Abstract
Rapid endocytosis, which takes only a few seconds, is widely observed in secretory cells. Although it is more efficient in recycling vesicles than slow clathrin-mediated endocytosis, its underlying mechanism, thought to be clathrin-independent, is largely unclear. Here we reported that cleavage of three SNARE proteins essential for exocytosis, including synaptobrevin, SNAP-25 and syntaxin, inhibited rapid endocytosis at the calyx of Held nerve terminal, suggesting the involvement of three SNARE proteins in rapid endocytosis. These SNARE proteins were also involved in slow endocytosis. In addition, SNAP-25 and syntaxin facilitated vesicle mobilization to the readily releasable pool, likely via their roles in endocytosis and/or exocytosis. We concluded that both rapid and slow endocytosis share the involvement of SNARE proteins. The dual role of three SNARE proteins in exo- and endocytosis suggests that SNARE proteins may be molecular substrates contributing to the exo-endocytosis coupling, which maintains exocytosis in secretory cells.
Introduction
Vesicle endocytosis recycles exocytosed vesicles and thus maintains exocytosis in secretory cells (Royle and Lagnado, 2003). Endocytosis may be slow with a time constant (τ) of ~10 - 60 s or rapid with a τ of ~1 - 3 s. Slow endocytosis, observed at most secretory cells examined (Royle and Lagnado, 2003), is mediated by the clathrin-dependent mechanism (Dittman and Ryan, 2009). Rapid endocytosis is observed at most cells where the membrane capacitance can be measured, such as retinal nerve terminals (Von Gersdorff and Matthews, 1994), calyx of Held (Sun et al., 2002; Wu et al., 2005), hippocampal mossy fiber terminals (Hallermann et al., 2003), pituitary nerve terminals (Hsu and Jackson, 1996), auditory hair cells (Beutner et al., 2001), and non-neuronal secretory cells (Artalejo et al., 1995; He et al., 2008). Optical imaging also reveals rapid endocytosis at small synapses (Zhang et al., 2009), although this issue remains debated (Granseth et al., 2009; He and Wu, 2007). Since rapid endocytosis is triggered by calcium influx during intense activity (Artalejo et al., 1995; Neves et al., 2001; Beutner et al., 2001; Wu et al., 2005; Wu et al., 2009; but see Von Gersdorff and Matthews, 1994), it may fulfill the need of faster vesicle recycling with a larger capacity when many vesicles are released by intense stimulation. It may also rapidly restore the normal membrane structure of secretory cells (Wu and Wu, 2009). Despite these important roles, its underlying molecular mechanism, which is clathrin-independent (Artalejo et al., 1995; Jockusch et al., 2005), remains elusive.
Here we determined whether three SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins essential for exocytosis, synaptobrevin, SNAP-25 and syntaxin (Sudhof, 2004), are involved in rapid and/or slow endocytosis at the calyx of Held nerve terminal. We found that all three SNARE proteins are involved in rapid and slow endocytosis, which may help to explain the tight coupling of exo-endocytosis.
Results
Rapid endocytosis in control
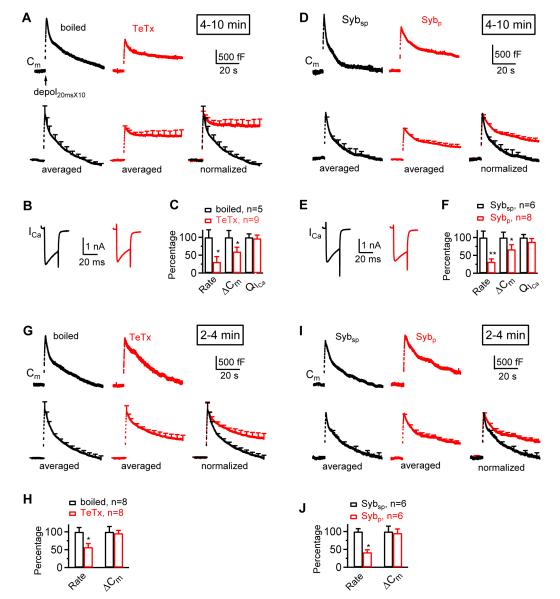
At calyces of 7 – 10 days old rats, we induced rapid endocytosis with 10 pulses of 20 ms depolarization from −80 to +10 mV at 10 Hz (depol20msX10) (Wu et al., 2005; Wu et al., 2009). With boiled tetanus toxin (TeTx, 2 μM) in the pipette serving the control for TeTx application, depol20msX10 induced a membrane capacitance (Cm) jump (ΔCm) of 1710 ± 325 fF, followed by a bi-exponential decay with time constants (τ) of 1.6 ± 0.3 s (amplitude: 22 ± 4%) and 21.0 ± 2.2 s (n = 5, Fig. 1A). The initial Cm decay after the ΔCm (Ratedecay), measured from the trace between 0.5 and 1 – 1.5 s after stimulation, was 237 ± 58 fF/s (n = 5). We did not measure the first 0.5 s after stimulation because it may contain Cm artifacts with a τ of ~200 ms (Wu et al., 2005; Yamashita et al., 2005). For two reasons, rapid Cm decay beyond 0.5 s reflects endocytosis. First, botulinum toxin C (BoNT/C), which cleaves syntaxin (Sakaba et al., 2005), abolishes the ΔCm induced by depol20msX10 (Wu et al., 2005). Thus, the ΔCm reflects SNARE-mediated exocytosis. Subsequent rapid Cm decay must reflect retrieval of exocytosed vesicles. Second, rapid Cm decay is mediated by dynamin- and calcium/calmodulin-dependent endocytosis because it is inhibited by various dynamin inhibitors (Xu et al., 2008), calcium buffers, and calmodulin inhibitors (Wu et al., 2009).
