Abstract
The impact of recent experiences of task performance on resting functional connectivity MRI (fcMRI) has important implications for the design of many neuroimaging studies, because, if an effect is present, the fcMRI scan then must be performed before any evoked fMRI or after a time gap to allow it to dissipate. The present study aims to determine the effect of simple button presses, which are used in many cognitive fMRI tasks as a response recording method, on later acquired fcMRI data. Human volunteers were subject to a 23-minute button press motor task. Their resting-state brain activity before and after the task was assessed with fcMRI. It was found that, compared to the pre-task resting period, the post-task resting fcMRI revealed a significantly higher (p=0.002, N=24) cross correlation coefficient (CC) between left and right motor cortices. These changes were not present in sham control studies that matched the paradigm timing but had no actual task. The amplitude of fcMRI signal fluctuation (AF) also demonstrated an increase in the post-task period compared to pre-task. These changes were observed using both the right-hand-only task and the two-hand task. Study of the recovery time course of these effects revealed that the CC changes lasted for about 5 minutes while the AF change lasted for at least 15 minutes. Finally, voxelwise analysis revealed that the pre/post-task differences were also observed in several other brain regions, including the auditory cortex, visual areas, and the thalamus. Our data suggest that the recent performance of the simple button press task can result in elevated fcMRI CC and AF in relevant brain networks and that fcMRI scan should be performed either before evoked fMRI or after a sufficient time gap following fMRI.
Keywords: resting state, functional connectivity MRI, motor cortex, blood oxygenation
Introduction
Resting state functional connectivity MRI (fcMRI) is now widely used in investigations of brain networks (Biswal et al., 1995; He et al., 2009; Raichle et al., 2001; Wig et al., 2011), neurological diseases such as Alzheimer's disease (Greicius et al., 2004; Wang et al., 2007; Wang et al., 2006), Traumatic Brain Injury (Mayer et al., 2011; Nakamura et al., 2009; Stevens et al., 2012; Tang et al., 2011), multiple sclerosis (De Luca et al., 2005; Lowe et al., 2002), and psychiatric disorders such as schizophrenia (Bluhm et al., 2007; Liang et al., 2006; Liu et al., 2006; Salvador et al., 2007; Zhou et al., 2007), as well as in cognitive aging (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Tomasi and Volkow, 2012). In many studies, fcMRI data are collected in the same scan session as evoked fMRI. However, it is not yet clear whether the results of fcMRI could be affected by task fMRI runs that were performed earlier in the same session.
There is some evidence in the literature that the recent experience of task performance could alter fcMRI data collected later. For example, Waites et al. provided early evidence in a sample of six subjects that performance of a five-minute language task resulted in an increased connectivity in fcMRI data acquired immediately before and after the task (5 minutes each), in particular in language regions such as left middle frontal gyrus (Waites et al., 2005). Stevens and colleagues showed that resting connectivity can be affected by the context of the task performed during the preceding fMRI runs. Specifically, connectivity in face visual regions (from a 9-minute fcMRI data) following a 15-min face classification task was significantly greater than that after a scene classification task (Stevens et al., 2010). Tambini et al. showed that connectivity (from 8-minute fcMRI data) between the lateral occipital lobe and the fusiform face area was enhanced following a 21-minute object-face associative encoding task, but not after a scene-face associative encoding task of identical duration (Tambini et al., 2010). The investigators also found that the former task corresponded to a better associative memory (from a surprise memory test after the scanning session); thus it was proposed that the enhanced post-task resting activity was related to memory consolidation (Tambini et al., 2010). Modulations of fcMRI results by physiologic states or other cognitive tasks have also been reported (Albert et al., 2009; Barnes et al., 2009; Fukunaga et al., 2008; Lewis et al., 2009).
The influence of motor task on resting fcMRI data has also been reported. Peltier and colleagues have shown that, after a 20-minute fatiguing right handgrip motor task (120 contracts at 50% maximal grip force), interhemispheric correlation of motor cortex in the fcMRI data (3 minutes and 20 seconds) decreased significantly (Peltier et al., 2005). Klingner et al. reported that resting connectivity (from 5-minute fcMRI data) between primary somatosensory and motor cortex was enhanced bilaterally following a 32-minute, right-side-only median nerve stimulation, with connectivity decreases in thalamus and higher order somatosensory areas (Klingner et al., 2012). Albert et al. noted that Independent-Component-Analysis (ICA) based connection strength (from 11-minute fcMRI data) increased following a 11-minute left-hand joystick target tracking task, which was attributed to neural plasticity associated with motor learning (Albert et al., 2009).
However, it is not yet clear whether simpler motor tasks such as button presses could also alter fcMRI network. We note that button presses are employed in virtually all fMRI studies as a means to obtain subject response, thus a significant effect of button presses on later fcMRI data would indicate that fcMRI scan should ideally be performed before fMRI scans in future study design.
In this study, we compared fcMRI data acquired before and after a 23-minute button press task (right-hand only). Sham control experiments were performed to ensure that the changes detected were not due to the subject becoming drowsy or sleepy after being inside the scanner for a while. The results were further verified by additional studies on a new cohort with a two-hand button press task. Moreover, in this cohort, three post-task fcMRI runs were performed to assess how long it takes for the fcMRI changes to dissipate. Voxelwise comparison was conducted in the entire brain to examine whether brain areas other than a priori regions-of-interest (ROI) (i.e. motor cortex) showed these changes.
