Summary
Objective
Matrix fragments, including fibronectin fragments (Fnf), accumulate during the development of osteoarthritis (OA) stimulating chondrocyte matrix metalloproteinase (MMP) production. The objective of this study was to determine the role of the small GTPase Rac1 in chondrocyte signaling stimulated by Fnf that results in MMP-13 production.
Methods
Normal human cartilage was from tissue donors and OA cartilage from knee arthroplasty specimens. Rac1 activity was modulated with a chemical inhibitor, siRNA knock-down, constitutively active (CA)-Rac or dominant negative (DN)-Rac adenovirus. Cells were treated with Fnf or without known Rac activators, epidermal growth factor (EGF) or transforming growth factorα (TGFα). Rac1 activity was measured with a colorometric activity ELISA, pulldown assay, and immunostaining with a monoclonal antibody against active Rac.
Results
Chemical inhibition of Rac1, as well as knockdown by siRNA and expression of DN-Rac blocked Fnf stimulated MMP-13 production while expression of CA-Rac increased MMP-13. Inhibition of Rho-associated kinase had no effect. EGF and TGFα, but not Fnf, increased Rac1 activity and promoted the increase in MMP-13 above that stimulated by Fnf alone. Active Rac was detected by immunostaining in OA cartilage.
Conclusion
Rac1 is required for Fnf induced signaling that results in increased MMP-13 production. EGF receptor ligands, which activate Rac, can promote this effect. The presence of active Rac in OA cartilage and the ability of Rac to stimulate MMP-13 production suggests that it could play a role in the cartilage matrix destruction seen in OA.
Destruction of the articular cartilage matrix by proteolytic enzymes produced by activated articular chondrocytes is thought to play a key role in the development of osteoarthritis (OA) (1). The matrix degrading enzymes include matrix metalloproteinases (MMPs), aggrecanses, and various cysteine and serine proteases (2). MMP-13 is a potent collagenase that degrades type II collagen, an abundant cartilage matrix protein that provides cartilage with its ability to withstand mechanical loads. Neuhold et al (3) demonstrated that transgenic overexpression of MMP-13 in mice results in pathological changes in articular cartilage similar to those observed in human osteoarthritis. A more recent study by Little et al (4) found that mice lacking MMP-13 are resistant to the cartilage erosion that is a hallmark of osteoarthritis. Thus, understanding mechanisms responsible for stimulation of chondrocyte MMP-13 production is important for a better understanding of OA.
Multiple factors appear to be capable of stimulating chondrocytes to produce MMP-13 including several pro-inflammatory cytokines, chemokines, and growth factors (1). Our focus has been on the role of fibronectin fragments (Fnf) that are generated by proteolytic cleavage and are found at elevated levels in osteoarthritic cartilage and synovial fluid (5, 6). These fragments, in particular the Fnf’s containing the cell-binding RGD sequence, can potentially bind to and stimulate the α5β1 integrin receptor resulting in production of MMP-13 as well as many of the other pro-inflammatory factors and MMPs found in OA cartilage (7–9). The cell signaling network activated by Fnf includes the mitogen-activated protein kinases (MAPK) and transcriptional regulators such as AP-1 and NFκB which are thought to play a role in OA (7–9).
The Rho family of small GTPases consists of the three family members RhoA, Rac1, and CDC42, which have been shown to mediate signaling events in other cell types but have not been well studied in chondrocytes (10). RhoA appears to promote stress fiber formation and inhibits chondrocyte differentiation while Rac1 and CDC42 promote chondrocyte hypertrophy (10–12). Rac has been well studied in fibroblasts and found to control many diverse cellular functions including actin cytoskeletal reorganization, production of reactive oxygen species, and transcription (13). Rac is activated by extracellular signals including growth factors, cytokines, and, most relevant to the present work, integrins (14). Mice with Rac1 deletion in chondrocytes were found to have severe skeletal deformities with disorganized growth plates (15). Expression of constitutively active Rac increased production of type X collagen and alkaline phosphatase as well as MMP-13 and promoted chondrocyte hypertrophy (11, 16).
OA chondrocytes exhibit some features of the hypertrophic phenotype which can include the production of MMP-13. Thus, the signaling molecules involved in chondrocyte hypertrophy are also likely to be involved in osteoarthritis. The present study was undertaken to examine the role of Rac in chondrocyte signaling that results in MMP-13 production when articular chondrocytes are stimulated with Fnf. We found that Rac1 was required for the increased MMP-13 expression but surprisingly could not demonstrate direct activation of Rac by Fnf. Instead, EGF receptor ligands, including EGF and TGFα, were discovered to activate chondrocyte Rac and to promote the ability of Fnf to stimulate MMP-13 production.
