to the neurobehavioral deficits observed in MRL/lpr Fn14WT mice, at least by magnetic resonance imaging we did not previously find brain structural abnormalities in the mice of this strain [17].
Another mechanism whereby the TWEAK/Fn14 pathway may promote NPSLE pathogenesis is through its regulation of BBB permeability. BBB dysfunction has been demonstrated in NPSLE patients [44]. Interestingly, an increased albumin quotient may be a risk factor for corticosteroid-induced psychiatric disorders as well [45]. Moreover, in longitudinal studies in lupus patients, improvement in CNS symptoms is associated with reversal of BBB dysfunction [44]. In MRL/lpr mice, several studies have demonstrated a C5a-mediated increase in BBB permeability that correlates with disease activity [6, 23, 46]. Furthermore, TWEAK is known to alter the permeability of the BBB in vitro [47]. Indeed, the Fn14 staining of brain endothelial cells in MRL/lpr mice demonstrated above would also point towards a possible BBB effect. By increasing the permeability of the BBB, as we demonstrated in MRL/lpr Fn14WT mice, TWEAK may promote the diffusion of pathogenic moieties from the circulation into the brain, including autoantibodies, activated lymphocytes and cytokines [48]. Thus, enhanced protection of the brain from the harmful effects of autoantibodies and cytokines coming from the circulation is likely to have contributed to preserved neuronal function and the improved neurobehavioral profile that we found in MRL/lpr Fn14KO mice. It would be interesting to directly compare the concentrations of autoantibodies and cytokines in the CSF of MRL/lpr Fn14WT and Fn14KO mice, and particularly those mediators which have been previously linked to NPSLE, although these additional studies have been restricted by the minuscule amounts of available CSF.
In this paper, we demonstrated a role for TWEAK/Fn14 signaling in the pathogenesis of NPSLE. It is important to point out that this pathway may not only contribute to the pathogenesis of central nervous system manifestations; rather, there is evidence for the role of TWEAK/Fn14 in lupus associated kidney and skin disease as well. Lu et al [49] found increased TWEAK and Fn14 mRNA expression in kidney biopsies from patients with lupus nephritis. Furthermore, we [50,51] and others [52,53] reported increased urinary TWEAK levels in lupus nephritis that correlated with disease activity. Experimentally, Fn14 deletion was protective in preliminary studies against nephritis [54] and cutaneous disease [55] in lupus mice. Nevertheless, significant proteinuria was not yet present when the MRL/lpr Fn14WT mice described herein first displayed neuropsychiatric abnormalities, indicating that the phenotype was primary rather than secondary to renal insufficiency. The mechanisms by which the TWEAK/Fn14 pathway is relevant to the pathogenesis of extra-cranial disease in lupus are important and will be different than those discussed here, and deserve careful and separate dissection in future studies.
5. Conclusions
We found that Fn14 deficiency protects against several neurobehavioral deficits that characterize MRL/lpr mice, including depression-like behavior and impaired cognition. Our study demonstrates for the first time the involvement of TWEAK/Fn14 pathway in the pathogenesis of NPSLE. Differences in peripheral autoantibody levels apparently did not contribute to the differential neuropsychiatric phenotype observed in the MRL/lpr Fn14WT mice. However, Fn14KO mice showed decreased brain expression of several mediators known to be instrumental in the pathogenesis of murine NPSLE, including RANTES, which may be directly stimulated by TWEAK, and C3. Fn14 deficient mice also exhibited improved BBB barrier integrity, thereby potentially restricting access of autoantibodies and soluble mediators to the CNS. Considering the similarities between murine NPSLE and human disease, our results point to the inhibition of the TWEAK/Fn14 pathway as a potential new target for the treatment of human lupus with brain involvement.
Highlights.
Fn14 deficient MRL/lpr lupus mice have attenuated neuropsychiatric disease.
Fn14 deficiency does not affect serum autoantibodies in MRL/lpr lupus mice.
Cytokine expression is decreased in brains of MRL/lpr Fn14 knockout mice.
Blood brain barrier integrity is preserved in MRL/lpr Fn14 knockout mice.
Acknowledgments
Funding This work was supported by NIH grants RO1 AR048693 and RO1 DK090319, and a research grant from Biogen Idec (to C.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest J.S.M was an employee of Biogen Idec when this work was performed. L.C.B is currently a full time employee of Biogen Idec. C.P. received a research grant from Biogen Idec.
