Abstract
Background
Theobromine, a methylxanthine related to caffeine and present in high levels in cocoa, may contribute to the appeal of chocolate. However, currently evidence for this is limited.
Objectives
We conducted a within-subjects placebo-controlled study of a wide range of oral theobromine doses (250, 500, and 1000 mg) using an active control dose of caffeine (200 mg) in 80 healthy participants.
Results
Caffeine had the expected effects on mood including feelings of alertness, and cardiovascular parameters. Theobromine responses differed according to dose: it showed limited subjective effects at 250 mg and negative mood effects at higher doses. It also dose-dependently increased heart rate. In secondary analyses we also examined individual differences in the drugs' effects in relation to genes related to their target receptors, but few associations were detected.
Conclusions
This study represents the highest dose of theobromine studied in humans. We conclude that theobromine at normal intake ranges may contribute to the positive effects of chocolate, but at higher intakes effects become negative.
Keywords: theobromine, caffeine, genetics, subjective, cognitive, healthy volunteers
Introduction
The sensory pleasures of chocolate products can explain much of their appeal. However, psychoactive ingredients have also been thought to play a role. Among other candidate psychoactive ingredients, chocolate contains two methylated xanthine derivatives, caffeine (1,3,7-trimethylxanthine) and theobromine (3,7-dimethylxanthine) that may contribute to its reinforcing effects. Dark chocolate contains about 25–35 mg of caffeine and 200–300 mg of theobromine per 40 g chocolate (Bruinsma and Taren 1999; The Hershey Company 2012; UK Joint Food Safety and Standards Group 1998): both compounds are therefore present in sufficient concentrations to potentially produce psychoactive effects.
The rewarding effects and human pharmacology of caffeine are well characterized. At moderate doses caffeine increases self-reported alertness and improves attention and psychomotor performance (Ruxton 2008). At higher doses, and in some individuals at moderate doses, caffeine also causes anxiety and other unpleasant effects (Brice and Smith 2002; Childs et al. 2008). Almost 90% of adults in the United States regularly consume caffeine-containing beverages, with an average intake of 193 mg/day (Frary et al. 2005). Individuals can discriminate 56 mg caffeine from placebo (Mumford et al. 1994), which is similar to the amount contained in 100 grams of dark chocolate (see above). Thus, it is plausible that the psychoactive effects of caffeine contribute to the appeal of chocolate.
The contributions of theobromine are less clear and its psychoactive effects appear subtle (reviewed in Smit 2011). Although two early studies failed to detect psychopharmacological activity (Brunk et al. 1973; Dorfman and Jarvik 1970), Mumford (1994) found that 5 of 7 participants were able to discriminate 560 mg theobromine from placebo or caffeine, suggesting that theobromine might be about one tenth as potent as caffeine. While theobromine did not significantly increase any subjective or behavioral measures in the Mumford et al. (1994) study when all subjects were combined, the compound increased alertness, headache, and irritability in some individuals, suggesting the possibility of individual differences in sensitivity. Using a higher dose, Mitchell et al. (2011) found that 700 mg theobromine lowered blood pressure, decreased self-report calmness and increased subjects' ratings of how interesting they found performance of study tasks.
Pharmacological assays confirm that theobromine is less active than caffeine. Theobromine has two- and threefold lower affinity than caffeine for the A1 and A2A receptors (Carney 1982; Carney et al. 1985; Ferré 2008; Fredholm 2007; Fredholm et al. 1999; Shi and Daly 1999; Snyder et al. 1981) and is apparently less efficacious as a phosphodiesterase inhibitor (Heim and Ammon 1969; Robinson et al. 1967). In addition, their pharmaokinetics differ substantially. Caffeine is highly water soluble, peaks in the blood 30–40 minutes after ingestion, and has a half-life of 2.5–5 hours, while theobromine is fat soluble, attains peak blood concentrations 2–3 hours after ingestion, and has an estimated half-life of 7 –12 hours (Drouillard et al. 1978; Lelo et al. 1986; Mumford et al. 1996; Tarka and Cornish 1982). Caffeine also penetrates the blood-brain barrier more readily than theobromine (Svenningsson et al. 1999). Theobromine, on the other hand, is a more potent cardiac stimulant than caffeine and was previously used in humans as a dilator of coronary arteries at daily doses of 300 – 600 mg (Moffat 1986). A previous report by van den Bogaard and colleagues (2010) found that 979 mg theobromine with cocoa, given daily for 3 weeks, lowered systolic blood pressure and raised heart rate.