MATERIALS AND METHODS
Reagents
Alexa488 fluorescent secondary antibody was from Invitrogen (Carlsbad, CA). Total Rac antibody and EGF receptor inhibitor AG1478 were from Cell Signaling (Beverly, MA). MMP-13 antibody was from Abcam (Cambridge, MA). MMP-13 ELISA and recombinant EGF were from R&D Systems (Minneapolis, MN). Recombinant TGFα was from Gemini Bioproducts (West Sacramento, CA). Control siRNA and smartpool siRNA against Rac1 was from Dharmacon (Lafayette, CO). Amaxa nucleofection reagents for transfection were from Lonza (Walkersville, MD). Predesigned MMP-13 real-time PCR primer was from SuperArray Biosciences (Frederick, MD). Rac inhibitor NSC23766 and ROCK inhibitor Y-27632 were from EMD Chemicals (Gibbstown, NJ). Rac inhibitor EHT1864 was from Tocris Biosciences (Bristol, UK). Recombinant fibronectin fragment containing the RGD cell binding domain (FN7-10) was a kind gift of Dr. Harold Erickson (Duke University, Durham, NC). Dominant negative and constitutively active Rac adenoviral constructs and a null control adenovirus were obtained from Cell Biolabs (San Diego, CA). Monoclonal antibody that recognizes active Rac1-GTP was from New East Biosciences (Malvern, PA).
Tissue acquisition and chondrocyte isolation
Normal human ankle articular cartilage was obtained from donors with no known history of arthritis from the National Disease Research Interchange (Philadelphia, PA) and from the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL) through the Department of Biochemistry at Rush University Medical Center (Chicago, IL). Diseased human knee cartilage was obtained from donors undergoing knee replacement surgery for osteoarthritis at the Wake Forest Baptist Hospital (Winston-Salem, NC). Chondrocytes were isolated with sequential pronase and collagenase digestion and plated in high density monolayers as previously described (7). All cells were used without passaging to ensure proper phenotype was retained and experiments were performed with confluent cultures after an overnight incubation in serum-free media unless otherwise stated. Cartilage explants were made by using a 4 mm biopsy punch to remove a small tissue piece from cartilage. Care was taken to remove only articular cartilage and not to punch into subchondral bone. Explants were cultured in individual wells of a 96 well plate containing media with 10% serum for two days as a recovery period and then switched to mini-ITS media for stimulation and experimentation.
Chondrocyte transfection
Chondrocytes were transfected by the nucleofection method, using a human chondrocyte nucleofection kit (Lonza) as described previously (9). Briefly, 2 million isolated cells were resuspended in transfection reagent and nucleofected with 1000 nm of siRNA for Rac1. After the recovery period of 48 h, cells were made serum-free overnight before stimulation. For viral infection, viruses were complexed with calcium chloride and then added to chondrocytes at a ratio of 4000 virus particles per cell. Infection was continued for 2 hours before virus was removed and replaced with fresh media. Stimulations were performed 48 hours after infection with the final 24 hours in serum-free conditions.
Immunoblotting
Conditioned media and cell lysates were collected and used for immunoblotting as previously described (7). Primary antibodies were diluted to 1:1000 and immunoreactive bands were detected using chemiluminescence. All immunoblotting experiments were repeated at least 3 times with cells from different donors.
Rac activity assay
Rac activity was measured by both colorometric ELISA and traditional pulldown assay. For the ELISA assay, chondrocytes were plated at a density of 1 × 106 cells per well in 12 well culture plates. Cells were allowed to recover 5–7 days before being switched to serum-free media overnight and then used in stimulation experiments. Cells were lysed and 50 µg protein was used to run an ELISA based Rac activity assay according to manufacturer’s instructions (Cytoskeleton, Inc., Denver, CO). For traditional pulldown assay, chondrocytes were plated at a density of 3.3 × 106 cells in 60 mm dishes. Cells were lysed and 500 µg protein was used in a pulldown assay with PAK-PBD beads. The resulting pulldown was then immunoblotted with Rac antibody according to the manufacturer’s instructions (Cytoskeleton, Inc., Denver, CO).
Quantitative real-time PCR
Two micrograms of total RNA was reverse transcribed for 1 h at 37 °C using an avian myeloblastosis virus reverse transcriptase and oligo(dT) primer. Then 2 µl of reverse transcriptase was combined in a reaction mixture with 1 µl of the specific primer pair, 12.5 µl of 2 µl of SYBR Green PCR Master Mix, and water to a final reaction volume of 25 µl. All specific primers were predesigned and validated commercial primers. Samples were run in triplicate, with 40 cycles of amplification, on an ABI Prism 7000 real time PCR instrument (Applied Biosystems, Foster City, CA). An amplification plot was generated using ABI software. The expression level of MMP-13 was normalized to the expression of TATA box-binding protein measured in parallel samples.