Figure 1.
Block of synaptobrevin inhibits endocytosis induced by depol20msX10
(A) Sampled (upper, single trace) and averaged (lower, mean + s.e.m.) capacitance changes (Cm) induced by depol20msX10 (arrow) with a pipette containing boiled TeTx (2 μM, n = 5 calyces, black) or TeTx (2 μM, n = 9 calyces, red). Averaged Cm traces (lower left two traces) are also normalized to the same amplitude and superimposed (lower right). Traces were taken at 4 – 10 min after whole-cell break in (applies to A-F).
(B) Sampled calcium currents (ICa) induced by the first 20 ms depolarization during depol20msX10 with a pipette containing boiled TeTx (black) or TeTx (red).
(C) Ratedecay_n (Rate), ΔCm, and QICa induced by depol20msX10 in the presence of boiled TeTx (2 μM, black, n = 5) or TeTx (2 μM, red, n = 9). Data were normalized to the mean of the boiled TeTx group. *: < 0.05; **: < 0.01.
(D-F) Similar to panels A-C, respectively, except that boiled TeTx and TeTx are replaced with Sybsp (2 mM, n = 6) and Sybp (2 mM, n = 8), respectively.
(G-J) Similar to panels A, C, D, and F, respectively, except that the data were obtained at 2 – 4 min after break in.
When the ΔCm was normalized to 1, the Ratedecay measured from 0.5 to 1 – 1.5 s after stimulation, now called Ratedecay_n, was 0.14 ± 0.03/s (n = 5). Similar results were obtained in the absence or presence of other boiled toxins or scrambled peptides used in this study. The Ratedecay or Ratedecay_n reflected mostly the rapid component of endocytosis (Wu et al., 2005; Wu et al., 2009), as confirmed below. Based on the averaged capacitance trace in control (Fig. 1A), the Ratedecay of the rapid component of endocytosis, which can be theoretically calculated as the ratio between the amplitude and the τ, was 235 fF/s (= 1710fF * 0.22/1.6 s), whereas the Ratedecay of the slow component of endocytosis was only ~64 fF/s (= 1710 fF*0.78/21 s). Thus, the rapid component represented ~79% (= 235/(235+64)) of the overall Ratedecay. In this study, Ratedecay or Ratedecay_n was measured directly from the trace, but not from theoretical calculation, because the block of endocytosis by toxins made it difficult for exponential fitting. Likewise, we did not use endocytosis τ for statistics.
Block of synaptobrevin inhibits rapid endocytosis
We included in the pipette either tetanus toxin (TeTx, 2 μM) to cleave synaptobrevin or a peptide containing the N-terminal proline-rich domain of synaptobrevin (Sybp, 2 mM) that blocks the interaction between synaptobrevin and other exocytosis proteins (Cornille et al., 1995). The corresponding control was boiled TeTx (2 μM) or scrambled Sybp (Sybsp, 2 mM). At 4 - 10 min after whole-cell break in, TeTx and Sybp significantly reduced the Ratedecay_n induced by depol20msX10 to ~31 - 32% and the ΔCm to ~60 - 67% of the corresponding control, but did not affect the calcium current amplitude or charge (QICa, Fig. 1A-F). Thus, reduction of Ratedecay_n was not due to QICa reduction (Fig. 1B-C, E-F).
Four sets of evidence indicate that the Ratedecay_n reduction was not caused by the ΔCm reduction. First, since ΔCm was normalized to 1, Ratedecay_n should be inversely proportional to Cm decay τ regardless of the ΔCm value (theoretically, Ratedecay_n = 1/τ). Thus, Ratedecay_n inversely reflects endocytosis τ and is independent of the ΔCm value. Second, there has been no report that an exocytosis decrease alone can prolong endocytosis or reduce Ratedecay_n. Instead, decrease of exocytosis is accompanied by a linear decrease or no change of endocytosis τ depending on whether the endocytic capacity is saturated or not (Wu and Betz, 1996; Sankaranarayanan and Ryan, 2000; Sun et al., 2002; Wu et al., 2005; Yamashita et al., 2005; Balaji et al., 2008). Accordingly, the ΔCm decrease itself may increase or not affect Ratedecay_n, but not decrease it. The decrease of Ratedecay_n is therefore a conservative estimate of endocytosis inhibition when ΔCm is decreased.