Individuals vary in their sensitivity to drugs, including caffeine and theobromine, and some of this variability appears to be genetic in origin (Hart et al. 2012; Yang et al. 2010). For example, individuals vary in the extent to which they experience anxiety after moderate doses of caffeine, and this anxiogenic response is associated with a single nucleotide polymorphism (SNP) in the A2A receptor gene (Alsene et al. 2003; Childs et al. 2008; Rogers et al. 2010; Yang et al. 2010). Whether this same polymorphism contributes to variations in responses to theobromine has not been investigated.
We conducted a controlled laboratory-based study of several doses of theobromine in healthy young adults. We characterized the effects of theobromine (250, 500 and 1000 mg), vs placebo (0 mg) and a positive control dose of caffeine (200 mg) on mood, cognitive performance, and associated physiological measures in a placebo-controlled, within-subjects, double blind design. We selected participants without extensive prior drug use or psychiatric problems, who reported very low regular use of caffeine and related methylxanthines, to minimize the effects of tolerance or withdrawal. We also examined the drugs' effects in relation to two polymorphisms in the adenosine receptor 2A gene that might contribution to the compounds' actions.
Methods
Study design
We used a randomized double-blind, placebo-controlled within-subjects Williams design. Eighty-four healthy adult volunteers participated in five 6-hour sessions separated by at least 48 hours. During each session, they received caffeine (200 mg), theobromine (250, 500, 1000 mg), or placebo in a randomized order. They were tested for recent drug use and pregnancy at the beginning of each session, and completed physiological, cognitive, and mood measures before and at regular intervals after capsule administration according to the schedule in Table 1.
Table 1.
Timing of measurements
| Actual time | Time relative to theobromine | Time relative to caffeine | Event |
|---|---|---|---|
| 9.00 | −90 min | −240 min | Urine, breath, and saliva (screening) |
| Baseline heart rate and blood pressure (HR&BP) | |||
| Baseline mood | |||
|
| |||
| 10.30 | 0 | −150 | First capsules: Placebo or Theobromine |
|
| |||
| 13.00 | 150 | 0 | Second capsules: Placebo or Caffeine |
|
| |||
| 13.30 | 180 | 30 | Mood, HR&BP |
|
| |||
| 13.40 | 190 | 40 | Cognitive battery (lasting 60 min) |
|
| |||
| 14.45 | 255 | 105 | Mood, HR&BP |
Participants
Healthy adults, aged 18–35, were recruited from the Chicago metropolitan area and surroundings by means of flyers and Internet postings. We screened potential participants with semi-structured psychiatric interview, electrocardiogram, and a physical examination. To minimize the influence of population stratification on genotypic linkage analysis (Freedman et al. 2004), only Caucasians were accepted into the study. Individuals taking any prescription medications, working night shift or not fluent in English were not accepted. Participants were required to report consuming low levels of dietary caffeine or other methylxanthines to reduce potential confounds from tolerance and withdrawal. Candidates consuming more than 5 cups of coffee per week, or large quantities of chocolate, were excluded.
Ethical considerations
The Institutional Review Board of the University of Chicago approved the study in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All persons gave their informed consent prior to inclusion in the study.
Experimental conditions
Each subject ingested two sets of capsules on each session of either caffeine with placebo, or theobromine with placebo. Because caffeine peak plasma times are around 30 minutes post-administration whilst those for theobromine are around 2–3 hours (Mumford et al. 1996), either theobromine or placebo was given first, followed 2.5 hours later by caffeine or placebo. This was to align Cmax for caffeine and theobromine, or, in other words, to ensure that caffeine and theobromine plasma concentrations would peak at approximately the same time. Study treatments, given in random order, were:
Placebo at 10:30, then Placebo at 13:00
Theobromine (250 mg) at 10:30, then Placebo at 13:00
Theobromine (500 mg) at 10:30, then Placebo at 13:00
Theobromine (1000 mg) at 10:30, then Placebo at 13:00
Placebo at 10:30, then Caffeine (200 mg) at 13:00
Pharmaceutical-grade theobromine and caffeine (Fagron BV, Netherlands) were prepared according to GMP standards with identity and stability confirmed by HPLC/GC (Unilever R&D Vlaardingen).