Immunohistochemistry
Cartilage slices from normal donors and osteoarthritis patients were fixed with 10% formalin, embedded in paraffin, and mounted onto charged slides. Tissue was deparaffinized and antigen retrieval was performed by proteinase K digestion for 15 minutes at 37°C. Tissue was blocked with 10% normal goat serum for 30 minutes. Tissue was then incubated overnight at 4°C with a 1:100 dilution of active Rac antibody. Nonspecific Mouse IgG was used as a negative control. Specificity of the active Rac antibody was also tested by pre-incubating cartilage explants prior to immunostaining with a Rac inhibitor (EHT1864) which displaces GTP from the Rac binding site. Following washing, tissue was incubated with 1:300 dilution of AlexaFluor 488 secondary antibody. Images were captured with a Zeiss Axioplan fluorescent microscope.
Statistical methods
Results were analyzed using SigmaStat version 3.5 (Systat Software Inc., Richmond, CA).. EGF and TGFα induced Rac activity at 3 minutes in articular chondrocytes were examined using a paired t-test. All other statistical comparisons utilized a two-way ANOVA with a Tukey’s post-hoc test to account for both treatment effects and the variance attributed to cells harvested from different donors. The threshold for significance for all tests was set at α ≤ 0.05.
RESULTS
Requirement for Rac in MMP-13 production after stimulation of chondrocytes with Fnf
In order to determine if activity of the small GTPases Rac or Rho are required for Fnf-mediated MMP-13 production, human articular chondrocytes were pre-treated in monolayer culture with the Rac1 inhibitor NSC23766 or the Rho-associated protein kinase (ROCK) inhibitor Y-27632 before stimulation with a recombinant Fnf. MMP-13, measured in the conditioned media, was significantly decreased by Rac inhibition while ROCK inhibition resulted in a modest increase in Fnf stimulated MMP-13 (Fig 1A). Rac inhibition also blocked Fnf mediated MMP-13 expression, whereas ROCK inhibition had no effect (Fig 1B), suggesting that Rac regulates MMP-13 at the level of transcription.
Figure 1.
Effects of Rac inhibition on fibronectin fragment (Fnf) stimulated MMP-13 production. (A), Pretreatment of chondrocytes with 100 µM Rac1 inhibitor (Rac1i) NSC23766 but not 10 µM ROCK inhibitor (Rocki) Y-27632, prior to 1 µM Fnf stimulation, blocked MMP-13 production as demonstrated by immunoblotting of conditioned media. MMP-2 does not change with Fnf and is shown as a loading control. (B), The Rac1 inhibitor but not the Rock inhibitor also blocked Fnf induced MMP-13 expression measured by real-time PCR analysis. (C), Knockdown of Rac1 using small interfering RNA (siRNA) blocked Fnf stimulated MMP-13 production measured by MMP-13 ELISA of conditioned media. Cell lysates were immunoblotted for Rac and actin as a control to demonstrate Rac knockdown. Results are representative of five experiments. (D), Adenoviral overexpression of constitutively active (CA) Rac induced MMP-13 production. The control cells were incubated with a null control adenovirus. Results are representative of three experiments. (E), Cartilage explants treated with Rac1 inhibitor showed reduced MMP-13 production both in the presence or absence of Fnf stimulation measured by MMP-13 ELISA of explant media. Values were corrected for wet weight of tissue explants and were performed in duplicate for each treatment condition. Results are representative of four experiments. Statistical analyses were performed using a two-way ANOVA (main effects were individual and dose) and a Tukey’s Post Hoc Test to identify between group differences.
The results using chemical inhibition of Rac were confirmed using siRNA to knock-down Rac expression and with an ELISA to quantify MMP-13 production. Transfection of chondrocytes with Rac1 siRNA significantly reduced Rac1 levels and resulted in inhibition of Fnf stimulated MMP-13 production (Fig 1C). To determine if Rac1 activity was sufficient to induce MMP-13 production, chondrocytes were infected with an adenovirus expressing constitutively active Rac1 which resulted in increased MMP-13 production (Fig 1D).
Since Rac is involved in cytoskeletal dynamics which could be altered in monolayer culture, the effects of Rac inhibition were also tested in cartilage explants. Treatment of explants with the Rac1 inhibitor decreased basal MMP-13 production and completely inhibited Fnf induced MMP-13 (Fig 1E), similar to the observations in monolayer cultures.