Third, we recently showed that various strong stimuli induced a ΔCm (~1500 fF) similar to that induced by depol20msX10, but different Ratedecay (or Ratedecay_n) that varies by ~8 times owing to the difference in calcium influx (Xue et al., 2012b). Various weaker stimuli induced a ΔCm of ~400 - 500 fF, a value similar to that induced by a 20 ms depolarization we used later, but ~2 - 10 times of differences in the Ratedecay or Ratedecay_n (Wu et al., 2009; Xue et al., 2012b). Thus, ΔCm has minimal effect on Ratedecay_n.
Fourth, at 2 - 4 min after whole-cell break in, TeTx and Sybp did not reduce the ΔCm, but the Ratedecay_n to 57 ± 12% and 42 ± 7% of the corresponding control, respectively (n = 6 - 8, p < 0.05, Fig. 1G-J). Thus, the Ratedecay_n decrease was not caused by the ΔCm decrease.
Reduction of the ΔCm by TeTx (2 μM, 6-8 min dialysis) was smaller than an earlier report (Sakaba et al., 2005), likely because the earlier report used a higher concentration (5 μM) for a longer dialysis time (8 - 12 min). Consistent with this possibility, less block was observed when a lower concentration was dialyzed for only 5-6 min from the same lab (Hosoi et al., 2009).
The decrease of Ratedecay_n by TeTx and Sybp suggests that synaptobrevin is involved in endocytosis (Fig. 1). However, if there are two vesicle populations, one more susceptible to TeTx and Sybp but better equipped with endocytosis machinery, the other in the opposite, the latter population would be left in the presence of TeTx or Sybp to mediate exocytosis and thus cause slower endocytosis. This scenario is highly unlikely for two reasons. First, it predicts that when TeTx or Sybp does not block exocytosis, endocytosis is not affected. In contrast, at ~2-4 min after break in, TeTx or Sybp did not reduce ΔCm, but reduced Ratedecay_n (Fig. 1G-J). Second, the scenario predicts that at the time endocytosis finishes in control with two vesicle populations, endocytosis in the presence of blockers, in which only the slower population is left, should also finish. This prediction is incorrect. At ~40 s after stimulation, Cm returned to baseline in control, but did not decay much in the presence of TeTx or Sybp (Fig. 1A, D, G, I). Similar results were found for other toxins and slow endocytosis described later (e.g., Figs. 2D; 4A, 4C, 4E, 4G, 4I). We concluded that synaptobrevin is involved in endocytosis.
Figure 2.
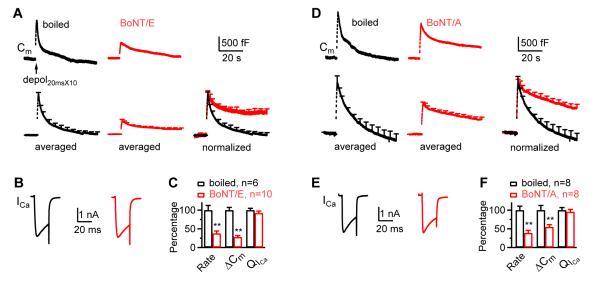
Block of SNAP-25 inhibits endocytosis induced by depol20msX10
(A-C) Similar arrangements as Fig. 1A-C, respectively, except using boiled BoNT/E (50 - 150 nM, n = 6) and BoNT/E (50 - 150 nM, n = 10). Data were taken at 4 – 10 min after break in (applies to A-F).
(D-F) Similar to panels A-C, respectively, except using boiled BoNT/A (1 μM, n = 8) and BoNT/A (1 μM, n = 8).
Figure 4.
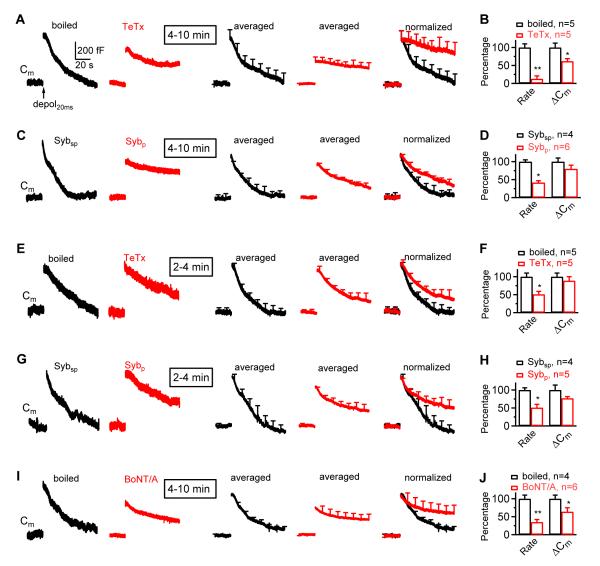
Block of synaptobrevin and SNAP-25 inhibits slow endocytosis induced by depol20ms
(A) Sampled (single traces, left two panels) and averaged (right three panels) Cm changes induced by depol20ms with a pipette containing boiled TeTx (2 μM, black; n = 5) or TeTx (2 μM, red; n = 5). The averaged traces are also normalized and superimposed (right). Traces were taken at 4 – 10 min after break in (applies to A-D, I-J). Scale bars apply to all Cm traces in Fig. 4 (except normalized traces).
