Abstract
Background
Antidepressants that act on two or more amine neurotransmitters may confer higher remission rates when first-line agents affecting a single neurotransmitter have failed. Pramipexole, a dopamine agonist, has antidepressant effects in patients with major depressive disorder (MDD). This pilot study examined the efficacy and safety of combination therapy with pramipexole and the selective serotonin reuptake inhibitor (SSRI) escitalopram in MDD.
Methods
In this double-blind, controlled, pilot study, 39 patients with DSM-IV MDD who had failed to respond to a standard antidepressant treatment trial were randomized to receive pramipexole (n=13), escitalopram (n=13), or their combination (n=13) for six weeks. Pramipexole was started at 0.375 mg/day and titrated weekly up to 2.25 mg/day; escitalopram dosage remained at 10 mg/day. The primary outcome measure was the Montgomery–Asberg Depression Rating Scale (MADRS).
Results
Subjects receiving pramipexole monotherapy had significantly lower MADRS scores than the combination group (p=0.01); no other primary drug comparisons were significant. The combination group had a substantially higher dropout rate than the escitalopram and pramipexole groups (69%, 15%, 15%, respectively). Only 15% of patients in the combination group tolerated regularly scheduled increases of pramipexole throughout the study, compared with 46% of patients in the pramipexole group.
Limitations
Group size was small and the treatment phase lasted for only six weeks.
Conclusions
The combination of an SSRI and a dopamine agonist was not more effective than either agent alone, nor did it produce a more rapid onset of antidepressant action. Combination therapy with escitalopram and pramipexole may not be well-tolerated.
Keywords: Depression, Escitalopram, Pramipexole, Combination treatment, Augmentation strategies
1. Introduction
While several treatments are available for major depressive disorder (MDD), the rate of remission associated with first-line antidepressants is as low as 30–40% (Nemeroff et al., 2008; Trivedi et al., 2006). Both newer and older antidepressants typically exert their primary biochemical effects by increasing intrasynaptic levels of monoamines, most often serotonin and/or norepinephrine (Berton and Nestler, 2006; Insel and Charney, 2003). Newer agents often have fewer side effects, but have not demonstrated improved efficacy over older, traditional antidepressants (e.g., tricyclic antidepressants) (Gartlehner et al., 2008, 2011). Some evidence suggests that newer antidepressants acting on two or more amine neurotransmitters may produce higher rates of remission when first-line agents that affect a single neurotransmitter have failed, but the remission rate remains below 40% (Baldomero et al., 2005; Lenox-Smith and Jiang, 2008; Papakostas et al., 2007; Poirier and Boyer, 1999; Rush et al., 2006).
Given the limited success associated with currently available antidepressants, it would be reasonable to investigate other classes of psychotropic drugs that affect processes outside the serotonin-norepinephrine dyad. A common strategy used to improve treatment response is the combination of a first-line treatment with one or more additional pharmacologic agents (American Psychiatric Association, 2010). Combining medications with dissimilar mechanisms of action may have a synergistic or additive effect that would be superior to that of an antidepressant that targets a single mechanism of action.
Escitalopram is an established selective serotonin reuptake inhibitor (SSRI) approved by the Food and Drug Administration (FDA) for the treatment of MDD in adults and adolescents, as well as Generalized anxiety disorder (Forest Laboratories, 2011). Studies have shown that it is highly selective for the serotonin transporter (Owens et al., 2001), and has been reported – though not established – to be one of the fastest acting SSRIs (Gorman et al., 2002; Montgomery et al., 2004). It also has a favorable side effect profile (Baldwin et al., 2006; Montgomery et al., 2004; Murdoch and Keam, 2005; Nierenberg et al., 2007).
Pramipexole, a dopamine agonist, has preference for the D3 receptor and less affinity for the D1, D2, and D4 receptors (Gerlach et al., 2003). The D3 receptor is densely distributed in the mesolimbic system and has been implicated in the motor and anhedonic symptoms of depression (Zarate et al., 2004). It is also known to have antioxidant activity, inhibits mitochondrial permeability transition, and up-regulates the expression of the neuroprotective factor B-cell lymphoma-2 (Bcl-2) (Cassarino et al., 1998, 2000; Le and Jankovic, 2001; Takata et al., 2000). Pramipexole is FDA-approved for the treatment of Parkinson’s disease and Restless Leg Syndrome (Antonini et al., 2010; Aurora et al., 2012); it has also shown antidepressant effects in patients with MDD, in patients with bipolar depression, and in Parkinson’s patients comorbid for MDD (Barone et al., 2006; Cassano et al., 2004; Corrigan et al., 2000; Goldberg et al., 2004; Inoue et al., 2010; Lattanzi et al., 2002; Moller et al., 2005; Perugi et al., 2001; Rektorova et al., 2003; Sporn et al., 2000; Zarate et al., 2004).
