Abstract
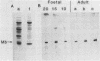
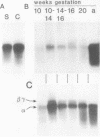
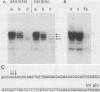
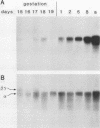
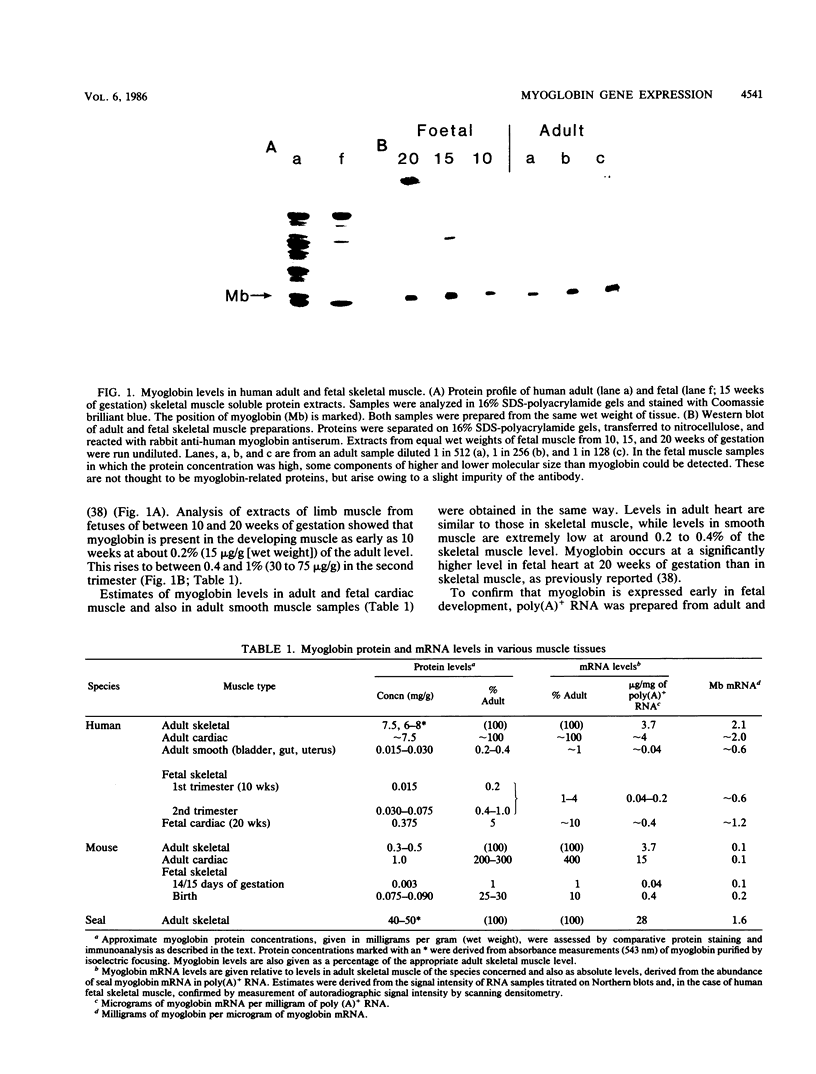
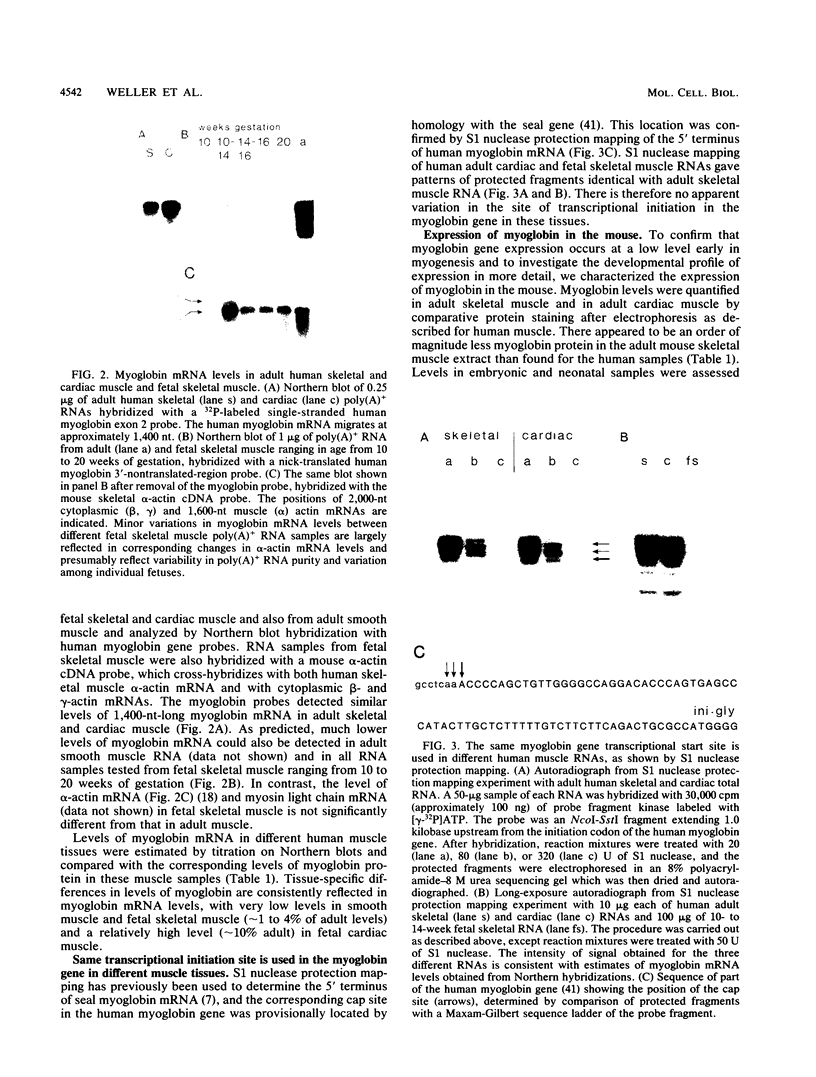
We showed that myoglobin gene transcription and the appearance of myoglobin occur very early in myogenesis, in both humans and mice. In contrast to the contractile protein genes, there is a subsequent increase of 50- to 100-fold in myoglobin mRNA and protein levels during later muscle development. Myoglobin and myoglobin mRNA are present at elevated levels in fetal heart and are also detectable at low levels in adult smooth muscle. The absolute level of myoglobin mRNA in highly myoglobinized seal muscle is very high [2.8% of the total population of poly(A)+ RNAs]. Levels of myoglobin in seal skeletal muscle and in various human muscle types appear to be determined by the size of the myoglobin mRNA pool. In contrast, low levels of myoglobin in mouse skeletal muscle are not apparently correlated with low levels of myoglobin mRNA. As expected from the early appearance of myoglobin mRNA in embryonic skeletal muscle, both rat and mouse embryonic myoblasts accumulate myoglobin mRNA on fusion and differentiation in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akeson A., Biörck G., Simon R. On the content of myoglobin in human muscles. Acta Med Scand. 1968 Apr;183(4):307–316. doi: 10.1111/j.0954-6820.1968.tb10482.x. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains W., Ponte P., Blau H., Kedes L. Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol Cell Biol. 1984 Aug;4(8):1449–1453. doi: 10.1128/mcb.4.8.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Blanchetot A., Wilson V., Wood D., Jeffreys A. J. The seal myoglobin gene: an unusually long globin gene. Nature. 1983 Feb 24;301(5902):732–734. doi: 10.1038/301732a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Jones D. R. The comparative physiology of diving in vertebrates. Adv Comp Physiol Biochem. 1982;8:179–364. doi: 10.1016/b978-0-12-011508-2.50012-5. [DOI] [PubMed] [Google Scholar]
- Christian C. N., Nelson P. G., Peacock J., Nirenberg M. Synapse formation between two clonal cell lines. Science. 1977 May 27;196(4293):995–998. doi: 10.1126/science.193191. [DOI] [PubMed] [Google Scholar]