(B) Ratedecay_n (Rate) and ΔCm induced by depol20ms in the presence of boiled TeTx (2 μM, black, n = 5) or TeTx (2 μM, red, n = 5). Data were normalized to the mean of the boiled TeTx group.
(C-D) Similar to panels A-B, respectively, except using Sybsp (2 mM, n = 4, black) and Sybp (2 mM, n = 6, red).
(E-H) Similar to panels A-D, respectively, except that the data were taken at 2-4 min after break in.
(I-J) Similar to panels A-B, respectively, except using boiled BoNT/A (1 μM; n = 4, black) and BoNT/A (1 μM; n = 6, red). Data were taken at 4-10 min after break in.
Block of SNAP-25 or syntaxin inhibits rapid endocytosis
We included botulinum neurotoxin E (BoNT/E, 50 - 150 nM) or BoNT/A (1 μM) in the pipette to cleave SNAP-25 (Niemann et al., 1994). The corresponding control was boiled BoNT/E (50 - 150 nM) or BoNT/A (1 μM). At 4 - 10 min after break in, BoNT/E and BoNT/A reduced the Ratedecay_n induced by depol20msX10 to 38 - 39% and the ΔCm to 28 - 55% of control on average, but did not affect QICa (n = 6 - 10 for each group, Fig. 2A-F). These results suggest the involvement of SNAP-25 in rapid endocytosis.
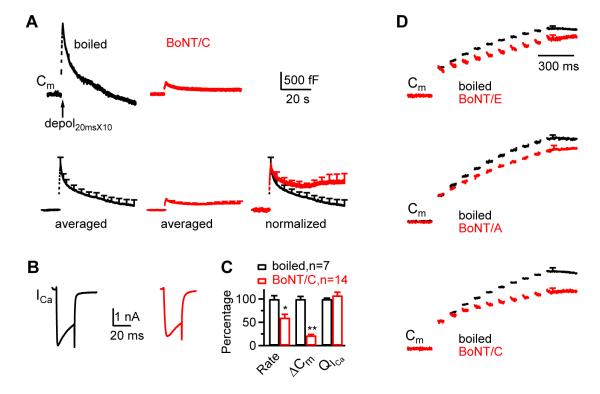
We included BoNT/C (200 - 400 nM) in the pipette to cleave syntaxin 1 (Niemann et al., 1994). The corresponding control was boiled BoNT/C. Although BoNT/C has a weak effect on SNAP-25 (Niemann et al., 1994), it cleaves syntaxin rather than SNAP-25 within ~10 min of dialysis at calyces (Sakaba et al., 2005). At 4 - 10 min after whole-cell break in, BoNT/C reduced the Ratedecay_n induced by depol20msX10 to 60% and the ΔCm to ~22% of control on average, but did not affect QICa (n = 7 - 14, Fig. 3A-C), suggesting the involvement of syntaxin in rapid endocytosis.
Figure 3.
Block of syntaxin inhibits endocytosis induced by depol20msX10 and block of SNAP-25 or syntaxin slows the RRP replenishment
(A-C) Similar arrangements as Fig. 1A-C, respectively, except using boiled BoNT/C (200 – 400 nM, n = 7, black) and BoNT/C (200 – 400 nM, n = 14, red). Data were taken at 4 – 10 min after break in.
(D) ΔCm induced by each 20 ms depolarization during depol20msX10 in the presence of BoNT/E (upper, red), BoNT/A (middle, red), BoNT/C (lower, red) and or their corresponding control (boiled toxin, black). Traces are taken from the averaged traces in Figs. 2A, 2D, and 3A respectively, and the ΔCm induced by the first 20 ms depolarization was normalized to be the same.
Block of SNAP25 or syntaxin inhibits replenishment of the readily releasable pool
Recent studies suggest that endocytosis may facilitate replenishment of the readily releasable pool (RRP) by clearance of fused vesicle membrane and proteins at the active zone of calyces (Wu et al., 2009; Hosoi et al., 2009). We noticed that BoNT/E, BoNT/A and BoNT/C slowed the RRP replenishment. The ΔCm induced by each 20 ms depolarization during depol20msX10 reflects the rate of RRP replenishment, because each 20 ms depolarization depleted the RRP (Wu et al., 2009). When the ΔCm induced by the first 20 ms depolarization was normalized to the same amplitude, it was evident that the ΔCm induced by the subsequent 9 depolarizing pulses during depol20msX10 decreased significantly in the presence of BoNT/E, BoNT/A or BoNT/C (Fig. 3D). We did not observe a consistent decrease for TeTx or Sybp. However, a small decrease below our detection limit remains possible. These results suggest that SNAP-25 and syntaxin facilitate the RRP replenishment, likely via their roles in endocytosis and/or exocytosis.