Randomized placebo-controlled trials demonstrated the efficacy of pramipexole in treating mood disorders. In an eight-week, double-blind, placebo-controlled study of 174 subjects with MDD without psychotic features, Corrigan et al. (2000) found that 1.0 mg/day of pramipexole was as effective as 20 mg/day of fluoxetine and superior to placebo. Another trial investigated treatment-resistant subjects with bipolar II depression (Zarate et al., 2004). After an initial six-week phase of treatment with either divalproex sodium or lithium, those who did not respond to the mood stabilizer were randomly assigned to receive augmentation with either pramipexole or placebo. The antidepressant effects of pramipexole began to differentiate from placebo at three weeks; significant symptom reduction was observed at week six. Mean Montgomery–Asberg Depression Rating Scale (MADRS) scores at the end of six weeks for pramipexole vs. placebo respectively were 17.2 ± 8.4 vs. 27.6 ± 7.7 (p=0.01), and mean 17-item Hamilton Depression Rating Scale (HDRS-17) scores at the end of six weeks for pramipexole vs. placebo were 14.1 ± 7.1 vs. 22.6 ± 8.6, respectively (p=0.02). Finally, Goldberg et al. (2004) compared flexible dosing of pramipexole to placebo as an augmentation strategy for patients with bipolar depression who were receiving mood stabilizers but who had not responded to at least two previous antidepressant trials. Eight (67%) of 12 patients taking pramipexole and two (20%) of 10 taking placebo had an improvement of at least 50% on the HDRS. The mean percentage of improvement from baseline HDRS was greater for patients taking pramipexole (48%) than for those taking placebo (21%, p=0.04).
The current pilot study examined the value of combined treatment with escitalopram and pramipexole under controlled, double-blind conditions. We hypothesized that combining an SSRI and a dopamine agonist would: (1) be more effective than treatment with either agent alone; and (2) lead to a more rapid onset of antidepressant effects in patients suffering from MDD. We selected the combination of escitalopram and pramipexole because escitalopram is an effective and well-tolerated SSRI, and because pramipexole is a dopamine agonist with antidepressant properties.
2. Methods
2.1. Subjects
This was a single-center, random assignment, double-blind, preliminary study conducted to assess the efficacy and safety of escitalopram, pramipexole, and the combination of escitalopram plus pramipexole in the treatment of MDD. Subjects with MDD without psychotic features currently experiencing a major depressive episode lasting at least four weeks were evaluated at baseline and weekly for six weeks of acute treatment. Diagnoses were established by clinical evaluation and by the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2001).
Subjects underwent a physical exam, psychiatric and medical history, routine laboratory analyses, pregnancy test, electrocardiogram (ECG), assessment of vital signs, and administration of the MADRS (Montgomery and Asberg, 1979). Subjects were excluded if they had any serious unstable medical disorders, such as uncontrolled diabetes, hypertension, or seizures. Other exclusion criteria included pregnancy or breastfeeding, history of substance abuse within the past three months, any history of psychotic disorder, bipolar disorder, or current active suicidal ideation. Subjects who had previously failed to respond to escitalopram or pramipexole, or those treated with clozapine or electroconvulsive therapy (ECT) within three months prior to the study were also excluded. Patients were not permitted to participate in psychotherapy for the duration of the study. All subjects provided written informed consent as approved by the National Institutes of Health (NIH) Combined Neuroscience Institutional Review Board.
2.2. Study phases
This study comprised two phases. During Phase 1, subjects underwent a prospective trial of a standard antidepressant for six weeks at therapeutic doses and were required to be non-responsive to this agent, defined as not achieving 50% or greater improvement in MADRS scores from baseline. Subsequently, subjects meeting non-response criteria were gradually tapered off any psychotropic medications and were medication-free for 14 days (five weeks if the patient was taking fluoxetine). In the final week of the two-week, drug-free period, subjects received a single-blind placebo lead-in (Fig. 1). If there was a 25% or greater improvement in MADRS scores compared to study entry at the end of the single-blind placebo week, or if the clinician judged a subject to be at acute risk of suicide, the subject was excluded and began standard treatment. All subjects were required to have a score of ≥20 on the MADRS at screening and at entry into Phase 2 of the study.
Fig. 1.