- Colling-Saltin A. S. Enzyme histochemistry on skeletal muscle of the human foetus. J Neurol Sci. 1978 Dec;39(2-3):169–185. doi: 10.1016/0022-510x(78)90121-1. [DOI] [PubMed] [Google Scholar]
- Czelusniak J., Goodman M., Hewett-Emmett D., Weiss M. L., Venta P. J., Tashian R. E. Phylogenetic origins and adaptive evolution of avian and mammalian haemoglobin genes. Nature. 1982 Jul 15;298(5871):297–300. doi: 10.1038/298297a0. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards Y. H., Lloyd J. C., McMillan S. L., Benham F. J. Human glyceraldehyde-3-phosphate dehydrogenase: mRNA levels and enzyme activity in developing muscle. Mol Cell Biol. 1985 Aug;5(8):2147–2149. doi: 10.1128/mcb.5.8.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasold H., Riedl G., Jaisle F. Evidence for an absence of myoglobin from human smooth muscle. Eur J Biochem. 1970 Jul;15(1):122–126. doi: 10.1111/j.1432-1033.1970.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichnik J. M., Bergsma D. J., Schwartz R. J. Tissue restricted and stage specific transcription is maintained within 411 nucleotides flanking the 5' end of the chicken alpha-skeletal actin gene. Nucleic Acids Res. 1986 Feb 25;14(4):1683–1701. doi: 10.1093/nar/14.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröschel-Stewart U., Jaroschik U., Schwalm H. Chicken gizzard, a myoglobin containing smooth muscle. Experientia. 1971 May 15;27(5):512–513. doi: 10.1007/BF02147568. [DOI] [PubMed] [Google Scholar]
- Humphries S. E., Whittall R., Minty A., Buckingham M., Williamson R. There are approximately 20 actin gene in the human genome. Nucleic Acids Res. 1981 Oct 10;9(19):4895–4908. doi: 10.1093/nar/9.19.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Longo L. D., Koos B. J., Power G. G. Fetal myoglobin: quantitative determination and importance for oxygenation. Am J Physiol. 1973 May;224(5):1032–1036. doi: 10.1152/ajplegacy.1973.224.5.1032. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melloul D., Aloni B., Calvo J., Yaffe D., Nudel U. Developmentally regulated expression of chimeric genes containing muscle actin DNA sequences in transfected myogenic cells. EMBO J. 1984 May;3(5):983–990. doi: 10.1002/j.1460-2075.1984.tb01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Minty A., Blau H., Kedes L. Two-level regulation of cardiac actin gene transcription: muscle-specific modulating factors can accumulate before gene activation. Mol Cell Biol. 1986 Jun;6(6):2137–2148. doi: 10.1128/mcb.6.