Block of synaptobrevin, SNAP-25 or syntaxin inhibits slow endocytosis
We induced slow endocytosis by a 20 ms depolarization (−80 to +10 mV, depol20ms) (Wu et al., 2005; Wu et al., 2009). In control with 2 μM boiled TeTx in the pipette, depol20ms induced a ΔCm of 531 ± 61 fF, followed by a decay with a τ of 12.4 ± 0.9 s and a Ratedecay of 40 ± 5 fF/s (n = 6, Fig. 4A), as measured within the first 4 s after stimulation. The Ratedecay_n was 0.08 ± 0.01/s (n = 6). Similar results were obtained with different boiled toxins or scrambled peptides.
At 4 - 10 min after whole-cell break in, TeTx and Sybp significantly reduced the Ratedecay_n induced by depol20ms to 13 - 42% and the ΔCm to 62 - 80% of control, on average (n = 4 - 6 for each group, Fig. 4A-D). As described above, reduction of the ΔCm itself could not decrease Ratedecay_n. In addition, at 2 - 4 min after break in, TeTx and Sybp did not significantly decrease the ΔCm, but reduced the Ratedecay_n to 51% of control (n = 4 - 5, Fig. 4E-H). These results suggest the involvement of synaptobrevin in slow endocytosis.
At 4 - 10 min after break in, BoNT/A reduced the Ratedecay_n and the ΔCm induced by depol20ms to 35 ± 7% and 64 ± 11% (n = 6) of control (n = 4, Fig. 4I-J). BoNT/E caused a near full block of the ΔCm induced by depol20ms, making it difficult to measure the Ratedecay_n. Nevertheless, the slow component of endocytosis after depol20msX10 was largely blocked by BoNT/E, as evident at 15 - 40 s after stimulation (Fig. 2A). These results suggest the involvement of SNAP-25 in slow endocytosis. Similarly, BoNT/C nearly abolished the ΔCm induced by depol20ms. The slow component of endocytosis after depol20msX10 was largely blocked by BoNT/C, as evident at 15 - 40 s after stimulation (Fig. 3A), suggesting the involvement of syntaxin in slow endocytosis.
Discussion
We found that cleavage of synaptobrevin, SNAP-25 and syntaxin blocked rapid endocytosis (Figs. 1 - 3), suggesting the involvement of three SNARE proteins in rapid endocytosis, which is suggested to be clathrin-independent (Artalejo et al., 1995; Jockusch et al., 2005). This finding provides new mechanistic information to the poorly understood rapid endocytosis. We also found that these three SNARE proteins are involved in slow endocytosis (Fig. 4), a clathrin-dependent form of endocytosis (Dittman and Ryan, 2009). Thus, two apparently different forms of endocytosis share a common mechanism – the dual role of three SNARE proteins in exo- and endocytosis.
Previously, three pioneering studies examined the involvement of SNARE proteins in endocytosis. One study insightfully implicates the involvement of synaptobrevin in rapid endocytosis at hippocampal synapses (Deak et al., 2004). However, the technique used to detect rapid endocytosis is indirect and controversial (He and Wu, 2007; Zhang et al., 2009; Granseth et al., 2009). A recent study shows that TeTx blocks slow endocytosis at calyces, suggesting the involvement of synaptobrevin in slow endocytosis (Hosoi et al., 2009). Knockout of SNAP-25 at cultured hippocampal synapses does not inhibit sucrose-induced FM dye uptake into synaptic vesicles, suggesting that SNAP-25 is not involved in endocytosis (Bronk et al., 2007). The present work extended over these previous studies in three aspects. First, by recording rapid endocytosis unequivocally with capacitance measurements (Wu et al., 2005; Xu et al., 2008; Xue et al., 2012c), we provided strong evidence showing that synaptobrevin is involved in rapid endocytosis. We consolidated the previous finding for the involvement of synaptobrevin in slow endocytosis by excluding the possibility that the block of endocytosis by TeTx is a side effect caused by reduction of exocytosis (Figs. 1G-H, 4E-F), which has not been addressed in the previous study (Hosoi et al., 2009). Second, we found that SNAP-25 was involved in both rapid and slow endocytosis in synapses. Unlike the previous study using sucrose to induce calcium-independent exo- and endocytosis (Bronk et al., 2007), we applied trains of depolarization to mimic physiological action potential stimuli (Wu et al., 2005; Xu et al., 2008; Wu et al., 2009), which induces calcium-dependent exo- and endocytosis. Third, we found that not only synaptobrevin, SNAP-25, but also syntaxin are involved in both rapid and slow endocytosis. More generally speaking, both vesicular and membrane targeted SNARE proteins are involved in endocytosis.