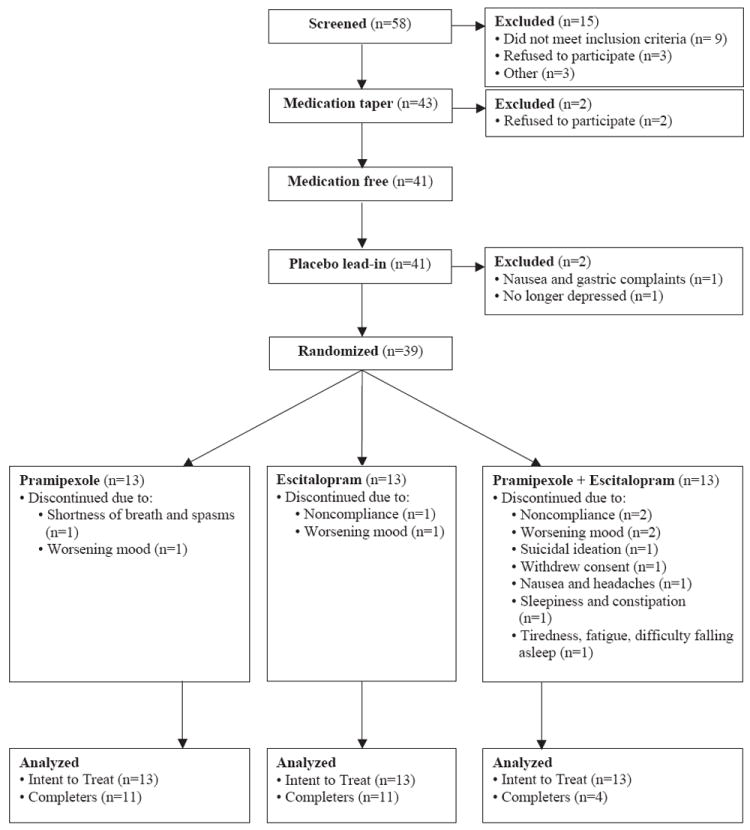
CONSORT diagram indicating patient recruitment, exclusion, and randomization.
During Phase 2, subjects who met study criteria were randomized to six-week, double-blind treatment. Subjects were randomized to one of the following three groups: pramipexole; escitalopram; or a combination of pramipexole plus escitalopram. The blind was maintained with the use of a double-dummy design. Two types of capsules were used; one type contained pramipexole or its placebo and the other contained escitalopram or its placebo. Subjects assigned to the pramipexole group took pramipexole capsules and placebo-escitalopram capsules; the escitalopram group took escitalopram capsules and placebo-pramipexole capsules; and the combination group took escitalopram capsules and pramipexole capsules. All groups received the same number of pills.
Subjects taking pramipexole or the combination of both drugs received 0.375 mg/day of pramipexole during week 1 (0.125 mg three times per day (TID)). The pramipexole dose was subsequently increased in a blinded fashion as follows: week 2=0.25 mg TID, week 3=0.5 mg TID, week 4=0.75 mg TID. The maximum pramipexole dose was 0.75 mg TID (2.25 mg per day). Dose escalations of pramipexole were continued according to the above schedule until one of the following occurred: (1) clinical remission was achieved; (2) intolerable side effects occurred; (3) the maximum daily dose of pramipexole (2.25 mg/day) was achieved at week 4 or later; or (4) the six-week study period ended. The dose regimen of pramipexole used here was drawn from our previous study (Zarate et al., 2004). Subjects were assessed weekly throughout the study.
The initial power analysis suggested 24 patients per group (72 total) would be needed to have 80% power to detect a 35% increase in remission rates for the combination treatment compared to monotherapy. The study ended prior to reaching the full study size because interim results indicated that improved response to the combination treatment could not be shown even with a complete sample.
2.3. Measures
The MADRS was our primary outcome measure. The HDRS-17 (Hamilton, 1980) was also administered. To monitor side effects, the Massachusetts General Hospital Sexual Functioning Questionnaire (MGH) and the NIMH Event Codes were used. The former comprises five items evaluating interest, arousal, orgasm, erection, and overall sexual satisfaction (Labbate and Lare, 2001), and the latter is a clinician-administered assessment comprising 87 items that evaluate possible side effects on a scale from zero (none) to three (severe). Vital signs were collected, and rating scales were administered, at baseline and during each weekly visit until the end of the study.
2.4. Statistics
A linear mixed model with restricted maximum likelihood estimation was used to examine depressive symptoms and sexual side effects over time. Fixed factors for drug, time, and a time by drug interaction were included in the model along with the intercept. No random factors were included because the subject factor did not contribute significantly to the model. Baseline symptoms were used as a covariate. Schwarz’s Bayesian criteria were used to determine the best fitting covariance structure that was a first order autoregressive model. Bonferroni adjusted simple effects tests were used post hoc to explore significant omnibus effects. Significance levels are reported after correction for post hoc analyses.
Chi-square analysis was used to examine categorical demographic variables, response rates, drop out, and general side effects by drug group. Continuous demographic factors were examined with one-way ANOVAs and Tukey post hoc tests. Demographic and side effect comparisons were not adjusted for multiple comparisons.
Results were considered significant when p ≤ 0.05, two tailed. IBM SPSS version 19.0 was used for all analyses.
3. Results
3.1. Demographics
Fifty-eight subjects were screened (see Fig. 1), and 39 were randomized to blinded medication arms. Thirteen received pramipexole, 13 received escitalopram, and 13 received the combination of pramipexole plus escitalopram. Table 1 describes the demographic and clinical characteristics of the subjects; no statistically significant differences were observed between the three groups.
Table 1.