Materials and Methods
General
The protocol was approved by University of Texas Southwestern Medical Center's Institutional Review Board and informed written consent was obtained from each participant. Healthy right-handed adults were recruited through flyers posted on the university campus. The subjects were divided into three substudies: a one-hand experiment, a sham experiment, and a two-hand experiment. There were no group differences in terms of age (p=0.35, one-way ANOVA) or gender (p=0.64). MR imaging was conducted using a 3 Tesla system (Philips, Best, The Netherlands). FcMRI and fMRI scans used identical imaging parameters: Blood-Oxygenation-Level-Dependent (BOLD) sequence, single-shot gradient-echo EPI, TR=1000ms, TE=25ms, Field-of-view (FOV) 220×220 mm2, matrix size 64×64, slice thickness 5 mm, voxel size 3.4×3.4×5mm3, 21 axial slices. Ten image volumes were discarded at the beginning of every run to allow the magnetization to reach a steady state.
One-hand motor experiment
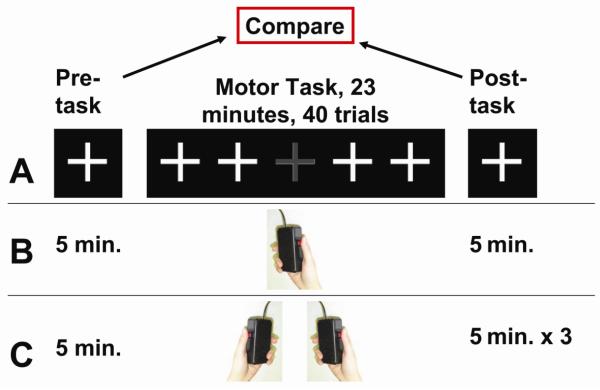
Twenty-four subjects (25±14 y.o., 13 males) were recruited. The scan session lasted approximately 33 minutes (Fig. 1A and B) and included a five-minute pre-task fcMRI run, a 23-minute motor task, and a five-minute post-task fcMRI run. The fcMRI runs were performed while the subject fixated on a white crosshair and was instructed to think of nothing in particular. During the motor task period, the subject performed button presses using their right hand. Specifically, they fixated on a white crosshair and, when the crosshair occasionally changed color to grey for 1000ms, they pressed a button in their right hand three times with their thumb. The response time (RT) was recorded using the E-Prime software (Version 2.0, Psychology Software Tools, Inc., Sharpsburg, PA). Only the first press (out of the three presses) of the right-hand was recorded for quantitative analysis. The color change occurred every 27–32 seconds with randomized intervals and there were a total of 40 trials during the motor task period.
Figure 1.
Experimental design. (A) The experiment started with a pre-task fcMRI, followed by a motor task in which the subject attended to a white crosshair and pressed a button when the color of the crosshair changed to grey for 1000ms. The session concluded with a post-task fcMRI. (B) In the one-hand motor experiment (N=24), the post-task fcMRI was five minutes in duration. (C) In the two-hand motor experiment (N=24), the post-task fcMRI was 15 minutes in duration.
Sham experiment
Ten healthy right-handed subjects (28±7 y.o., 6 males) underwent a 35-minute scan session consisting of seven fcMRI runs of five minutes each. During the scan session, the subject fixated on a white crosshair and held a button box in their right hand, thereby matching the condition of the fcMRI runs during the real experiment. No button presses took place in the sham experiment. FcMRI imaging parameters were identical to those used in the real experiment.
Two-hand motor experiment
Twenty-four healthy right-handed subjects (30±10 y.o., 9 male) were recruited for the two-hand button press experiment. The experimental procedure was identical to that of the one-hand experiment with two exceptions (Fig. 1C). First, the subject held one button box in each hand and was instructed to press both button boxes simultaneously three times. Second, we performed three post-task fcMRI runs (five minutes each) in these subjects, which allowed us to examine how long the changes may last.
Data preprocessing
The data were analyzed using the software AFNI (National Institutes of Health, Bethesda, MD). All image volumes were coregistered to the first volume of the first fcMRI run. Physiologic fluctuations in the signal time course were removed by regressing out time courses of the whole brain white matter, cerebral spinal fluid, and six motion vectors (Chang and Glover, 2009; He and Liu, 2012). The time series were then bandpass-filtered to 0.01–0.1 Hz using the AFNI command 3dBandpass, which is the bandwidth commonly used in fcMRI processing (Cordes et al., 2000; Damoiseaux et al., 2006). To further identify the bandwidth in which the changes are most pronounced, we further bandpass-filtered the time series to 0.01–0.05 Hz for a lower band, and from 0.05–0.1 Hz for a higher band (Baria et al., 2011).
Region-of-Interest (ROI) definitions
ROI analysis was conducted on the hand area of the left/right sensorimotor cortex. Given that these ROIs are functionally defined areas and standard anatomic atlas would not be applicable, we collected additional data to define these ROI masks (with the same imaging parameters) and used these masks in all three groups (one-hand, two-hand, and sham) of subjects. We recruited back the 24 subjects from the one-hand task group for a separate scan session (at least 24 hours later), in which they performed a simple bilateral finger tapping task (40 seconds OFF, 20 seconds ON, eight repetitions, imaging parameters were identical to the fcMRI sessions, total duration 8 minutes and 40 seconds). The data were processed with AFNI scripts to detect activated voxels (false positive corrected p<0.003, minimal cluster size 50 voxels). To standardize the ROI size, we selected the top 1000 voxels in the left/right motor areas in the group activation map as the final masks (cutoff t thresholds were 6.08 and 6.15 for the left and right motor regions, respectively). These masks then served as the motor template for the rest of the analyses.