References
- [1].The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- [2].Gulinello M, Putterman C. The MRL/lpr mouse strain as a model for neuropsychiatric systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:207504. doi: 10.1155/2011/207504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Petri M, Naqibuddin M, Carson KA, Wallace DJ, Weisman MH, Holliday SL, et al. Depression and cognitive impairment in newly diagnosed systemic lupus erythematosus. J Rheumatol. 2010;37:2032–38. doi: 10.3899/jrheum.091366. [DOI] [PubMed] [Google Scholar]
- [4].Sakic B, Denburg JA, Denburg SD, Szechtman H. Blunted sensitivity to sucrose in autoimmune MRL-lpr mice: a curve-shift study. Brain Res Bull. 1996;41:305–11. doi: 10.1016/s0361-9230(96)00190-6. [DOI] [PubMed] [Google Scholar]
- [5].Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it’s the antibodies. Nature Rev Immunol. 2009;9:449–56. doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alexander JJ, Quigg RJ. Systemic lupus erythematosus and the brain: what mice are telling us. Neurochem Int. 2007;50:5–11. doi: 10.1016/j.neuint.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [7].Michaelson JS, Wisniacki N, Burkly LC, Putterman C. Role of TWEAK in lupus nephritis: A bench-to-bedside review. J Autoimmunity. 2012;39:130–42. doi: 10.1016/j.jaut.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Desplat-Jego S, Varriale S, Creidy R, Terra R, Bernard D, Khrestchatisky M, et al. TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity. J Neuroimmunol. 2002;133:116–23. doi: 10.1016/s0165-5728(02)00368-5. [DOI] [PubMed] [Google Scholar]
- [9].Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, et al. TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia. 2000;32:102–7. [PubMed] [Google Scholar]
- [10].Campbell S, Michaelson J, Burkly LC, Putterman C. The role of TWEAK/Fn14 in the pathogenesis of inflammation and systemic autoimmunity. Frontiers in Bioscience. 2004;8:2273–84. doi: 10.2741/1395. [DOI] [PubMed] [Google Scholar]
- [11].Yepes M. TWEAK and the central nervous system. Molecular Neurobiol. 2007;35:255–65. doi: 10.1007/s12035-007-0024-z. [DOI] [PubMed] [Google Scholar]
- [12].Trysberg EBL, Su L, Michaelson J, Putterman C. Cerebrospinal fluid (CSF) TWEAK: A novel biomarker for neuropsychiatric SLE? Arthritis Rheum. 2007;56:S750. [Google Scholar]
- [13].Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–60. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–66. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- [15].Deocharan B, Zhou Z, Antar K, Siconolfi-Baez L, Angeletti RH, Hardin J, et al. Alpha-actinin immunization elicits anti-chromatin autoimmunity in nonautoimmune mice. J Immunol. 2007;179:1313–21. doi: 10.4049/jimmunol.179.2.1313. [DOI] [PubMed] [Google Scholar]
- [16].Khan P, Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, et al. Prevention of murine anti-phospholipid syndrome by BAFF blockade. Arthritis Rheum. 2008;58:2824–2834. doi: 10.1002/art.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gao HX, Campbell SR, Cui MH, Zong P, Hee-Hwang J, Gulinello M, et al. Depression is an early disease manifestation in lupus-prone MRL/lpr mice. J Neuroimmunol. 2009;207:45–56. doi: 10.1016/j.jneuroim.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gao HX, Sanders E, Tieng AT, Putterman C. Sex and autoantibody titers determine the development of neuropsychiatric manifestations in lupus-prone mice. J Neuroimmunol. 2010;229:112–22. doi: 10.1016/j.jneuroim.2010.07.020. [DOI] [PubMed] [Google Scholar]
- [19].Sakic B, Szechtman H, Talangbayan H, Denburg SD, Carbotte RM, Denburg JA. Disturbed emotionality in autoimmune MRL-lpr mice. Physiol Behav. 1994;56:609–17. doi: 10.1016/0031-9384(94)90309-3. [DOI] [PubMed] [Google Scholar]
- [20].Katzav A, Solodeev I, Brodsky O, Chapman J, Pick CG, Blank M, et al. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum. 2007;56:938–48. doi: 10.1002/art.22419. [DOI] [PubMed] [Google Scholar]
- [21].Frisch P, Bilkei-Gorzo A, Racz I, Zimmer A. Modulation of the CRH system by substance P/NKA in an animal model of depression. Behav Brain Res. 2010;213:103–8. doi: 10.1016/j.bbr.2010.04.044. [DOI] [PubMed] [Google Scholar]