Demographic and illness characteristics for patients receiving pramipexole, escitalopram, or pramipexole plus escitalopram.
| Sample Characteristics | Pramipexole (n=3) N (%) | Escitalopram (n=13) N (%) | Combination (n=13) N (%) | χ2, p |
|---|---|---|---|---|
| Sex (female) | 7 (54) | 9 (69) | 10 (77) | 1.62, 0.45 |
| Ethnicity | 2.82, 0.59 | |||
| • Caucasian | 5 (38) | 9 (69) | 6 (46) | |
| • Black | 3 (23) | 2 (17) | 3 (25) | |
| • Hispanic | 5 (38) | 2 (17) | 4 (33) | |
| Co-morbidity | ||||
| • Anxiety disorders | 5 (38) | 7 (58) | 7 (58) | 0.82, 0.66 |
| • Lifetime substance abuse or dependence | 4 (31) | 4 (31) | 4 (31) | 0.00, 1.00 |
| Mean (SD) | Mean (SD) | Mean (SD) | F, p | |
|
| ||||
| Age | 44.9 (10.1) | 45.6 (13.6) | 46.1 (12.5) | 0.04, 0.96 |
| Age of onset | 24.3 (11.0) | 19.6 (10.2) | 27.1 (16.3) | 1.14, 0.33 |
| Duration of current episode (months) | 21.9 (30.0) | 34.3 (65.1) | 19.8 (24.8) | 0.42, 0.66 |
| Lifetime antidepressant trials | 1.92(1.12) | 2.54(1.39) | 1.83(1.03) | 1.32, 0.28 |
| Failed antidepressant trials | 1.45(1.13) | 2.00(1.28) | 1.36(0.51) | 1.28, 0.29 |
| Baseline | ||||
| • MADRS | 33.5 (4.8) | 30.1 (5.4) | 31.4 (5.6) | 0.70, 0.50 |
| • HDRS-17 | 20.1 (5.1) | 18.0 (3.8) | 21.0 (5.0) | 1.00, 0.37 |
Abbreviations: MADRS, Montgomery Asberg Depression Rating Scale; HDRS-17, 17-Item Hamilton Depression Rating Scale.
3.2. Efficacy
Mean ± SE MADRS scores for the three treatment groups over the six weeks of the study are presented in Fig. 2. The linear mixed model with MADRS as the outcome measure found significant drug and time main effects but no significant interaction (drug: F=4.45, df=2,42, p=0.02; time: F=2.39, df=5,133, p=0.04; drug × Time: F=0.68, df=10,133, p=0.74; Fig. 2). Baseline MADRS score was a significant covariate (F=7.88, df=1,41, p=0.008). Post hoc tests indicated significantly lower MADRS scores with pramipexole compared to the combination treatment (p=0.01), but no other comparisons were significant (pramipexole vs. escitalopram: p=0.35; escitalopram vs. combination; p=0.45).
Fig. 2.
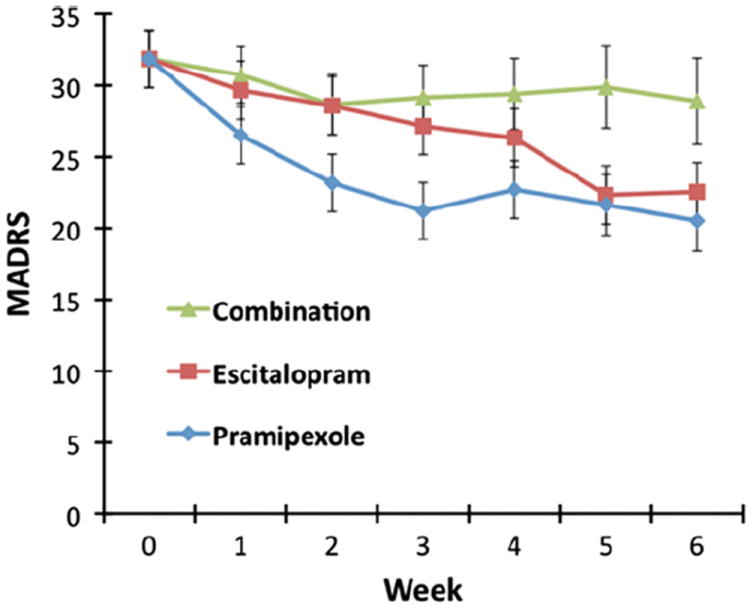
Mean depression score on the Montgomery Asberg Depression Rating Scale (MADRS) over six weeks. Linear mixed models using MADRS score as the outcome produced significant drug and time main effects (F=4.45, df=2,42, p=0.02 and F=2.39, df=5,122, p=0.04, respectively).
Response was defined as 50% or better improvement at the six-week endpoint relative to baseline. Response rates were 31% (4/13) for subjects receiving pramipexole, 15% (2/13) for subjects receiving escitalopram, and 8% (1/13) for those receiving the combination treatment; no significant difference was noted in response rates (χ2=2.44, df=2, p=0.30). Remission was defined as 75% improvement from baseline in MADRS scores as well as a final MADRS score of 9 or below. Remission rates for each group were 8% (1/13) for pramipexole, 15% (2/13) for escitalopram, and 0% (0/13) for the combination. No significant difference in remission rates was seen between the treatment groups (χ2=2.17, df=2, p=0.34).