Group-level ROIs were also defined for left/right parietal cortex that is part of the default-mode-network (DMN), using the resting data of the 24 subjects in the one-hand cohort. A seed ROI (size=0.73 cm3) was positioned at bilateral posterior cingulate cortices based on Talairach coordinates (Hong et al., 2009; Xu et al., 2011). The cross correlation coefficient (CC) between these seed voxels and all other voxels was calculated to generate a correlation map (using both pre-task and post-task fcMRI data). Then, a Fisher-z transform was employed and a group analysis was performed on the z-maps of all subjects to identify the DMN, which included the posterior cingulate cortex, the medial frontal cortex, and the left/right parietal cortex. The final left (right) parietal mask is then defined as the top 500 voxels according to the z-score in the left (right) parietal cluster (cutoff t thresholds were 4.60 and 3.38 for the left and right parietal regions, respectively). These masks then served as the DMN template for the rest of the analysis. Note that even though the ROIs were defined using the one-hand cohort, they can be used for all subjects (one-hand, two-hand, and sham) because these are group-level activation results.
Statistical analysis
Spatially averaged time courses (after removal of white matter, CSF, and motion vectors, and bandpass filtered as stated above) were obtained for each ROI. Two fcMRI indices were computed. CC between bilateral ROIs was calculated using Pearson correlation. Amplitude of fluctuation (AF) of the time course was calculated as the temporal standard deviation divided by mean signal intensity × 100% (Yang et al., 2007). This index is defined for each of the left and right sides.
Two-tail paired Student's t tests were used for comparisons of pre-task and post-task indices. For the sham control fcMRI data, linear mixed effect model and one-way ANOVA were used since multiple time points were available.
For the two-hand-task cohort in which we acquired three post-task time points, we used a One-way ANOVA in which time points (pre1, post1, post2, and post3) were categorical variables and pre1 was used as the reference point. The analysis provided a comparison between pre1 and each of the post-task time points.
Interaction analyses were also performed for time × group effect and for time × region effect. For the time × group interaction analysis, pre- and post-task data were treated as two time points within the same subject. The sham and task (combined one-hand and two-hand) data were considered as two groups of different subjects. For the time × region interaction analysis, the pre- and post-task data were treated as two time points within the same subject. Motor and DMN CC's were considered as two regions within the same subject. Given the a priori hypothesis of expected changes, a one-tailed p<0.05 is considered a significant effect.
Voxel based analysis
Aside from regional analysis based on a priori hypothesis, we also conducted voxel wise analysis to examine the whole brain for any clusters with significant pre/post-task differences. Motor cortex (including both left and right) was used as the seed region and averaged BOLD time courses were obtained. A voxel-by-voxel map of CC between the time course of each voxel and the seed time course was then computed and the pre- and post-task CC values were compared. AF was calculated on a voxel-by-voxel basis to generate spatial maps, which were then compared between pre-task and post-task conditions. To limit the number of false positive voxels, we only displayed clusters that showed significantly pre/post-task difference in both one-hand and two-hand datasets. Specifically, a voxelwise threshold of p<0.005 was applied on the one-hand-task group comparison to generate a preliminary mask. Another preliminary mask was generated for the two-hand-task data. An “AND” operation was performed between the two masks and a cluster size of 1350mm3 (corresponding to a FDR p<0.05) were used to delineate significant clusters (Xu et al., 2007).
Results
Behavioral data
All participants followed the task instructions and none missed any button presses. The trial-averaged RT was 470.8±129.9 ms (mean±SD across subjects) and 483.6±145.1 ms for the right-hand-only and bilateral task cohorts, respectively, with no statistical differences (p=0.15, two-sample t test between the right-hand group, N=24, and bilateral group, N=24). Within the 23-minute task period, there was no change of RT as a function of time. The effect of trial index on RT (linear mixed effect model with trial index as a fixed effect and subject as a random effect) was p=0.82 and p=0.52 for right-hand-only and bilateral task cohorts, respectively.
CC between bilateral motor cortices was significantly greater in post-task fcMRI
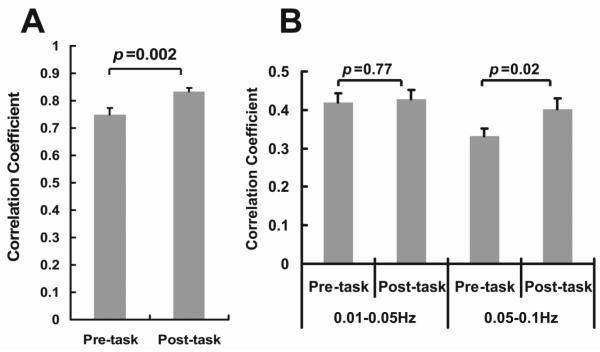
CC between left and right motor BOLD time courses was 0.75±0.02 (mean±SEM, N=24) in the pre-task fcMRI data, consistent with previous literature that bilateral motor cortices show strong resting-state connectivity (Biswal et al., 1995; Damoiseaux et al., 2006; van den Heuvel et al., 2008). The post-task fcMRI data showed a CC value of 0.83±0.02, which was significantly greater (p=0.002, two-tail paired t test) than that of the pre-task data (Fig. 2A). Next, we used a frequency band division approach proposed in the literature (Baria et al., 2011) to separate the data into a lower (0.01–0.05 Hz) and a higher band (0.05–0.1 Hz). This allows us to determine the frequency range that contributed most to the correlation increase. It was found that the increase in CC was specific to the higher frequency band (p=0.02) (Fig. 2B), whereas the lower frequency band did not show a significant change in CC (p=0.77) (Fig. 2B). This is in agreement with the notion that the 0.01–0.05 Hz band signal is more variable and the 0.05–0.1 Hz band is more tightly distributed (Baria et al., 2011). We therefore focused on the higher frequency band in the remainder of the analyses.
Figure 2.
Correlation coefficient (CC) between left and right motor cortex before and after the one-hand motor task (N=24). (A) CC values calculated using fcMRI time series that were bandpass-filtered to 0.01–0.1 Hz. (B) CC values calculated using time series further filtered to 0.01–0.05 Hz and 0.05–0.1 Hz, respectively. Error bar = standard error of mean across subjects.