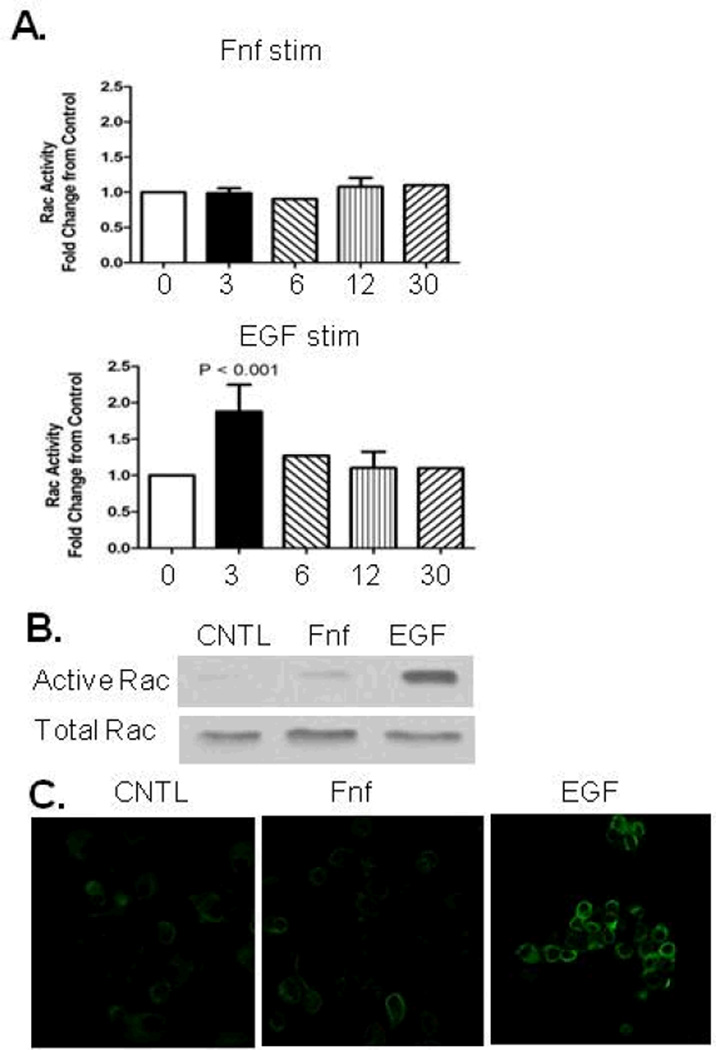
Stimulation of chondrocytes with Fnf does not directly induce Rac activation
A previous study using synovial fibroblasts indicated that Rac is activated in response to α5β1 integrin stimulation mediated by antibodies to the α5 integrin (14). However, when Rac activity was measured by ELISA after chondrocytes were stimulated with Fnf, no change in activity was seen out to 30 minutes. EGF, a known inducer of Rac activity, increased activity within 3 minutes (Fig. 2A). A traditional pull-down assay showed similar results, with EGF but not Fnf stimulating Rac activity at 3 minutes (Fig. 2B). Additionally, Rac activity was measured by immunostaining cells with an antibody that recognizes GTP-bound Rac which is the active form. Cells treated with EGF demonstrated greater staining than control, whereas cells treated with Fnf displayed no visible increase in staining (Fig. 2C).
Figure 2.
Effects of fibronectin fragment (Fnf) and EGF on Rac activity. (A), 1 µM Fnf stimulation of chondrocytes did not induce Rac activation at time points out to 30 minutes as determined by an activity based ELISA. 10ng/mL EGF stimulation significantly increased Rac activation at 3 minutes. Results from 3 and 12 minutes represent three experiments. (B), Stimulation of chondrocytes for 3 minutes with EGF increased Rac activity as determined by active Rac pulldown assay. Fnf had no effect. (C), Stimulation of chondrocytes for 3 minutes with EGF but not Fnf increased immunostaining for active Rac in cultured chondrocytes.
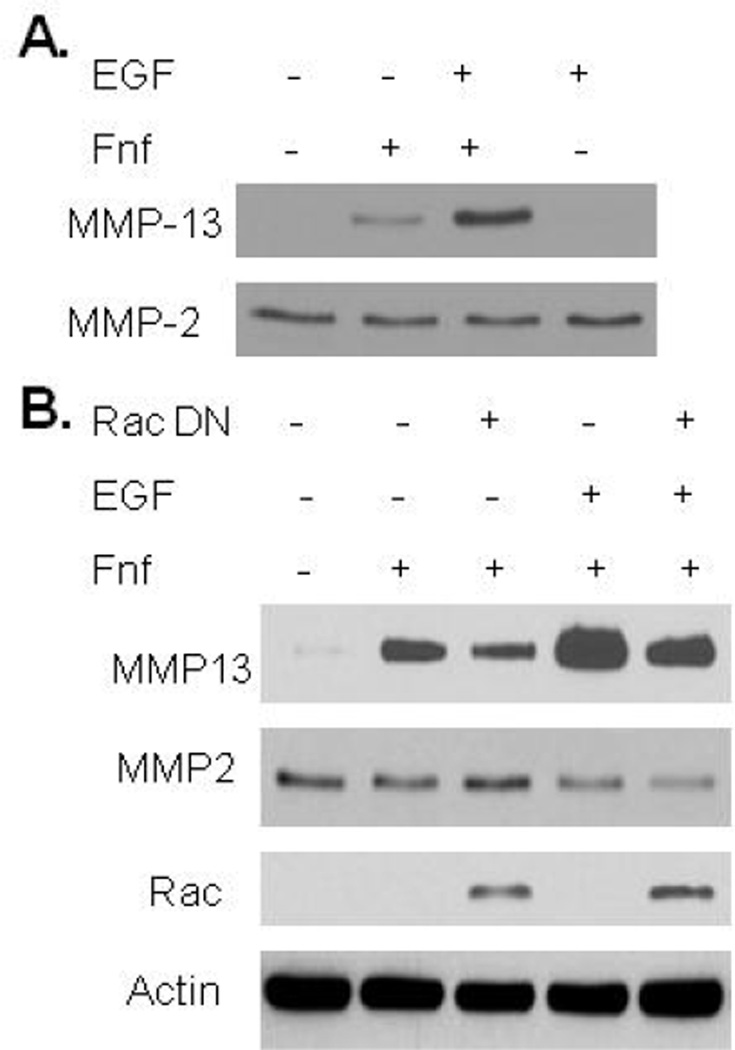
EGF treatment enhances Fnf production of MMP-13 through Rac activation
Unlike Fnf, EGF by itself did not stimulate MMP-13 production but it was noted to promote an increase in Fnf stimulated MMP-13 above that seen with Fnf alone (Fig 3A). A requirement for Rac in the combined stimulation of MMP-13 production by EGF and Fnf was demonstrated using an adenovirus expressing a construct that expressed dominant negative Rac (Fig 3B). Similar results were seen using the chemical Rac inhibitor (data not shown).