Procedure
Within 2 weeks of the first session, participants attended a one-hour visit to practice the tasks and provide a blood sample for DNA analysis. They were instructed to refrain from consuming caffeine- or theobromine- containing foods, supplements, and beverages (coffee, tea, cola, chocolate, energy drinks), 24 hours prior to each session. Participants were informed that they might receive a stimulant/appetite suppressant, a sedative/tranquilizer, or placebo.
Participants completed five 6-hour sessions (each from 8–10 am to 2–4 pm) separated by at least 48 hours. They arrived for each session at 9 am and were tested individually in comfortably furnished rooms located in a hospital. Compliance with pre-study restrictions was verified using breath alcohol (Alcosensor III, Intoximeters Inc., St. Louis, MO) and urine tests for commonly used drugs (ToxCup, Branan Medical Corporation, Irvine, CA). Females were urine tested for pregnancy (AimStrip, Germaine Laboratories, San Antonio, TX), which was an exclusion criterion for the study. Participants verbally confirmed that they had consumed a standard breakfast before 8 am and had refrained from caffeine and theobromine containing products. A heart rate monitor (Minilogger Inc) provided a continuous measure of heart rate during the session. A research assistant measured blood pressure hourly.
Participants completed mood and cognitive measures according to the schedule in Table 1. When not being tested, participants could engage in quiet activities including reading or watching selected neutral movies. They were not allowed to bring their work, talk on their cell phones, use the internet, or leave the laboratory.
Measures
Physiological measures
Heart rate and blood pressure were measured using a heart rate monitor (Minilogger Inc). Participants were seated for at least 5 min, were required to be still and silent, and have both feet on the ground during each measurement.
Mood measures
Addiction Research Center Inventory (ARCI)
This inventory is a true–false questionnaire that consists of empirically derived scales sensitive to the effects of a variety of classes of psychoactive drugs (Haertzen 1966). In the current study we used a 49-item version, which yields scores for five scales: stimulant-like effects (amphetamine (A) and benzedrine (BG)), euphoric effects (morphine-benzedrine group (MBG)), sedative effects (pentobarbital–chlorpromazine–alcohol group (PCAG)), and somatic and dysphoric effects (lysergic acid diethylamide (LSD)).
Drug Effects Questionnaire (DEQ)
This form contains five questions; participants indicate on 100-mm visual analog scales whether they (a) are currently feeling any effects (from none at all to a lot), (b) like the effects they feel (from not at all to very much), (c) dislike the effects they feel (from not at all to very much) (d) are high (from not at all to very much), and (e) want more of the treatment (from not at all to very much). Caffeine (450 mg) has been shown to increase ratings of “feel treatment” and “feel high” (Childs and de Wit 2006).
Profile of Mood States (POMS)
We assessed current mood states using the 72-item version of the Profile of Mood States (POMS; (McNair et al. 1971), which is widely used to assess mood in responses to drugs (de Wit and Griffiths 1991; Fischman and Foltin 1991). Participants indicate how they feel at the moment in relation to each of the 72 adjectives on a 5-point scale from “not at all” (0) to “extremely” (4). Eight clusters of items have been separated empirically using factor analysis (Anxiety, Depression, Anger, Vigor, Fatigue, Confusion, Friendliness, Elation). Two additional scales are derived from the other scales as follows: Arousal = (Anxiety + Vigor) − (Fatigue + Confusion) and Positive Mood = Elation − Depression (Johanson and Uhlenhuth 1980).
Cognitive measures
Digit Span test
The Digit Span test (Wechsler 1958) is a test of short-term memory in which participants are read a progressively longer series of numbers ranging from two to nine digits and then asked to repeat the series, forward and backward. A trial ends when the participant misses both trials at one sequence length. Five versions of the test were used to avoid learning across trials. Outcome measures are the number of digits remembered in the forward series and the number remembered in the backward series. Caffeine (450 mg) has been shown to decrease the number of digits remembered backward (Childs and de Wit 2006).