Sham control experiment showed no changes in CC in motor cortices
To provide evidence that the motor task was causal of the observed CC changes, we performed a sham experiment where the subject remained at rest during the entire duration of the experiment (no motor task) while seven fcMRI runs were performed consecutively. CC values between the bilateral motor cortices showed no changes with time regardless of the type of statistical analysis used (linear mixed effect model on all seven runs, p=0.86; one-way ANOVA on all seven runs, p=0.73; paired t test between run 1 and run 7, p=0.96).
The fcMRI changes were region specific
To examine whether the fcMRI changes were spatially specific or they were a whole brain phenomenon, we studied another brain region that also has strong bilateral connectivity but is unrelated to the motor task, specifically the left and right parietal cortices, which are parts of the DMN. The left and right parietal cortices showed a significant correlation as expected, but there was no difference between the pre-task and post-task CC values (0.01–0.1 Hz band: 0.68±0.02 and 0.67±0.03 for pre- and post-task (p=0.86), respectively; 0.01–0.05 Hz band: 0.42±0.02 and 0.41±0.02 for pre- and post-task (p=0.78), respectively; 0.05–0.1 Hz band: 0.26±0.02 for both pre- and post-task, p=0.72).
Amplitude of Fluctuation (AF) of fcMRI signal also increased in post-task period
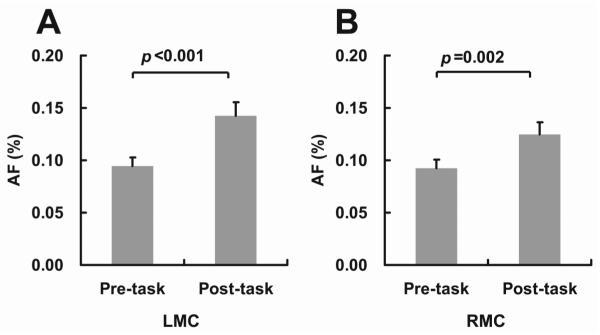
AF is another quantitative index from the fcMRI data. When physiologic fluctuations have been removed by WM/CSF/motion regressors and random noise is minimized by spatial averaging, AF is thought to be predominantly originated from spontaneous neural activity (Luchinger et al., 2012; Yang et al., 2007). Figures 3A and 3B show the AF indices during the pre-task and post-task periods. It was found that AF increased following the motor task. Interestingly, both the left motor cortex (LMC) (p<0.001, paired t test, N=24) and the right motor cortex (RMC) (p=0.002) showed a significant task-related AF increase although the right-hand task used in this study was expected to primarily activate the LMC. In terms of the effect size, LMC did manifest a significantly (p=0.003, paired t test, N=24) greater effect (51% increase comparing post-task AF to pre-task AF) compared to RMC (35% increase comparing post-task AF to pre-task AF).
Figure 3.
FcMRI amplitude of fluctuation (AF) in the bilateral motor cortices before and after the one-hand motor task (N=24). (A) Left Motor Cortex (LMC). (B) Right Motor Cortex (RMC). Error bar = standard error of mean across subjects.
Reproducing the results in a new cohort with a two-hand motor task
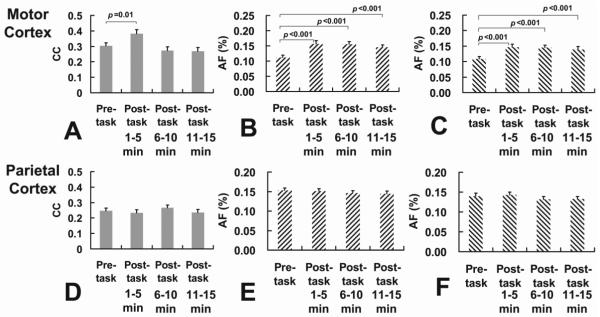
In a new cohort of subjects (N=24), the experiments were repeated but the motor task now involved button presses of both hands. The lower frequency band (0.01–0.05 Hz) showed no changes in motor CC (p=0.229), consistent with the results of the one-hand study. For the higher frequency band (0.05–0.1 Hz), the CC between bilateral motor cortices showed a significant increase (p=0.01, paired t test) following the task (Fig. 4A). AF showed an increase in both LMC (p<0.001, Figure 4B) and RMC (p<0.001, Fig. 4C). The effect sizes in LMC and RMC were not different (p=0.233) in the two-hand study, presumably because the two-hand task strongly activates both sides of the cortex. Left and right parietal cortices showed no pre/post-task differences in either CC or AF (Fig. 4D, E and F).
Figure 4.
Results of two-hand motor experiment (N=24) in motor cortex (top panels) and parietal cortex (bottom panels). (A) CC between LMC and RMC during the pre-task period and during three segments of the post-task periods. (B) AF in LMC during the pre-task period and during three segments of the post-task periods. (C) AF in RMC during the pre-task period and during three segments of the post-task periods. (D) CC between LPC and RPC during the pre-task period and during three segments of the post-task periods. (E) AF in LPC during the pre-task period and during three segments of the post-task periods. (F) AF in RPC during the pre-task period and during three segments of the post-task periods. CC values were based on 0.05–0.1 Hz data. p values of less than 0.05 are marked in the plots. Error bar = standard error of mean across subjects.
Interaction analyses using all data (one-hand, two-hand, and sham) confirmed the pre/post-task CC changes by revealing a significant (F(1,56)=3.84, p=0.027, one-tail) time × group interaction effect. Similarly, a significant (F(1,94)=7.52, p=0.004, one-tail) time × region interaction effect was observed, i.e. the pre/post-task CC's were different in a region specific manner.