Figure 3.
Effects of EGF and fibronectin fragment (Fnf) on MMP-13 production. (A), Fnf increased MMP-13 production as determined by immunoblotting of conditioned media. MMP-13 production was enhanced by co-stimulation with 10ng/mL EGF. EGF alone did not cause any increase in MMP-13. MMP-2 was used as a loading control. (B), Adenoviral overexpression of dominant negative (DN) Rac1 decreased Fnf stimulated MMP-13 production and production in response to EGF + Fnf as determined by immunoblotting of conditioned media. Control wells received null control adenovirus. Cell lysates were immunoblotted for Rac and for actin as a loading control to demonstrate overexpression of Rac DN.
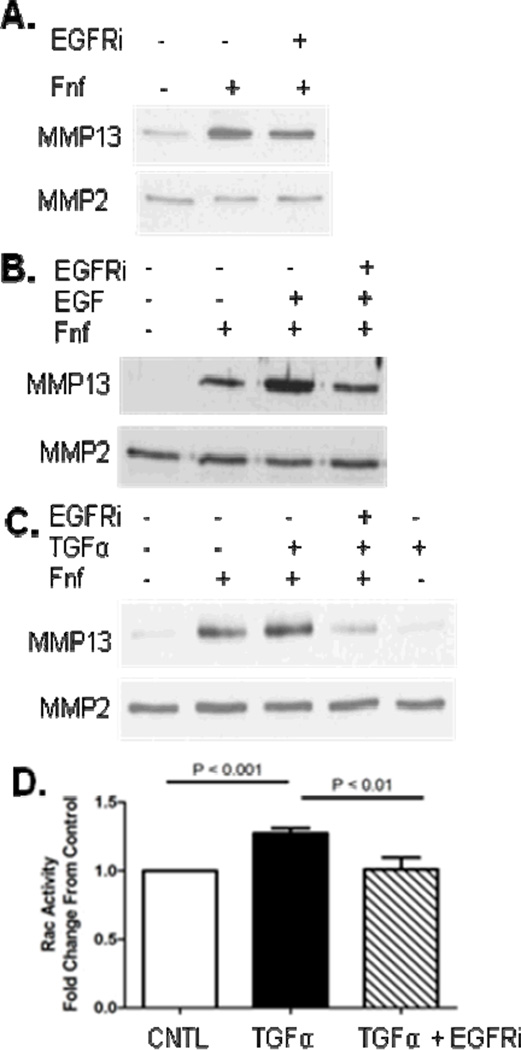
After demonstrating that Rac activation downstream of EGF can enhance Fnf mediated MMP-13 production, we next wanted to examine the role of the EGF receptor in this process. Pretreatment of chondrocytes with the EGF receptor inhibitor AG1478 minimally inhibited MMP-13 production stimulated by Fnf indicating that EGFR is not required for Fnf stimulation of MMP-13 (Fig 4A). However, pretreatment of chondrocytes with the EGF receptor inhibitor prior to combined stimulation with EGF and Fnf reduced MMP-13 production back down to the level noted with Fnf alone (Fig 4B). Co-stimulation of chondrocytes with Fnf and TGFα, an EGF receptor ligand that has previously been shown to be increased in OA (17), also showed an increase in MMP-13, while treatment with TGFα alone did not affect MMP-13 levels (Fig 4C). Similar to EGF, the ability of TGFα to promote Fnf stimulated MMP-13 was inhibited by the EGF receptor inhibitor. Stimulation of chondrocytes with TGFα significantly increased Rac activity, an effect which was blocked when cells were pretreated with EGF receptor inhibitor (Fig 4D).
Figure 4.