3.3. Drop out
The dropout rate for the combination treatment group (9/13, 69%) was significantly higher than for the escitalopram (2/13, 15%) or pramipexole groups (2/13, 15%) (χ2=11.308, df=2, p=0.004). On post hoc testing, the dropout rate remained significantly higher for the combination group than for either of the other two groups (vs. pramipexole: p=0.02; vs. escitalopram: p=0.004). In the pramipexole group, one patient was discontinued from the study due to worsening depression and another for shortness of breath and muscle spasms. In the escitalopram group one patient had worsening of depressive symptoms and one patient was non-compliant with medication. However, in the combination group, nine patients dropped out. One patient withdrew consent, one developed suicidal ideation, two experienced worsening mood, two were non-compliant with medication, and three had severe side effects (one had nausea and headaches, one had sleepiness and constipation, and one had tiredness, fatigue, and difficulty falling asleep).
3.4. Sexual functioning
Linear mixed models were used to examine each of the five items of the MGH (Fig. 3). Main effects of drug were significant for lack of sexual interest (F=3.72, df=2,29, p=0.04), lack of sexual arousal (F=4.93, df=2,32, p=0.01), inability to orgasm (F=4.52, df=2,29, p=0.02), difficulty maintaining an erection (F=5.82, df=2,9, p=0.02), and sexual dissatisfaction (F=9.03, df=2,33, p=0.001). No significant interactions with time were observed (lack of sexual interest: F=0.91, df=4,45, p=0.47; lack of sexual arousal: F=0.88, df=4,49, p=0.48; inability to orgasm: F=1.57, df=4,47, p=0.20; difficulty maintaining an erection: F=0.82, df=4,14, p=0.53; sexual dissatisfaction: F=0.79, df=4,49, p=0.54). Post hoc tests indicated significantly more symptoms in the combination group compared to the pramipexole group for all of the scales (p’s < 0.03) except lack of sexual interest, where there were only marginal group differences (p=0.053). The combination group had significantly more sexual dissatisfaction than the escitalopram group (p=0.01), but there were no significant differences with other symptoms (p’s > 0.59).
Fig. 3.
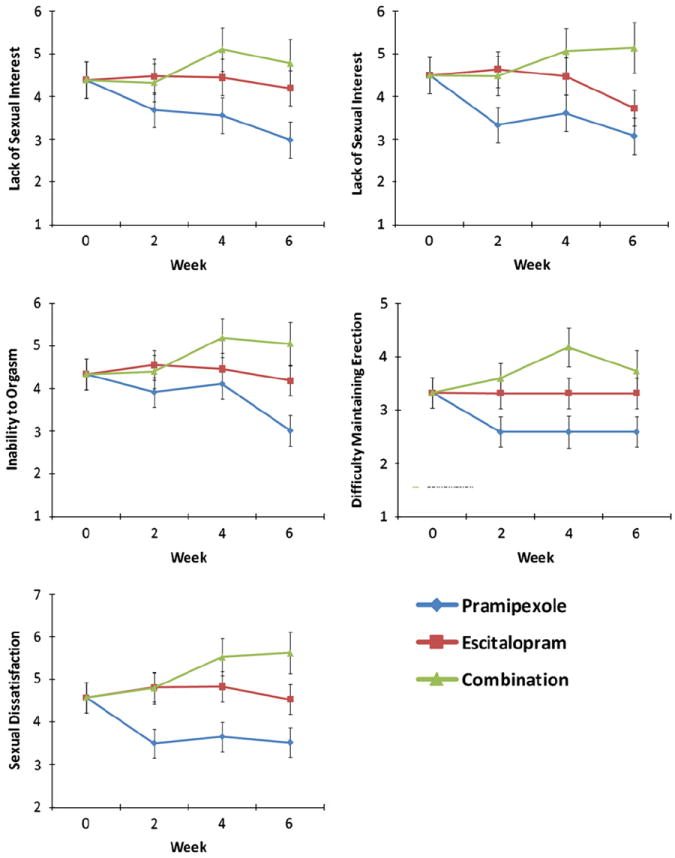
Mean score for each of the five items of the Massachusetts General Hospital Sexual Functioning Questionnaire across six visits. Linear mixed models produced significant main effects of drug for all items: sexual interest (F=3.72, df=2,29, p=0.04), lack of sexual arousal (F=4.93, df=2,32, p=0.01), inability to orgasm (F=4.52, df=2,29, p=0.02), difficulty maintaining an erection (F=5.82, df=2,9, p=0.02), and sexual dissatisfaction (F=9.03, df=2,33, p=0.001).
3.5. Side effects
Patients experiencing moderate or severe levels of a given side effect one or more weeks after randomization were coded as having experienced that side effect; a greater proportion of patients receiving escitalopram alone experienced sedation (55%) compared to patients receiving the combination treatment (0%) (p=0.009). No other side effects evaluated were significantly different. No major changes in laboratory results or ECG were observed during the study.