How long do the changes last?
In the two-hand task cohort, we conducted three consecutive post-task fcMRI runs (for a total of 15 minutes) which allowed us to investigate how long the changes lasted. As can be seen in Figures 4A, B and C, CC and AF showed different time courses. Specifically, CC value returned to the pre-task level (One-way ANOVA p=0.38) in the second post-task fcMRI (i.e. 5–10 minutes after the task had ended) and stayed at this value (One-way ANOVA p=0.32) during the third run (10–15 minutes post-task) (Fig. 4A). In contrast, AF showed continued elevation during second and third post-task fcMRI runs (p<0.001 for all comparisons). These findings suggested that fcMRI changes in AF remained for at least 15 minutes after the task had ended. For the parietal cortices, both CC and AF stayed unchanged during the entire post-task period (Fig. 4D, E and F), as expected.
Voxel Based Analysis
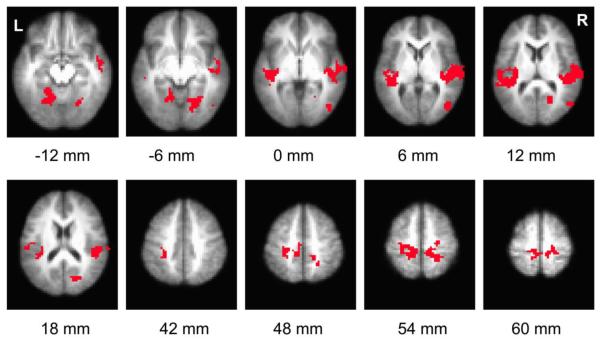
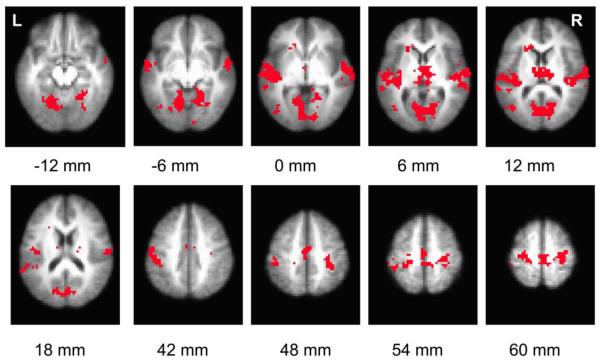
Figure 5 shows regions that revealed significant increases in brain connectivity to motor cortex, when comparing post-task to pre-task periods. Table 1 listed the coordinates of these clusters. Figure 6 illustrates clusters that showed significant post-task AF increase in both one-hand-task and two-hand-task data. Table 2 listed the coordinates of the clusters. No brain regions showed a significant decrease in either CC or AF.
Figure 5.
Voxel based map illustrating regions with significant increases in post-task resting CC with respect to the averaged motor time course. Statistical threshold: p<0.05 FDR corrected, minimum cluster size 1350 mm3. Only voxels delineated in both one-hand and two-hand experiments are shown. The label below each slice denotes its location relative to anterior commissure-posterior commissure (AC-PC) line.
Table 1.
Regions that show significant increases in CC during the post-task period.
| Region | x (mm) | y (mm) | z (mm) |
|---|---|---|---|
| Right Auditory Cortex (BA41) | −54.9 | −20.7 | 6.2 |
| Left Auditory Cortex (BA41) | 43.8 | −27.4 | 8.9 |
| Left Motor Cortex (BA4) and Left Sensorimotor Area (BA3) | 18.4 | −31 | 52.2 |
| Right Motor Cortex (BA4) and Right Sensorimotor Area (BA3) | −19.2 | −36.5 | 54.5 |
| Left Visual Area (BA19) | 16.9 | −54.2 | −9.2 |
| Right Visual Area (BA18) | −20.4 | −66.4 | 16.1 |
| Right Visual Area (BA19) | −22.4 | −61.7 | −5.8 |
| Right Visual Area (BA18) | −48 | −70.7 | 4.6 |
RAI/DICOM coordinates of the center-of-mass correspond to the space of TT-Daemon atlas provided by AFNI (“3dclust” command in AFNI). Voxel size 3×3×3mm3. p<0.05 FDR corrected. Minimum cluster size: 1350 mm3. Brodmann Area designations are indicated in parenthesis.
Figure 6.
Voxel based map illustrating regions with significant increases in post-task resting AF. Statistical threshold: p<0.05 FDR corrected, minimum cluster size 1350 mm3. Only voxels delineated in both one-hand and two-hand experiments are shown. The label below each slice denotes its location relative to anterior commissure-posterior commissure (AC-PC) line.
Table 2.
Brain regions with significant increases in AF comparing post-task to pre-task periods as was shown in Figure 6.
| Region | x (mm) | y (mm) | z (mm) |
|---|---|---|---|
| Left Sensorimotor Area (BA3) Left Primary Motor Cortex (BA4) | 49.5 | −22.2 | 40.4 |
| Right Sensorimotor Area (BA3) Left Primary Motor Cortex (BA4) | −30.7 | −26.2 | 54.5 |
| Left and Right premotor area and SMA (BA6) | −1.7 | −20.3 | 53.2 |
| Left Auditory Cortex (BA41) | 50.3 | −20.2 | 6 |
| Right Auditory Cortex (BA41) | −59.2 | −15 | 7.3 |
| Thalamus, Left and Right | −2.6 | −14 | 9.3 |
| Cuneus (BA30) / Secondary Visual Cortex (BA18) | 2.9 | −68.3 | 5.2 |
| Right Parahippocampal Gyrus (BA19) | −19.9 | −51.5 | −6.8 |
| Left Middle Temporal Gyrus (BA37) | 43.6 | −67.4 | 5.1 |
| Left Caudate/Anterior Cingulate | 19.4 | 25.6 | 7.7 |
RAI/DICOM coordinates of the center-of-mass correspond to the space of TT-Daemon atlas provided by AFNI (“3dclust” command in AFNI). Voxel size 3×3×3mm3. p<0.05 FDR corrected. Minimum cluster size: 1350 mm3. Brodmann Area designations are indicated in parenthesis.