- [22].Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF. Olfactory deficits in mice overexpressing human wildtype alpha-synuclein. Eur J Neurosci. 2008;28:247–56. doi: 10.1111/j.1460-9568.2008.06346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sidor MM, Sakic B, Malinowski PM, Ballok DA, Oleschuk CJ, Macri J. Elevated immunoglobulin levels in the cerebrospinal fluid from lupus-prone mice. J Neuroimmunol. 2005;165:104–13. doi: 10.1016/j.jneuroim.2005.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zandman-Goddard G, Chapman J, Shoenfeld Y. Autoantibodies involved in neuropsychiatric SLE and antiphospholipid syndrome. Sem Arth Rheum. 2007;36:297–315. doi: 10.1016/j.semarthrit.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [25].Szechtman H, Sakic B, Denburg JA. Behaviour of MRL mice: an animal model of disturbed behaviour in systemic autoimmune disease. Lupus. 1997;6:223–9. doi: 10.1177/096120339700600302. [DOI] [PubMed] [Google Scholar]
- [26].Shoenfeld N, Agmon-Levin N, Flitman-Katzevman I, Paran D, Katz BS, Kivity S, et al. The sense of smell in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1484–7. doi: 10.1002/art.24491. [DOI] [PubMed] [Google Scholar]
- [27].Walker SE, Wright DC, O’Sullivan FX, Johnson GC, Besch-Willif6ord CL, Vogelweid CM. Memory, learning ability, and neuropathologic status of mice with systemic lupus erythematosus. Annals NY Acad Sci. 1997;823:107–15. doi: 10.1111/j.1749-6632.1997.tb48383.x. [DOI] [PubMed] [Google Scholar]
- [28].Ballok DA. Neuroimmunopathology in a murine model of neuropsychiatric lupus. Brain Res Rev. 2007;54:67–79. doi: 10.1016/j.brainresrev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ziporen L, Shoenfeld Y, Levy Y, Korczyn AD. Neurological dysfunction and hyperactive behavior associated with antiphospholipid antibodies. J Clin Invest. 1997;100:613–9. doi: 10.1172/JCI119572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Katzav A, Ben-Ziv T, Chapman J, Blank M, Reichlin M, Shoenfeld Y. Anti-P ribosomal antibodies induce defect in smell capability in a model of CNS-SLE (depression) J Autoimmunity. 2008;31:393–8. doi: 10.1016/j.jaut.2008.09.002. [DOI] [PubMed] [Google Scholar]
- [31].Santer DM, Yoshio T, Minota S, Moller T, Elkon KB. Potent induction of IFN-alpha and chemokines by autoantibodies in the cerebrospinal fluid of patients with neuropsychiatric lupus. J Immunol. 2009;182:1192–201. doi: 10.4049/jimmunol.182.2.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xia Y, Campbell SR, Broder A, Herlitz L, Abadi M, Wu P, et al. Inhibition of the TWEAK/Fn14 pathway attenuates renal disease in nephrotoxic serum nephritis. Clinical Immunology. 2012;145:108–21. doi: 10.1016/j.clim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Okamoto H, Kobayashi A, Yamanaka H. Cytokines and chemokines in neuropsychiatric syndromes of systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:268436. doi: 10.1155/2010/268436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Baraczka K, Nekam K, Pozsonyi T, Szuts I, Ormos G. Investigation of cytokine (tumor necrosis factor-alpha, interleukin-6, interleukin-10) concentrations in the cerebrospinal fluid of female patients with multiple sclerosis and systemic lupus erythematosus. Eur J Neurol. 2004;11:37–42. doi: 10.1046/j.1351-5101.2003.00706.x. [DOI] [PubMed] [Google Scholar]
- [35].Fragoso-Loyo H, Richaud-Patin Y, Orozco-Narvaez A, Davila-Maldonado L, Atisha-Fregoso Y, Llorente L, et al. Interleukin-6 and chemokines in the neuropsychiatric manifestations of systemic lupus erythematosus. Arthritis Rheum. 2007;56:1242–50. doi: 10.1002/art.22451. [DOI] [PubMed] [Google Scholar]
- [36].Katsumata Y, Harigai M, Kawaguchi Y, Fukasawa C, Soejima M, Takagi K, et al. Diagnostic reliability of cerebral spinal fluid tests for acute confusional state (delirium) in patients with systemic lupus erythematosus: interleukin 6 (IL-6), IL-8, interferon-alpha, IgG index, and Q-albumin. J Rheum. 2007;34:2010–7. [PubMed] [Google Scholar]
- [37].Lu XY, Zhu CQ, Qian J, Chen XX, Ye S, Gu YY. Intrathecal cytokine and chemokine profiling in neuropsychiatric lupus or lupus complicated with central nervous system infection. Lupus. 2010;19:689–95. doi: 10.1177/0961203309357061. [DOI] [PubMed] [Google Scholar]