Effects of EGF receptor inhibition on fibronectin fragment (Fnf), EGF, and TGFα stimulated MMP-13 production. (A), Fnf increased MMP-13 production measured by immunoblotting of conditioned media and this was modestly inhibited by pretreatment of cells with 250 nM EGF receptor inhibitor (EGFRi) AG1478. MMP2 was used as a loading control. (B), Co-stimulation of chondrocytes with Fnf and 10ng/mL EGF increased MMP-13 production above that of Fnf alone. The co-stimulation of MMP13 was inhibited by pretreatment of cells with EGFRi. (C), Co-stimulation of chondrocytes with Fnf, 20 ng/ml TGFα, or both with and without EGFRi. (D), Stimulation of chondrocytes for 3 minutes with TGFα increased Rac activity as determined by an activity assay. This stimulation was blocked by pretreatment of cells with EGFRi. Results of immunoblots are representative of four independent experiments and the Rac activity assay results are the mean and sd of three independent experiments.
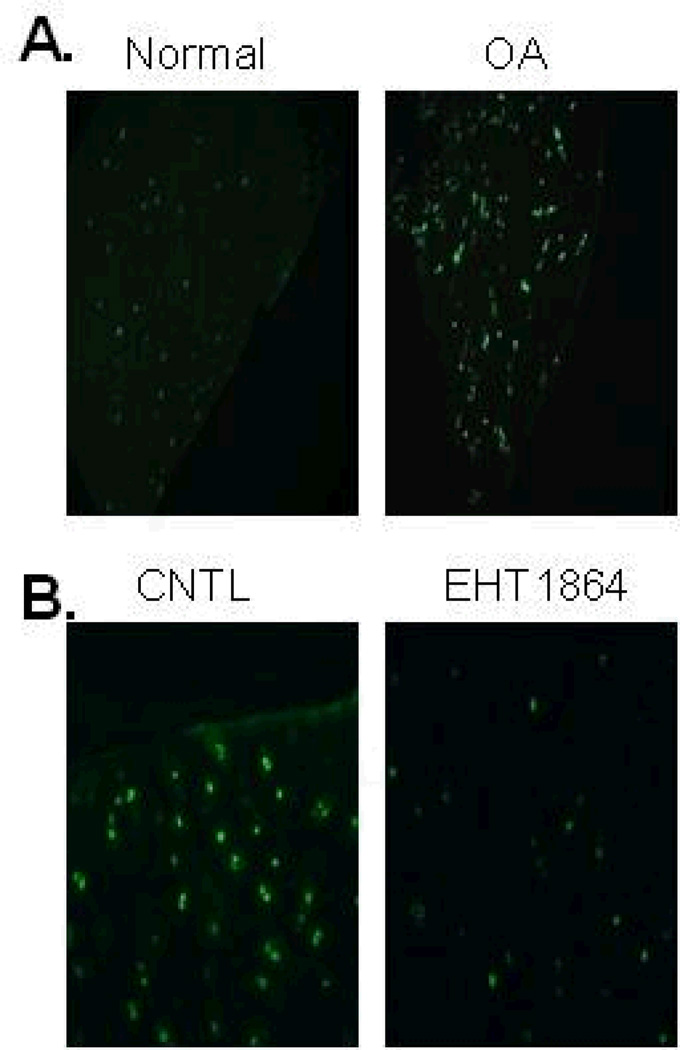
Rac activity is increased in OA cartilage
Since MMP-13 production is increased in OA and we found that Rac activity promoted MMP-13 production, we decided to examine Rac activity in OA cartilage. Immunohistochemical staining using an antibody for active Rac that detects GTP-bound Rac noted more activity in OA cartilage when compared to normal (Fig. 5A). Since the NSC23766 compound used to inhibit Rac in the cell culture experiments would not be expected to affect Rac that was already GTP bound, specificity of the antibody was demonstrated by treating cartilage samples with EHT1864, which has been shown previously to disrupt binding of GTP to Rac (18). OA cartilage treated with EHT1864 showed much lower immunostaining for active Rac than untreated control (Fig. 5B).
Figure 5.
Rac activity is increased in OA cartilage. (A), Immunofluorescence staining with an antibody to active Rac was performed on cartilage samples from normal and OA cartilage. Rac1 activity was higher in OA tissue samples relative to normal tissue samples. Results are representative of staining from four different donors for each condition. (B), OA cartilage explants were treated with Rac inhibitor EHT1864 (25 µM) overnight prior to immunostaining with active Rac1 antibody. Rac1 activity was reduced with EHT1864 treatment.
DISCUSSION
In this study, we demonstrate an important role for the small GTPase Rac1 in cell signaling that results in chondrocyte production of MMP-13. Signals stimulated by Fnf required Rac1 activity even though Fnf did not appear to directly activate Rac. This suggests a basal level of Rac1 is present in normal chondrocytes and this basal activity appeared to be increased in cells from OA cartilage. Furthermore, two EGF receptor ligands, EGF and TGFα, which did activate Rac but did not stimulate MMP-13 production by themselves, promoted higher levels of MMP-13 production when added with Fnf. These results are consistent with the notion of an active catabolic signaling network in OA cartilage resulting from the presence of matrix fragments and the autocrine and paracrine effects of factors such as EGF receptor ligands, as well as a host of other pro-inflammatory mediators, all acting in concert to promote cartilage matrix destruction.