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller P., Sylvén C. Myoglobin in human skeletal muscle. Scand J Clin Lab Invest. 1981 Sep;41(5):479–482. doi: 10.3109/00365518109090486. [DOI] [PubMed] [Google Scholar]
- Nudel U., Greenberg D., Ordahl C. P., Saxel O., Neuman S., Yaffe D. Developmentally regulated expression of a chicken muscle-specific gene in stably transfected rat myogenic cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3106–3109. doi: 10.1073/pnas.82.10.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipler T. D., Edwards Y. H., Hopkinson D. A. Developmental changes in the protein profiles of human cardiac and skeletal muscle. Ann Hum Genet. 1978 May;41(4):409–418. doi: 10.1111/j.1469-1809.1978.tb00911.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Phillips E. Specific changes in cellular glycoproteins and surface proteins during myogenesis in clonal muscle cells. Dev Biol. 1981 Jan 30;81(2):229–237. doi: 10.1016/0012-1606(81)90286-4. [DOI] [PubMed] [Google Scholar]
- Weller P., Jeffreys A. J., Wilson V., Blanchetot A. Organization of the human myoglobin gene. EMBO J. 1984 Feb;3(2):439–446. doi: 10.1002/j.1460-2075.1984.tb01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Butler-Browne G. S., Schwartz K., Bouveret P., Pinset-Härstöm I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981 Aug 27;292(5826):805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B., Caldwell P. R. Role of myoglobin in the oxygen supply to red skeletal muscle. J Biol Chem. 1975 Dec 10;250(23):9038–9043. [PubMed] [Google Scholar]
- Wittenberg J. B. Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol Rev. 1970 Oct;50(4):559–636. doi: 10.1152/physrev.1970.50.4.559. [DOI] [PubMed] [Google Scholar]
- Wood D., Blanchetot A., Jeffreys A. J. Molecular cloning of seal myoglobin mRNA. Nucleic Acids Res. 1982 Nov 25;10(22):7133–7144. doi: 10.1093/nar/10.22.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydro R. M., Nguyen H. T., Gubits R. M., Nadal-Ginard B. Characterization of sarcomeric myosin heavy chain genes. J Biol Chem. 1983 Jan 10;258(1):670–678. [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]