DISCUSSION
The present study demonstrated that recent use of motor cortex can alter resting-state functional connectivity and these changes can take place following simple tasks that do not necessarily involve motor learning. These findings suggest that, in neuroimaging studies where both resting and evoked functional MRI are planned, it is preferable to collect fcMRI data before the fMRI runs.
Comparison with previous literature on fcMRI modulation by task or training
It has been shown that resting-state functional connectivity may be altered after days or weeks of a task or training. For example, Lewis et al. conducted visual perception training for 2–9 days until the subject reached a performance accuracy of 80% and compared the subject's functional connectivity pattern before and after the training (Lewis et al., 2009). The authors observed a strengthening of connections between the visual and attention brain regions (Lewis et al., 2009). This task modulation effect has also been observed following minutes of training (see Introduction for descriptions of a few examples) (Albert et al., 2009; Klingner et al., 2012; Peltier et al., 2005; Tambini et al., 2010; Waites et al., 2005).
The present study provides further evidence that functional connectivity can be altered on the scale of minutes, such that one can detect the effect in the same imaging session. Furthermore, all previous studies on motor network modulations have used study specific tasks and devices, i.e. medial nerve stimulation in Klingner et al., fatiguing gripping task in Peltier et al., and joystick tracking in Albert et al. The present study used a more generic motor task of button pressing, with standard fMRI button boxes. Thus our findings may have more direct implications for the potential effect of evoked fMRI task on later acquired fcMRI data.
The total duration of the button pressing task and the total number of button presses were 23 minutes and 120, respectively, in the present study. Typical fMRI studies use 2–4 runs of fMRI tasks with each run lasting for approximately 5 minutes. Thus, the total duration of fMRI task (10–20 minutes) in typical studies may be slightly shorter than that used in the present study. On the other hand, the inter-stimulus interval (ISI) in typical fMRI studies is likely to be shorter than the 27–32 seconds used in the present study. Assuming an average ISI of 8 second in event-related design, the total number of button presses in typical studies will be between 75–150, which is comparable to the number, 120, used in the current study. Thus, it is plausible that the task performance in many fMRI runs could have a similar effect on fcMRI data.
Possible physiologic underpinnings of the fcMRI differences observed in the present study
Given that the task used in our study is a simple button press and there exists little learning or improvement in performance in such a task, one plausible reason for the observed fcMRI differences is a passive “echo” or “ripple effect” that occurs automatically within the previously activated brain regions, as proposed by Stevens et al. (2010). Indeed, Hoffman and McNaughton (2002) reported in macaque monkey that repeated somatosensory and motor activation resulted in a higher synchronization of local field potentials (LFP) between left and right motor cortices during the 30 minutes following the task, compared to the pre-task resting period (Hoffman and McNaughton, 2002). This type of enhancement in resting connectivity was reported in other brain networks too. Hasson and colleagues showed that passive listening to linguistic material can increase the functional connectivity of superior temporal gyrus and left precentral gyrus during the post-task period (Hasson et al., 2009). The reason that there is a passive “ripple effect” from previous activation could be due to an aggregation of neurotransmitter receptors in the synaptic terminal or an enlargement in the dendritic spine due to the recent use (Cingolani et al., 2008; Lushnikova et al., 2009; Yoshida et al., 2009).
While previous studies have reported that sleepiness could also increase the signal fluctuation in fcMRI data (Fukunaga et al., 2008), we do not believe that the observed differences can be solely attributed to a reduced alertness level for two reasons. First, we conducted a sham control study with similar session duration but without the motor task, and we observed no changes in fcMRI data comparing the end to the beginning of the session. Second, sleepiness-induced fcMRI changes are known to involve all of gray matter in the brain (Fukunaga et al., 2008), but only selective regions are observed in the present study, most notably in regions activated during the task period.
Are our results a case of imagery activation during the post-task period? Imagery activation has been shown to share some similarities with actual execution in term of neural representation (Binkofski et al., 2000; Boecker et al., 2002; Chen et al., 2009; Dechent et al., 2004; Deiber et al., 1998; Hanakawa et al., 2002; Iseki et al., 2008; Kasess et al., 2008; la Fougere et al., 2010; Leonardo et al., 1995; Munzert and Zentgraf, 2009; Nair et al., 2003; Rao et al., 1993; Sharma et al., 2006; Stephan et al., 1995). However, several pieces of evidence in our data suggest that imagery activation is unlikely to explain all of our results. First, the auditory cortices also show significant CC and AF increases during the post-task rest. Since our task does not involved auditory tasks, we see no reason that the subject would think of the sound more in the post-task period than in the pre-task period. Second, in our one-hand-task group, fcMRI changes were observed in both left and right motor cortices. Since motor task (and imagery) predominantly activates the contralateral side of the cortex, motor activation by imagery (of the right hand) cannot account for signal changes in the right motor cortex (Stinear et al., 2006). Third, the subjects were specifically instructed not to think of anything in particular during the resting scans, and any occasional thinking about the motor task could not have accounted for the large (35–51%) increase in signal fluctuation.