A previous report indicated that expression of CA-Rac is sufficient to induce MMP-13 production in cultured chick sternal chondrocytes (16), a finding confirmed here in adult human articular chondrocytes. It appeared that culturing chondrocytes in monolayer may have promoted basal Rac activity but, unlike CA-Rac, this level of Rac activity was not sufficient to stimulate MMP-13 production unless cells were also treated with Fnf which generates additional signals, such as MAP kinase activation, that promote MMP production. We also demonstrated a requirement for Rac in Fnf mediated MMP-13 production in explants indicating that chondrocytes in their native matrix also have sufficient Rac activity to mediate MMP production when further stimulated. Importantly, we extended this work to demonstrate the presence of active Rac in OA cartilage at levels higher than normal cartilage, using immunohistochemistry and a unique antibody to GTP-bound Rac.
A recent study has identified the EGF receptor as a mediator of MMP-13 production in hypertrophic chondrocytes (19) but the effects of EGFR ligands in adult cartilage have not been well studied. We did not detect an increase in MMP-13 production when adult human articular chondrocytes were treated with EGF alone, which indicates the cell signaling activated by EGF is not sufficient to induce MMP-13 in adult articular chondrocytes. Instead, we found that EGF promoted Fnf stimulation of MMP-13 and this was completely inhibited when Rac1 was blocked. We also tested a second EGFR ligand, TGF-α. TGF-α was previously found to be upregulated in a rat model of osteoarthritis (17) and to stimulate rat chondrocyte MMP-13 expression (20). Like EGF, we found that TGF-α alone did not stimulate human chondrocytes to produce MMP-13 but it did promote the effect of Fnf and this was Rac dependent.
The regulation of MMP-13 production by Rac1 may be related, at least in part, to increased production of ROS which we have shown are required for Fnf stimulation of MMP-13 expression (21). Rac1 is part of the NADPH oxidase complex and we have found that blocking NADPH oxidase reduces MMP-13 production (unpublished results). Activation of the α5β1 integrin on synovial fibroblasts was shown to activate Rac1 and increase production of MMP-1 through production of ROS (14). However, in fibroblasts a mitochondrial source of ROS, rather than from NADPH oxidase, was suggested (22). We have not ruled out a potential mitochondrial source of ROS in the activation of MMP-13 expression stimulated by Fnf and future studies will explore this possibility.
It is also important to note that, although we are studying the effects of a Fnf, FN7-10 (23), that contains the RGD cell binding region known to bind the α5β1 integrin, we have not directly proven that the effects of Fnf stimulation are due solely to α5β1-mediated signaling. We have found this very difficult to study because antibodies that effectively block the α5β1 integrin will activate cell signaling in a similar fashion as Fnf (7) and we were not able to effectively knockdown the α5 subunit with siRNA (unpublished observation) possibly because α5β1 promotes chondrocyte survival (24) and the cells with the most efficient knock-down died. However, previous studies by other investigators using either synthetic RGD peptides or Fnf containing the RGD region have supported our findings of stimulation of chondrocyte MMP production and found activation of similar signaling pathways (25–27). Also in support of our results, the same FN7-10 fragment used in our studies did not stimulate Rac activation in tumor cells (28). In OA cartilage, a number of different Fnf have been identified and it is likely that these various fragments will act in concert through more than one type of cell surface receptor (5, 6).
The previous studies connecting Rac1 to the promotion of chondrocyte hypertrophy (11, 16) and our finding that Rac1 mediates chondrocyte MMP-13 expression are consistent with the hypothesized association between the hypertrophic phenotype found in the growth plate and the OA chondrocyte phenotype in adult cartilage. It has also been shown that ROS promote chondrocyte hypertrophy in the growth plate and inhibition of ROS blocks production of MMP-13 (29). Given the level of matrix degrading enzymes present in the hypertrophic zone, production of matrix fragments, including Fnf, would be expected but their role in promoting hypertrophy has not been studied. Further studies will be also be needed to determine if the ability of EGF and TGFα to promote Fnf induced MMP-13 production is also due to increased production of ROS or a different effect of active Rac. These studies should elucidate new targets for modulating the OA chondrocyte phenotype and slowing the progression of OA.
ACKNOWLEDGEMENTS
The authors thank Mary Zhao for technical assistance and would like to acknowledge the Gift of Hope Organ & Tissue Donor Network (Elmhurst, IL), the National Disease Research Interchange (Philadelphia, PA), and the Department of Orthopedic Surgery along with the surgical pathology lab at Wake Forest Baptist Hospital for providing tissue. We also thank Drs. Susan Chubinskaya and Arkady Margulis as well as Arnavaz Hakimiyan at Rush University (Chicago, IL) for assistance with obtaining normal donor tissue from the Gift of Hope.