For subjects in the pramipexole and combination groups, the pramipexole dose was raised in weekly increments starting at 0.375 mg/day and leading to 2.25 mg/day. Only 15% of the patients in the combination group were able to adhere to the regularly scheduled pramipexole increases throughout the study, compared to 46% of patients in the pramipexole monotherapy group. The mean dose (±SD) of pramipexole in the pramipexole monotherapy group vs. the combination group was 1.55 mg±0.56 vs. 1.16 mg±0.74, respectively; this difference was statistically marginal. Thus, the pramipexole monotherapy group was able to tolerate an almost 33% higher mean dose than the combination group.
4. Discussion
Contrary to our hypothesis, combining an SSRI and a dopamine agonist was not more effective than either agent alone, nor did it produce a more rapid onset of antidepressant action. Although six weeks of treatment with escitalopram had an antidepressant effect similar to that of pramipexole, this effect was not significantly different from the combination group. This lack of robust antidepressant effect from escitalopram, a proven antidepressant, could be attributed to the treatment resistance of the cohort or other factors.
This study also found that the dopamine agonist pramipexole had antidepressant effects; however, the fact that combining it with a serotonergic agent did not result in greater antidepressant effects raises questions as to whether combining serotonergic agents with dopamine agonists is a dual mechanism worth pursuing, largely because of intolerability issues. One possibility for the lack of significant findings could have been that the combination therapy group had a greater number of intolerable side effects than either monotherapy group, leading to greater drop-out rates. Indeed, it is important to note that most patients in the combination group dropped out complaining of severe side effects. In contrast, no patients in the escitalopram monotherapy group dropped out (Fig. 1) despite the comparatively higher rate of sedation in that group. However, we cannot determine how the combination group side effect profile would have evolved if they had remained in the study.
A non-tolerability factor associated with the combination of escitalopram and pramipexole remains a likely possibility, as shown by the number of patients in the combination group who were not able to tolerate increases in the total daily dose of pramipexole when compared to subjects receiving pramipexole alone. Several open and controlled studies have used pramipexole as an augmentation strategy to treat patients, but in most studies pramipexole was added to an antidepressant or mood stabilizer after that agent was already being administered at therapeutic doses (Goldberg et al., 2004; Inoue et al., 2010; Lattanzi et al., 2002; Sporn et al., 2000; Zarate et al., 2004). In our study both escitalopram and pramipexole treatment were started at the same time; it is possible that the simultaneous activation of both the serotonergic and dopaminergic systems contributed to the high dropout rates from the combination group. Considering the evidence of low initiation of selected treatments and low adherence to them (Serna et al., 2010), it is important to understand the implications of these findings in order to avoid iatrogenic treatment discontinuation.
The symptom improvement observed in the pramipexole group is consistent with the notion that the dopaminergic system plays a role in treating MDD (Guiard et al., 2009; Yadid and Friedman, 2008). Nevertheless, the fact that combination therapy did not improve depressive symptoms and showed poor tolerability calls into question the interaction between the serotonergic and dopaminergic systems in the brain. It may be the case that agonism of dopamine receptors might be an effective augmentation strategy but only after the serotonergic system has been stabilized with a serotonergic agent.
Another interesting finding was that pramipexole monotherapy was associated with significantly fewer sexual side effects than escitalopram monotherapy; no such protective effect against sexual side effects was seen in the combination group. Indeed, the favorable sexual side effect profile was only seen in patients receiving pramipexole monotherapy, not when this medication was used in combination with escitalopram. In addition, the high dropout rate in the combination group did not seem to be due to the increased incidence of sexual adverse events.
The study was associated with several limitations and, as a result, the results must be interpreted with caution. Most notably, group size was small and the treatment phase lasted for only six weeks. More time may be required to observe greater response in the monotherapy groups.
In conclusion, this preliminary study investigated combined treatment with an SSRI and a dopamine agonist in patients with MDD and found that this combination was not well tolerated and did not lead to increased efficacy. Our findings emphasize the need to better understand the role of the simultaneous activation of the serotonergic and dopaminergic systems in order to develop more effective combination strategies for treating MDD. Larger and longer controlled studies are clearly warranted to better assess the treatment role of dopamine agonists in MDD.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH), Grant number(s): ZIAMH002856, and ZIA MH002927. Ioline Henter, MA (NIMH) provided invaluable editorial assistance.
Role of funding source
The NIMH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. Government but will share a percentage of any royalties that may be received by the Government. This work was initiated at the NIMH prior to Dr. Mallinger’s official duties with the Office of the Inspector General. The views expressed in this paper do not necessarily represent the views of the Office of Inspector General, the Department of Veterans Affairs, or the United States Government.
Footnotes
Conflict of interest
All other authors report no conflict of interest, financial or otherwise.