Given that SNARE proteins mediate exocytosis at all nerve terminals and many non-neuronal secretory cells, the dual role of three SNARE proteins in exo- and endocytosis is likely to have wide implication in secretory cells. The dual role in exo- and endocytosis suggests that after mediating vesicle fusion, three SNARE proteins participate in the subsequent endocytosis, likely at the initiation step. Three SNARE proteins may thus be the molecular substrate underlying the tight coupling between exo- and endocytosis, that is, exocytosis is quickly followed by endocytosis with a similar amount - a widely observed phenomenon essential in recycling vesicles and maintaining the membrane homeostasis.
The need of SNARE proteins in endocytosis may prevent futile endocytosis when the action potential-induced calcium influx, which triggers rapid, slow and bulk endocytosis (Wu et al., 2009; Hosoi et al., 2009; Clayton and Cousin, 2009; but see Von Gersdorff and Matthews, 1994; Leitz and Kavalali, 2011), fails to evoke SNARE-mediated exocytosis at nerve terminals with a low release probability. Thus, we suggest that calcium influx is not the only requirement for initiating endocytosis, SNARE proteins are also needed.
Many studies provide clues as to how SNARE proteins are involved in endocytosis. For example, the N-terminal half of the SNARE motif of synaptobrevin binds to ANTH domain of endocytic adaptors AP180 and clathrin assembly lymphoid myeloid leukemia (CALM), both of which are involved in endocytosis (Koo et al., 2011; Miller et al., 2011). SNAP-25 binds to intersectin, an endocytic protein, as strong as its binding with syntaxin (Okamoto et al., 1999). This binding is significantly weakened by BoNT/A or BoNT/E that cleaves SNAP-25 (Okamoto et al., 1999), which might explain why BoNT/A and BoNT/E blocked endocytosis (Figs. 2, 4). Syntaxin may bind dynamin, a GTPase mediating vesicle fission (Galas et al., 2000). It would be of great interest to understand how SNARE proteins participate in endocytosis in the future.
We showed that in the presence of TeTx or BoNTs that cleave SNARE proteins, the remaining SNARE proteins support the remaining exocytosis, but not endocytosis. Here we consider two mechanisms that may account for this observation. First, cleavage of SNARE proteins may not cause an all-or-none block of exocytosis. When SNAP-25 or synaptobrevin is cleaved at calyces, the release probability of RRP vesicles is reduced (Sakaba et al., 2005). However, all RRP vesicles can be released by 20 - 50 ms depolarization, suggesting that fewer copies of SNARE proteins per vesicle could still support exocytosis (Sakaba et al., 2005). Indeed, two copies of synaptobrevin are sufficient to support exocytosis (Sinha et al., 2011). If endocytosis needs more copies of SNARE complexes than exocytosis, TeTx and BoNTs may reduce the SNARE protein number per vesicle and thus inhibit endocytosis, but not necessarily the ΔCm induced by depol20ms or depol20msX10 that depletes the RRP. Second, TeTx and BoNTs cleave only free SNAREs, but not the SNARE complex (Niemann et al., 1994). After mediating exocytosis, the SNARE complex is disassembled into individual SNARE proteins (Sudhof, 2004), which becomes accessible to TeTx and BoNTs. Cleavage of these individual SNARE proteins after exocytosis by TeTx and BoNTs may thus cause inhibition of endocytosis without affecting the preceding exocytosis. These two mechanisms may explain why TeTx, Sybp or BoNTs inhibited endocytosis in the presence of exocytosis, including at early dialysis times when ΔCm was not reduced (Figs. 1G-J, 4E-H).
Our finding that block of SNAP-25 or syntaxin slowed down the RRP replenishment (Fig. 3D) suggests the involvement of SNARE proteins in the RRP replenishment. This mechanism might be mediated via the involvement of SNARE proteins in endocytosis, because endocytosis may facilitate the RRP replenishment by clearance of the exocytosed vesicle membrane and proteins at the active zones (Kawasaki et al., 2000; Hosoi et al., 2009; Wu et al., 2009). Alternatively, it might be due to a direct role of SNARE proteins in docking and priming of vesicles. Recent studies show that a pre-existing vesicle pool can be readily retrieved at hippocampal synapses (Fernandez-Alfonso et al., 2006; Wienisch and Klingauf, 2006; Hua et al., 2011) and calyces (Xue et al., 2012a). Our finding that block of SNARE proteins may nearly abolish endocytosis (e.g., Figs. 1A-C, 4A-B, 4I-J) supports the possibility that SNARE proteins are involved in retrieving readily retrievable vesicles. It would be of interest to further explore this possibility in the future.