Supported by the National Institute on Arthritis, Musculoskeletal and Skin Diseases (R37 AR49003-11).
REFERENCES
- 1.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2011;1824:133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Homandberg GA, Wen C, Hui F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage. 1998;6:231–244. doi: 10.1053/joca.1998.0116. [DOI] [PubMed] [Google Scholar]
- 6.Zack MD, Arner EC, Anglin CP, Alston JT, Malfait AM, Tortorella MD. Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthritis Rheum. 2006;54:2912–2922. doi: 10.1002/art.22045. [DOI] [PubMed] [Google Scholar]
- 7.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 8.Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem. 2003;278:24577–24585. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, et al. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beier F, Loeser RF. Biology and pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling pathways in chondrocytes. J Cell Biochem. 2010;110:573–580. doi: 10.1002/jcb.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Beier F. Rac1/Cdc42 and RhoA GTPases antagonistically regulate chondrocyte proliferation, hypertrophy, and apoptosis. J Bone Miner Res. 2005;20:1022–1031. doi: 10.1359/JBMR.050113. [DOI] [PubMed] [Google Scholar]
- 12.Novakofski K, Boehm A, Fortier L. The small GTPase Rho mediates articular chondrocyte phenotype and morphology in response to interleukin-1 alpha and insulin-like growth factor-I. J Orthop Res. 2009;27:58–64. doi: 10.1002/jor.20717. [DOI] [PubMed] [Google Scholar]
- 13.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: a "Rac" of all trades. Cell Mol Life Sci. 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Woods A, Agoston H, Ulici V, Glogauer M, Beier F. Genetic ablation of Rac1 in cartilage results in chondrodysplasia. Dev Biol. 2007;306:612–623. doi: 10.1016/j.ydbio.2007.03.520. [DOI] [PubMed] [Google Scholar]
- 16.Kerr BA, Otani T, Koyama E, Freeman TA, Enomoto-Iwamoto M. Small GTPase protein Rac-1 is activated with maturation and regulates cell morphology and function in chondrocytes. Exp Cell Res. 2008;314:1301–1312. doi: 10.1016/j.yexcr.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appleton CT, Pitelka V, Henry J, Beier F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 2007;56:1854–1868. doi: 10.1002/art.22711. [DOI] [PubMed] [Google Scholar]
- 18.Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J Biol Chem. 2007;282:35666–35678. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Siclari VA, Lan S, Zhu J, Koyama E, Dupuis HL, et al. The critical role of the epidermal growth factor receptor in endochondral ossification. J Bone Miner Res. 2011;26:2622–2633. doi: 10.1002/jbmr.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appleton CT, Usmani SE, Mort JS, Beier F. Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab Invest. 2010;90:20–30. doi: 10.1038/labinvest.2009.111. [DOI] [PubMed] [Google Scholar]
- 21.Del Carlo M, Schwartz D, Erickson EA, Loeser RF. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic Biol Med. 2007;42:1350–1358. doi: 10.1016/j.freeradbiomed.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werner E, Werb Z. Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. J Cell Biol. 2002;158:357–368. doi: 10.1083/jcb.200111028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leahy DJ, Aukhil I, Erickson HP. 2.0 A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 24.Pulai JI, Del Carlo M, Jr, Loeser RF. The alpha5beta1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum. 2002;46:1528–1535. doi: 10.1002/art.10334. [DOI] [PubMed] [Google Scholar]
- 25.Arner EC, Tortorella MD. Signal transduction through chondrocyte integrin receptors induces matrix metalloproteinase synthesis and synergizes with interleukin-1. Arthritis Rheum. 1995;38:1304–1314. doi: 10.1002/art.1780380919. [DOI] [PubMed] [Google Scholar]
- 26.Ding L, Guo D, Homandberg GA. The cartilage chondrolytic mechanism of fibronectin fragments involves MAP kinases: comparison of three fragments and native fibronectin. Osteoarthritis Cartilage. 2008;16:1253–1262. doi: 10.1016/j.joca.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Ding L, Guo D, Homandberg GA. Fibronectin fragments mediate matrix metalloproteinase upregulation and cartilage damage through proline rich tyrosine kinase 2, c-src, NF-kappaB and protein kinase Cdelta. Osteoarthritis Cartilage. 2009;17:1385–1392. doi: 10.1016/j.joca.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Wei Y, Tang CH, Kim Y, Robillard L, Zhang F, Kugler MC, et al. Urokinase receptors are required for alpha 5 beta 1 integrin-mediated signaling in tumor cells. J Biol Chem. 2007;282:3929–3939. doi: 10.1074/jbc.M607989200. [DOI] [PubMed] [Google Scholar]
- 29.Morita K, Miyamoto T, Fujita N, Kubota Y, Ito K, Takubo K, et al. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med. 2007;204:1613–1623. doi: 10.1084/jem.20062525. [DOI] [PMC free article] [PubMed] [Google Scholar]