- [38].Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine. 2007;40:1–16. doi: 10.1016/j.cyto.2007.09.007. [DOI] [PubMed] [Google Scholar]
- [39].Burkly LC, Michaelson JS, Zheng TS. TWEAK/Fn14 pathway: an immunological switch for shaping tissue responses. Immunol Rev. 2011;244:99–114. doi: 10.1111/j.1600-065X.2011.01054.x. [DOI] [PubMed] [Google Scholar]
- [40].Sakic B, Lacosta S, Denburg JA, Szechtman H. Altered neurotransmission in brains of autoimmune mice: pharmacological and neurochemical evidence. J Neuroimmunol. 2002;129:84–96. doi: 10.1016/s0165-5728(02)00171-6. [DOI] [PubMed] [Google Scholar]
- [41].Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psych. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- [42].Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–36. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ainiala H, Hietaharju A, Dastidar P, Loukkola J, Lehtimaki T, Peltola J, et al. Increased serum matrix metalloproteinase 9 levels in systemic lupus erythematosus patients with neuropsychiatric manifestations and brain magnetic resonance imaging abnormalities. Arthritis Rheum. 2004;50:858–65. doi: 10.1002/art.20045. [DOI] [PubMed] [Google Scholar]
- [44].Abbott NJ, Mendonca LL, Dolman DE. The blood-brain barrier in systemic lupus erythematosus. Lupus. 2003;12:908–15. doi: 10.1191/0961203303lu501oa. [DOI] [PubMed] [Google Scholar]
- [45].Nishimura K, Harigai M, Omori M, Sato E, Hara M. Blood-brain barrier damage as a risk factor for corticosteroid-induced psychiatric disorders in systemic lupus erythematosus. Psychoneuroendocrinology. 2008;33:395–403. doi: 10.1016/j.psyneuen.2007.12.007. [DOI] [PubMed] [Google Scholar]
- [46].Jacob A, Hack B, Chiang E, Garcia JG, Quigg RJ, Alexander JJ. C5a alters blood-brain barrier integrity in experimental lupus. FASEB J. 2010;24:1682–8. doi: 10.1096/fj.09-138834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Polavarapu R, Gongora MC, Winkles JA, Yepes M. Tumor necrosis factor-like weak inducer of apoptosis increases the permeability of the neurovascular unit through nuclear factor-kappa B pathway activation. J Neurosci. 2005;25:10094–100. doi: 10.1523/JNEUROSCI.3382-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tomita M, Khan RL, Blehm BH, Santoro TJ. The potential pathogenetic link between peripheral immune activation and the central innate immune response in neuropsychiatric systemic lupus erythematosus. Med Hypotheses. 2004;62:325–35. doi: 10.1016/j.mehy.2003.10.009. [DOI] [PubMed] [Google Scholar]
- [49].Lu J, Kwan BC, Lai FM, Choi PC, Tam LS, Li EK, et al. Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis. Nephrology. 2011;16:426–32. doi: 10.1111/j.1440-1797.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- [50].Schwartz N, Su L, Burkly LC, Mackay M, Aranow C, Kollaros M, et al. Urinary TWEAK and the activity of lupus nephritis. J Autoimmun. 2006;27:242–50. doi: 10.1016/j.jaut.2006.12.003. [DOI] [PubMed] [Google Scholar]
- [51].Schwartz N, Rubinstein T, Burkly LC, Collins CE, Blanco I, Su L, et al. Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res Ther. 2009;11:R143. doi: 10.1186/ar2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].El-Shehaby A, Darweesh H, El-Khatib M, Momtaz M, Marzouk S, El-Shaarawy N, et al. Correlations of urinary biomarkers, TNF-like weak inducer of apoptosis (TWEAK), osteoprotegerin (OPG), monocyte chemoattractant protein-1 (MCP-1), and IL-8 with lupus nephritis. J Clin Immunol. 2011;31:848–56. doi: 10.1007/s10875-011-9555-1. [DOI] [PubMed] [Google Scholar]
- [53].Xuejing Z, Jiazhen T, Jun L, Xiangqing X, Shuguang Y, Fuyou L. Urinary TWEAK level as a marker of lupus nephritis activity in 46 cases. J Biomed Biotech. 2012;2012:359647. doi: 10.1155/2012/359647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xia Y, Wen J, Michaelson JS, Burkly LC, Putterman C. Deficiency of the TWEAK receptor Fn14 is protective in the MRL/lpr mouse model of lupus nephritis (abstract) Arthritis Rheum. 2011;63:S212. [Google Scholar]
- [55].Xia Y, Blecher K, Wen J, Michaelson JS, Burkly LC, Friedman A, Putterman C. TWEAKing cutaneous manifestations in MRL-lpr/lpr lupus prone mice (abstract) Arthritis Rheum. 2011;63:S713. [Google Scholar]