Materials and Methods
Slice preparation, capacitance recordings and solutions
Slice preparation and capacitance recordings were described previously (Wu et al., 2009). Briefly, parasagittal brainstem slices (200 μm thick) containing calyces of Held were prepared from 7 - 10 days old male or female Wistar rats. Whole-cell capacitance measurements were made with the EPC-9 amplifier (HEKA, Lambrecht, Germany). The sinusoidal stimulus frequency was 1000 Hz with a peak-to-peak voltage ≤60 mV. We pharmacologically isolated Ca2+ currents with a bath solution (~22 - 24 °C) containing (in mM): 105 NaCl, 20 TEA-Cl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 glucose, 0.4 ascorbic acid, 3 myo-inositol, 2 sodium pyruvate, 0.001 tetrodotoxin (TTX), 0.1 3,4-diaminopyridine, 300 - 310 mOsm, pH 7.4 when bubbled with 95% O2 and 5% CO2. The pipette contained (in mM): 125 Cs-gluconate, 20 CsCl, 4 MgATP, 10 Na2-phosphocreatine, 0.3 GTP, 10 HEPES, 0.05 BAPTA, 310 - 320 mOsm, pH 7.2, adjusted with CsOH. Synaptobrevin peptide (SATAATVPPAAPAGEFFPPAPPPNLT) and scrambled synaptobrevin peptide (FPTAPAPASNPALPFTGPTAPAVEAP) were purchased from 21st Century Biochemicals, Inc. (Marlboro, MA, USA).
Data analysis
The statistical test was t-test. Means are presented as ± s.e.m. Ratedecay and Ratedecay_n were measured during 0.5 – 1.5 s after depol20msX10 and during 0.5 – 4 s after depol20ms. For comparison of the Ratedecay_n in the presence of a toxin or a boiled toxin, data were normalized to the mean Ratedecay_n for the boiled-toxin group. Cm baseline drift rate was usually less than 5 – 10% of the Ratedecay, and thus did not significantly affect the Ratedecay measurement. If the drift was >20%, which was infrequent, data were discarded.
Highlights.
Synaptobrevin, SNAP-25 and syntaxin are involved in rapid endocytosis
These SNARE proteins are also involved in slow endocytosis
SNAP-25 and syntaxin facilitate replenishment of the readily releasable pool
SNARE proteins may be the molecular substrate for exo-endocytosis coupling
Acknowledgements
We thank Dr. Peter Wen for reading of the manuscript. This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Artalejo CR, Henley JR, McNiven MA, Palfrey HC. Rapic endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc. Natl. Acad. Sci. USA. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J, Armbruster M, Ryan TA. Calcium control of endocytic capacity at a CNS synapse. J. Neurosci. 2008;28:6742–6749. doi: 10.1523/JNEUROSCI.1082-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Bronk P, Deak F, Wilson MC, Liu X, Sudhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+ -dependent and Ca2+ -independent neurotransmission. J Neurophysiol. 2007;98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- Clayton EL, Cousin MA. The molecular physiology of activity-dependent bulk endocytosis of synaptic vesicles. J. Neurochem. 2009;111:901–914. doi: 10.1111/j.1471-4159.2009.06384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornille F, Deloye F, Fournie-Zaluski MC, Roques BP, Poulain B. Inhibition of neurotransmitter release by synthetic proline-rich peptides shows that the N-terminal domain of vesicle-associated membrane protein/synaptobrevin is critical for neuro-exocytosis. J Biol. Chem. 1995;270:16826–16832. doi: 10.1074/jbc.270.28.16826. [DOI] [PubMed] [Google Scholar]
- Deak F, Schoch S, Liu X, Sudhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat. Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- Dittman J, Ryan TA. Molecular Circuitry of Endocytosis at Nerve Terminals. Annu. Rev. Cell Dev. Biol. 2009 doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- Fernandez-Alfonso T, Kwan R, Ryan TA. Synaptic Vesicles Interchange Their Membrane Proteins with a Large Surface Reservoir during Recycling. Neuron. 2006;51:179–186. doi: 10.1016/j.neuron.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Galas MC, Chasserot-Golaz S, rrig-Grosch S, Bader MF. Presence of dynamin--syntaxin complexes associated with secretory granules in adrenal chromaffin cells. J Neurochem. 2000;75:1511–1519. doi: 10.1046/j.1471-4159.2000.0751511.x. [DOI] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Comment on “The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles”. Science. 2009;325:1499. doi: 10.1126/science.1175790. [DOI] [PubMed] [Google Scholar]
- Hallermann S, Pawlu C, Jonas P, Heckmann M. A large pool of releasable vesicles in a cortical glutamatergic synapse. Proc. Natl. Acad. Sci. USA. 2003;100:8975–8980. doi: 10.1073/pnas.1432836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Wu LG. The debate on the kiss-and-run fusion at synapses. Trends Neurosci. 2007;30:447–455. doi: 10.1016/j.tins.2007.06.012. [DOI] [PubMed] [Google Scholar]