References
- American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3. American Psychiatric Association; Arlington, VA: 2010. [PubMed] [Google Scholar]
- Antonini A, Barone P, Ceravolo R, Fabbrini G, Tinazzi M, Abbruzzese G. Role of pramipexole in the management of Parkinson’s disease. CNS Drugs. 2010;24:829–841. doi: 10.2165/11585090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, Lamm CI, Tracy SL, Rosenberg RS. The treatment of restless legs syndrome and periodic limb movement disorder in adults-an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an american academy of sleep medicine clinical practice guideline. Sleep. 2012;35:1039–1062. doi: 10.5665/sleep.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldomero EB, Ubago JG, Cercos CL, Ruiloba JV, Calvo CG, Lopez RP. Venlafaxine extended release versus conventional antidepressants in the remission of depressive disorders after previous antidepressant failure: ARGOS study. Depression and Anxiety. 2005;22:68–76. doi: 10.1002/da.20080. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Cooper JA, Huusom AK, Hindmarch I. A double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorder. International Clinical Psychopharmacology. 2006;21:159–169. doi: 10.1097/01.yic.0000194377.88330.1d. [DOI] [PubMed] [Google Scholar]
- Barone P, Scarzella L, Marconi R, Antonini A, Morgante L, Bracco F, Zappia M, Musch B. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. Journal of Neurology. 2006;253:601–607. doi: 10.1007/s00415-006-0067-5. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nature Review Neuroscience. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Cassano P, Lattanzi L, Soldani F, Navari S, Battistini G, Gemignani A, Cassano GB. Pramipexole in treatment-resistant depression: an extended follow-up. Depression and Anxiety. 2004;20:131–138. doi: 10.1002/da.20038. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Fall CP, Smith TS, Bennett JP., Jr Pramipexole reduces reactive oxygen species production in vivo and in vitro and inhibits the mitochondrial permeability transition produced by the parkinsonian neurotoxin methylpyridinium ion. Journal of Neurochemistry. 1998;71:295–301. doi: 10.1046/j.1471-4159.1998.71010295.x. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Halvorsen EM, Swerdlow RH, Abramova NN, Parker WD, Jr, Sturgill TW, Bennett JP., Jr Interaction among mitochondria, mitogen-activated protein kinases, and nuclear factor-kappaB in cellular models of Parkinson’s disease. Journal of Neurochemistry. 2000;74:1384–1392. doi: 10.1046/j.1471-4159.2000.0741384.x. [DOI] [PubMed] [Google Scholar]
- Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL. Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depression and Anxiety. 2000;11:58–65. doi: 10.1002/(sici)1520-6394(2000)11:2<58::aid-da2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams AR. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research; New York: 2001. [Google Scholar]
- Forest Laboratories, I. Lexapro Prescribing Information 2011 [Google Scholar]
- Gartlehner G, Gaynes BN, Hansen RA, Thieda P, DeVeaugh-Geiss A, Krebs EE, Moore CG, Morgan L, Lohr KN. Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians. Annals of Internal Medicine. 2008;149:734–750. doi: 10.7326/0003-4819-149-10-200811180-00008. [DOI] [PubMed] [Google Scholar]
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux L, Van Noord M, Mager U, Thieda P, Gaynes BN, Wilkins T, Strobelberger M, Lloyd S, Reichenpfader U, Lohr KN. Comparative benefits and harms of second-generation anti-depressants for treating major depressive disorder: an updated meta-analysis. Annals of Internal Medicine. 2011;155:772–785. doi: 10.7326/0003-4819-155-11-201112060-00009. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Double K, Arzberger T, Leblhuber F, Tatschner T, Riederer P. Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum. Journal of Neural Transmission. 2003;110:1119–1127. doi: 10.1007/s00702-003-0027-5. [DOI] [PubMed] [Google Scholar]
- Goldberg JF, Burdick KE, Endick CJ. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. American Journal of Psychiatry. 2004;161:564–566. doi: 10.1176/appi.ajp.161.3.564. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Korotzer A, Su G. Efficacy comparison of escitalopram and citalopram in the treatment of major depressive disorder: pooled analysis of placebo-controlled trials. CNS Spectrums. 2002;7:40–44. doi: 10.1017/s1092852900028595. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Blier P. Prospect of a dopamine contribution in the next generation of antidepressant drugs: the triple reuptake inhibitors. Currents Drug Targets. 2009;10:1069–1084. doi: 10.2174/138945009789735156. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Rating depressive patients. Journal of Clinical Psychiatry. 1980;41:21–24. [PubMed] [Google Scholar]
- Inoue T, Kitaichi Y, Masui T, Nakagawa S, Boku S, Tanaka T, Suzuki K, Nakato Y, Usui R, Koyama T. Pramipexole for stage 2 treatment-resistant major depression: an open study. Progress in NeuroPsychopharmacology and Biological Psychiatry. 2010;34:1446–1449. doi: 10.1016/j.pnpbp.2010.07.035. [DOI] [PubMed] [Google Scholar]