Spatial distribution of the post-task changes
From the voxelwise map, it is clear that the pre/post-task differences are not a whole brain phenomenon. Instead, they are distributed in specific brain regions. The changes were primarily observed in areas activated during the task, such as sensorimotor cortices. Visual areas also showed changes, which can be attributed to the color change of the fixation crosshair during the task period. As for the auditory network, it is known that direct connections exist from auditory regions in the superior temporal gyrus to the motor areas (Chavis and Pandya, 1976). Functional imaging literature also suggests that motor, auditory and somatosensory regions of the human brain show functional coactivations. Zhang et al used fcMRI in human to show that posterior supplementary motor area (SMA) is functionally connected to auditory cortex (Zhang et al., 2012). Baumann et al showed that the auditory cortex was activated even though the subjects were moving their right hand fingers on a mock keyboard without auditory feedback (Baumann et al., 2005).
Practical implications for scan orders in an MRI session
The findings from the present study suggest that button press response that is commonly used in cognitive fMRI tasks can have a significant impact on subsequent fcMRI data. Therefore, it is recommended that fcMRI data be acquired before the evoked fMRI runs. If the experimental design stipulates that fMRI must be acquired before fcMRI (for example, due to outcome priority reasons), a time gap of 15 minutes or longer should be placed between them to allow the effect of recent use to dissipate. Anatomic scans can be performed during this gap period. The present study has focused on the effect of recent motor use on fcMRI data. Given previous literature that argues that recent use of other brain regions can also affect the corresponding fcMRI networks, it seems reasonable to suggest that, as a general rule of thumb, fcMRI run should be performed before fMRI.
Limitations of the study
The findings from the present study should be interpreted in view of a few limitations. First, the sham control study did not maximally match that of the actual task study. Specifically, the sham study did not have the visual and monitoring components that are present in the task study. It would have been more useful if the fixation cross in the sham study had changed color in the same fashion as the task study and the subject had responded with alternative means (e.g. verbal). Second, to further rule out the possibility that the observed increase in CC is an imagery effect (i.e. the subject was still thinking about the button press task in the post-task period), it would be useful to conduct another control study in which a short “distracting” task is inserted between the motor task and the post-task scan, so that the attention of the subject is effectively shifted away from the earlier motor task. Finally, although our hypothesized mechanism has focused on the finger press task, the curling of the hand itself may activate certain proprioceptive receptors and therefore the somatosensory cortex. It remains to be tested whether finger press without curling of the hand would induce a similar effect.
Conclusions
The findings in the present study suggest that recent use of motor cortex, even outside the context motor learning, can cause a change in subsequent fcMRI data by increasing interhemispheric correlation and BOLD signal fluctuation. It is recommended that, in an MRI session with both fcMRI and evoked fMRI tasks, fcMRI data should be collected either before evoked fMRI or after a sufficient time gap following the fMRI.
Highlights
-
●
CC between left and right motor cortices increased following a button-press task.
-
●
Sham control study without button-press revealed no changes in CC.
-
●
CC between bilateral parietal cortices did not change following button-press task.
-
●
Amplitude of fluctuation in motor cortex also increased in post-task period.
-
●
Several other regions activated during the task also showed fcMRI changes.
Acknowledgements
This study was supported in part by NIH grants R01 MH084021 (to HL), R01 NS067015 (to HL), R01 AG042753 (to HL), R21 NS078656 (to HL), and R21 AG034318 (to HL). The authors are grateful to Dr. Janet Jerrow for scientific editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest None declared.
References
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011;31:7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A, Bullmore ET, Suckling J. Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One. 2009;4:e6626. doi: 10.1371/journal.pone.0006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann S, Koeneke S, Meyer M, Lutz K, Jancke L. A network for sensory-motor integration: what happens in the auditory cortex during piano playing without acoustic feedback? Ann N Y Acad Sci. 2005;1060:186–188. doi: 10.1196/annals.1360.038. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Amunts K, Stephan KM, Posse S, Schormann T, Freund HJ, Zilles K, Seitz RJ. Broca's region subserves imagery of motion: a combined cytoarchitectonic and fMRI study. Hum Brain Mapp. 2000;11:273–285. doi: 10.1002/1097-0193(200012)11:4<273::AID-HBM40>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker H, Ceballos-Baumann AO, Bartenstein P, Dagher A, Forster K, Haslinger B, Brooks DJ, Schwaiger M, Conrad B. A H(2)(15)O positron emission tomography study on mental imagery of movement sequences--the effect of modulating sequence length and direction. Neuroimage. 2002;17:999–1009. [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis DA, Pandya DN. Further observations on corticofrontal connections in the rhesus monkey. Brain Res. 1976;117:369–386. doi: 10.1016/0006-8993(76)90089-5. [DOI] [PubMed] [Google Scholar]