- He Z, Fan J, Kang L, Lu J, Xue Y, Xu P, Xu T, Chen L. Ca2+ triggers a novel clathrin-independent but actin-dependent fast endocytosis in pancreatic beta cells. Traffic. 2008;9:910–923. doi: 10.1111/j.1600-0854.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- Hosoi N, Holt M, Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Hsu S-F, Jackson MB. Rapid exocytosis and endocytosis in nerve terminals of the rat posterior pituitary. J. Physiol. 1996;492:539–553. doi: 10.1113/jphysiol.1996.sp021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Sinha R, Thiel CS, Schmidt R, Huve J, Martens H, Hell SW, Egner A, Klingauf J. A readily retrievable pool of synaptic vesicles. Nat. Neurosci. 2011;14:833–839. doi: 10.1038/nn.2838. [DOI] [PubMed] [Google Scholar]
- Jockusch WJ, Praefcke GJ, McMahon HT, Lagnado L. Clathrin-dependent and clathrin-independent retrieval of synaptic vesicles in retinal bipolar cells. Neuron. 2005;46:869–878. doi: 10.1016/j.neuron.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Hazen M, Ordway RW. Fast synaptic fatigue in shibire mutants reveals a rapid requirement for dynamin in synaptic vesicle membrane trafficking. Nat. Neurosci. 2000;3:859–860. doi: 10.1038/78753. [DOI] [PubMed] [Google Scholar]
- Koo SJ, Markovic S, Puchkov D, Mahrenholz CC, Beceren-Braun F, Maritzen T, Dernedde J, Volkmer R, Oschkinat H, Haucke V. SNARE motif-mediated sorting of synaptobrevin by the endocytic adaptors clathrin assembly lymphoid myeloid leukemia (CALM) and AP180 at synapses. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13540–13545. doi: 10.1073/pnas.1107067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitz J, Kavalali ET. Ca(2) influx slows single synaptic vesicle endocytosis. J. Neurosci. 2011;31:16318–16326. doi: 10.1523/JNEUROSCI.3358-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SE, Sahlender DA, Graham SC, Honing S, Robinson MS, Peden AA, Owen DJ. The molecular basis for the endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell. 2011;147:1118–1131. doi: 10.1016/j.cell.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Gomis A, Lagnado L. Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells. Proc. Natl. Acad. Sci. USA. 2001;98:15282–15287. doi: 10.1073/pnas.261311698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann H, Blasi J, Jahn R. Clostridial neurotoxins: new tools for dissecting exocytosis. Trends in Cell Biology. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Schoch S, Sudhof TC. EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis? J Biol. Chem. 1999;274:18446–18454. doi: 10.1074/jbc.274.26.18446. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Lagnado L. Endocytosis at the synaptic terminal. J. Physiol. 2003;553:345–355. doi: 10.1113/jphysiol.2003.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Stein A, Jahn R, Neher E. Distinct kinetic changes in neurotransmitter release after SNARE protein cleavage. Science. 2005;309:491–494. doi: 10.1126/science.1112645. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat. Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- Sinha R, Ahmed S, Jahn R, Klingauf J. Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14318–14323. doi: 10.1073/pnas.1101818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- Wienisch M, Klingauf J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat. Neurosci. 2006;9:1019–1027. doi: 10.1038/nn1739. [DOI] [PubMed] [Google Scholar]
- Wu LG, Betz WJ. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 1996;17:769–779. doi: 10.1016/s0896-6273(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Wu W, Xu J, Wu XS, Wu LG. Activity-dependent acceleration of endocytosis at a central synapse. J Neurosci. 2005;25:11676–11683. doi: 10.1523/JNEUROSCI.2972-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat. Neurosci. 2009;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Wu LG. Rapid endocytosis does not recycle vesicles within the readily releasable pool. J. Neurosci. 2009;29:11038–11042. doi: 10.1523/JNEUROSCI.2367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, McNeil B, Wu W, Nees D, Bai L, Wu LG. GTP-independent rapid and slow endocytosis at a central synapse. Nat. Neurosci. 2008;11:45–53. doi: 10.1038/nn2021. [DOI] [PubMed] [Google Scholar]
- Xue L, McNeil BD, Wu XS, Luo F, He L, Wu LG. A Membrane Pool Retrieved via Endocytosis Overshoot at Nerve Terminals: A Study of Its Retrieval Mechanism and Role. J. Neurosci. 2012a;32:3398–3404. doi: 10.1523/JNEUROSCI.5943-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, zhang Z, McNeil BD, Luo F, Wu XS, Sheng J, Shin W, Wu LG. Voltage-dependent calcium channels at the plasma membrane, but not vesicular channels, couple exocytosis to endocytosis. Cell Reports. 2012b;1:632–638. doi: 10.1016/j.celrep.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, zhang Z, McNeil BD, Luo F, Wu XS, Sheng J, Shin W, Wu LG. Voltage-dependent calcium channels at the plasma membrane, but not vesicular channels, couple exocytosis to endocytosis. Cell Rep. 2012c;1:632–638. doi: 10.1016/j.celrep.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Hige T, Takahashi T. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307:124–127. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323:1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]