- Insel TR, Charney DS. Research on major depression: strategies and priorities. Journal of the American Medical Association. 2003;289:3167–3168. doi: 10.1001/jama.289.23.3167. [DOI] [PubMed] [Google Scholar]
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts general hospital sexual functioning questionnaire. Psychotheraphy and Psychosomatics. 2001;70:221–225. doi: 10.1159/000056257. [DOI] [PubMed] [Google Scholar]
- Lattanzi L, Dell’Osso L, Cassano P, Pini S, Rucci P, Houck PR, Gemignani A, Battistini G, Bassi A, Abelli M, Cassano GB. Pramipexole in treatment-resistant depression: a 16-week naturalistic study. Bipolar Disorder. 2002;4:307–314. doi: 10.1034/j.1399-5618.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- Le WD, Jankovic J. Are dopamine receptor agonists neuroprotective in Parkinson’s disease? Drugs Aging. 2001;18:389–396. doi: 10.2165/00002512-200118060-00001. [DOI] [PubMed] [Google Scholar]
- Lenox-Smith AJ, Jiang Q. Venlafaxine extended release versus citalopram in patients with depression unresponsive to a selective serotonin reuptake inhibitor. International Clinical Psychopharmacology. 2008;23:113–119. doi: 10.1097/YIC.0b013e3282f424c2. [DOI] [PubMed] [Google Scholar]
- Moller JC, Oertel WH, Koster J, Pezzoli G, Provinciali L. Long-term efficacy and safety of pramipexole in advanced Parkinson’s disease: results from a European multicenter trial. Movement Disorders. 2005;20:602–610. doi: 10.1002/mds.20397. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Huusom AK, Bothmer J. A randomised study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorder. Neuropsychobiology. 2004;50:57–64. doi: 10.1159/000078225. [DOI] [PubMed] [Google Scholar]
- Murdoch D, Keam SJ. Escitalopram: a review of its use in the management of major depressive disorder. Drugs. 2005;65:2379–2404. doi: 10.2165/00003495-200565160-00013. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Entsuah R, Benattia I, Demitrack M, Sloan DM, Thase ME. Comprehensive analysis of remission (COMPARE) with venlafaxine versus SSRIs. Biological Psychiatry. 2008;63:424–434. doi: 10.1016/j.biopsych.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Greist JH, Mallinckrodt CH, Prakash A, Sambunaris A, Tollefson GD, Wohlreich MM. Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study. Current Medical Research and Opinion. 2007;23:401–416. doi: 10.1185/030079906X167453. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biological Psychiatry. 2001;50:345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents. Biological Psychiatry. 2007;62:1217–1227. doi: 10.1016/j.biopsych.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Perugi G, Toni C, Ruffolo G, Frare F, Akiskal H. Adjunctive dopamine agonists in treatment-resistant bipolar II depression: an open case series. Pharmacopsychiatry. 2001;34:137–141. doi: 10.1055/s-2001-15872. [DOI] [PubMed] [Google Scholar]
- Poirier MF, Boyer P. Venlafaxine and paroxetine in treatment-resistant depression. Double-blind, randomised comparison. British Journal of Psychiatry. 1999;175:12–16. doi: 10.1192/bjp.175.1.12. [DOI] [PubMed] [Google Scholar]
- Rektorova I, Rektor I, Bares M, Dostal V, Ehler E, Fanfrdlova Z, Fiedler J, Klajblova H, Kulist’ak P, Ressner P, Svatova J, Urbanek K, Veliskova J. Pramipexole and pergolide in the treatment of depression in Parkinson’s disease: a national multicentre prospective randomized study. European Journal of Neurology. 2003;10:399–406. doi: 10.1046/j.1468-1331.2003.00612.x. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, Ritz L, Biggs MM, Warden D, Luther JF, Shores-Wilson K, Niederehe G, Fava M. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. New England Journal of Medicine. 2006;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- Serna MC, Cruz I, Real J, Gasco E, Galvan L. Duration and adherence of antidepressant treatment (2003 to 2007) based on prescription database. European Psychiatry. 2010;25:206–213. doi: 10.1016/j.eurpsy.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Sporn J, Ghaemi SN, Sambur MR, Rankin MA, Recht J, Sachs GS, Rosenbaum JF, Fava M. Pramipexole augmentation in the treatment of unipolar and bipolar depression: a retrospective chart review. Annals of Clinical Psychiatry. 2000;12:137–140. doi: 10.1023/a:1009060800999. [DOI] [PubMed] [Google Scholar]
- Takata K, Kitamura Y, Kakimura J, Kohno Y, Taniguchi T. Increase of bcl-2 protein in neuronal dendritic processes of cerebral cortex and hippocampus by the antiparkinsonian drugs, talipexole and pramipexole. Brain Research. 2000;872:236–241. doi: 10.1016/s0006-8993(00)02493-8. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. American Journal of Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Progress in Brain Research. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD, Charney DS, Manji HK. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biological Psychiatry. 2004;56:54–60. doi: 10.1016/j.biopsych.2004.03.013. [DOI] [PubMed] [Google Scholar]