- Chen H, Yang Q, Liao W, Gong Q, Shen S. Evaluation of the effective connectivity of supplementary motor areas during motor imagery using Granger causality mapping. Neuroimage. 2009;47:1844–1853. doi: 10.1016/j.neuroimage.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Smith S, De Stefano N, Federico A, Matthews PM. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp Brain Res. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- Dechent P, Merboldt KD, Frahm J. Is the human primary motor cortex involved in motor imagery? Brain Res Cogn Brain Res. 2004;19:138–144. doi: 10.1016/j.cogbrainres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Honda M, Sadato N, Raman R, Hallett M. Cerebral processes related to visuomotor imagery and generation of simple finger movements studied with positron emission tomography. Neuroimage. 1998;7:73–85. doi: 10.1006/nimg.1997.0314. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, de Zwart JA, van Gelderen P, Balkin TJ, Braun AR, Duyn JH. Metabolic origin of BOLD signal fluctuations in the absence of stimuli. J Cereb Blood Flow Metab. 2008;28:1377–1387. doi: 10.1038/jcbfm.2008.25. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Sawamoto N, Okada T, Yonekura Y, Fukuyama H, Shibasaki H. The role of rostral Brodmann area 6 in mental-operation tasks: an integrative neuroimaging approach. Cereb Cortex. 2002;12:1157–1170. doi: 10.1093/cercor/12.11.1157. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci U S A. 2009;106:10841–10846. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Liu TT. A geometric view of global signal confounds in resting-state functional MRI. Neuroimage. 2012;59:2339–2348. doi: 10.1016/j.neuroimage.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, Tang H, Zhu C, Gong Q, Zang Y, Evans AC. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One. 2009;4:e5226. doi: 10.1371/journal.pone.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science. 2002;297:2070–2073. doi: 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki K, Hanakawa T, Shinozaki J, Nankaku M, Fukuyama H. Neural mechanisms involved in mental imagery and observation of gait. Neuroimage. 2008;41:1021–1031. doi: 10.1016/j.neuroimage.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Kasess CH, Windischberger C, Cunnington R, Lanzenberger R, Pezawas L, Moser E. The suppressive influence of SMA on M1 in motor imagery revealed by fMRI and dynamic causal modeling. Neuroimage. 2008;40:828–837. doi: 10.1016/j.neuroimage.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Klingner CM, Hasler C, Brodoehl S, Axer H, Witte OW. Perceptual plasticity is mediated by connectivity changes of the medial thalamic nucleus. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50:1589–1598. doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]
- Leonardo M, Fieldman J, Sadato N, Campbell G, Ibanez V, Cohen L, P. DM, Jezzard P, Pons T, Turner R, Le Bihan D, Hallet M. A functional magnetic resonance imaging study of cortical regions associated with motor task execution and motor ideation in humans. Human Brain Mapping. 1995;3:83–92. [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, Yi Y, Xu L, Jiang T. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224:184–192. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- Luchinger R, Michels L, Martin E, Brandeis D. Brain state regulation during normal development: Intrinsic activity fluctuations in simultaneous EEG-fMRI. Neuroimage. 2012;60:1426–1439. doi: 10.1016/j.neuroimage.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Lushnikova I, Skibo G, Muller D, Nikonenko I. Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus. 2009;19:753–762. doi: 10.1002/hipo.20551. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32:1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzert J, Zentgraf K. Motor imagery and its implications for understanding the motor system. Prog Brain Res. 2009;174:219–229. doi: 10.1016/S0079-6123(09)01318-1. [DOI] [PubMed] [Google Scholar]
- Nair DG, Purcott KL, Fuchs A, Steinberg F, Kelso JA. Cortical and cerebellar activity of the human brain during imagined and executed unimanual and bimanual action sequences: a functional MRI study. Brain Res Cogn Brain Res. 2003;15:250–260. doi: 10.1016/s0926-6410(02)00197-0. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hillary FG, Biswal BB. Resting network plasticity following brain injury. PLoS One. 2009;4:e8220. doi: 10.1371/journal.pone.0008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier SJ, LaConte SM, Niyazov DM, Liu JZ, Sahgal V, Yue GH, Hu XP. Reductions in interhemispheric motor cortex functional connectivity after muscle fatigue. Brain Res. 2005;1057:10–16. doi: 10.1016/j.brainres.2005.06.078. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, et al. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- Salvador R, Martinez A, Pomarol-Clotet E, Sarro S, Suckling J, Bullmore E. Frequency based mutual information measures between clusters of brain regions in functional magnetic resonance imaging. Neuroimage. 2007;35:83–88. doi: 10.1016/j.neuroimage.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Sharma N, Pomeroy VM, Baron JC. Motor imagery: a backdoor to the motor system after stroke? Stroke. 2006;37:1941–1952. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Lovejoy D, Kim J, Oakes H, Kureshi I, Witt ST. Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging Behav. 2012;6:293–318. doi: 10.1007/s11682-012-9157-4. [DOI] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb Cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Fleming MK, Byblow WD. Lateralization of unimanual and bimanual motor imagery. Brain Res. 2006;1095:139–147. doi: 10.1016/j.brainres.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Ge Y, Sodickson DK, Miles L, Zhou Y, Reaume J, Grossman RI. Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology. 2011;260:831–840. doi: 10.1148/radiol.11110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012;17:471, 549–458. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Hulshoff Pol H. Normalized cut group clustering of resting-state FMRI data. PLoS One. 2008;3:e2001. doi: 10.1371/journal.pone.0002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T. Altered functional connectivity in early Alzheimer's disease: a resting-state fMRI study. Hum Brain Mapp. 2007;28:967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wig GS, Schlaggar BL, Petersen SE. Concepts and principles in the analysis of brain networks. Ann N Y Acad Sci. 2011;1224:126–146. doi: 10.1111/j.1749-6632.2010.05947.x. [DOI] [PubMed] [Google Scholar]
- Xu F, Uh J, Brier MR, Hart J, Jr., Yezhuvath US, Gu H, Yang Y, Lu H. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J Cereb Blood Flow Metab. 2011;31:58–67. doi: 10.1038/jcbfm.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Antuono PG, Jones J, Xu Y, Wu G, Ward D, Li SJ. Perfusion fMRI detects deficits in regional CBF during memory-encoding tasks in MCI subjects. Neurology. 2007;69:1650–1656. doi: 10.1212/01.wnl.0000296941.06685.22. [DOI] [PubMed] [Google Scholar]
- Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Satoh T, Nakamura KC, Kaneko T, Hata Y. Cortical activity regulates corticothalamic synapses in dorsal lateral geniculate nucleus of rats. Neurosci Res. 2009;64:118–127. doi: 10.1016/j.neures.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li CS. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex. 2012;22:99–111. doi: 10.1093/cercor/bhr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]