Hopkins Verbal Learning Test (HVLT)
The Hopkins Verbal Learning Task (Brandt 1991) is comprised of 12 items from three semantic categories, presented over three consecutive learning trials. During each trial, the experimenter reads the list and the participant repeats as many words as s/he can remember. Twenty minutes later, the participant is required to recall as many words as possible, and then to recognize them within another word list interspersed with 12 distracters. We assessed learning (slope from trial one to three), delayed recall and delayed recognition after 20–25 min.
Attention Network Task (ANT)
Attention was measured using the Attention Network Task (Fan et al. 2005), a computerized measure based on Posner and Petersen's model of attention (1990). The model includes three functionally and anatomically separate attentional networks (alerting, orienting, and executive control). The ANT consists of three blocks of 96 trials during which participants respond to an arrow flanked by other arrows presented on the screen. The alerting component can be assessed by comparing the effectiveness of cued trials versus non-cued trials. The orienting component can be measured via the added value of a special cue that guides the participant's response. For the executive component, participants are presented with conflicting information (flanking arrows in the opposite direction compared to the central arrow) that must be ignored. The output is the subject's performance on all three comparisons, plus a response time and accuracy measure. We used an accuracy criterion (80%) for including individuals' data, which is commonly done to ensure measures derived from task performance remain interpretable indices of specific cognitive domains (e.g., MacLeod et al. 2010). The task is sensitive to caffeine which improved the alerting and conflicting component without affecting the orienting function (Brunyé et al. 2010a).
Simple Reaction Time
Simple reaction time was measured using the simple reaction time task from the Automated Neuropsychological Assessment Metrics (ANAM) battery (Reeves et al. 2006). Participants were required to press a key as quickly as possible upon presentation of a symbol presented on the screen at variable intervals. Two measures of attention were derived from the simple reaction time task: a simple measure of Go reaction time, and a measure of lapses of attention based on long reaction times. Lapses in attention are defined by the positive skew in a distribution of simple reaction times. This form of inattention results in an inability to focus on completion of a specific task for extended periods of time, or perhaps a tendency to be distracted by other stimuli. To calculate “lapses in attention” we averaged the deviation of each reaction time from the modal reaction time, which provides an index of skewness.
Genotyping
We collected a blood sample during the orientation session for DNA extraction and genotyping. DNA was extracted by the General Clinical Research Center of the University of Chicago. For four participants from whom blood was not available, we extracted DNA from saliva samples with the Oragene OG-250 kit (Oragene, DNA Genotek, Kanata, Ontario). We determined single nucleotide polymorphisms (SNPs) for ADORA2A (rs4822492 and rs5751876) with Applied Biosystems Custom TaqMan® SNP Genotyping Assay.
Data Analyses
We excluded as outliers data exceeding the 4.5* interquartile range. Measures made more than once were expressed as change from baseline (time 0). For data from the drug questionnaires and physiological measures, maximum change from baseline (Emax) were calculated, using the baseline, 180, and 255 min measures. We analyzed data using mixed-effects models in R 2.15.2 (R Development Core Team 2012) with Condition as a fixed effect and Participant as a random effect using a 2-tailed test with α = 0.05. When the F-test showed a main effect of Condition, conditions were compared pairwise with post-hoc comparisons using Tukey's HSD test corrected for multiple comparisons using the single-step method.
For analyzing genotype, we first checked that each genetic marker was in Hardy-Weinberg equilibrium (HWE). To limit the number of statistical tests involving genotype, we restricted analyses to physiological measurements, two attention tasks (simple reaction time from the ANAM and the three attentional indices from the ANT), and two mood scales (POMS anxiety and depression, based on our previous results). We also conducted an analysis using a principal components analysis constructed from self-report measures. However, this did not reveal any effects of genotype and is not reported here.
Results
Participants
Eighty-four participants (48F, 37M) aged 23.3 ± 3.5 y (mean ± SD; range: 18 – 34 y) completed the study. New participant enrollment ended when 80 participants had completed, although those who had begun testing completed the study and we included their data in analyses. They had completed 15.5 ± 1.4 (range: 12–18) years of education. Participants reported typically having in a week: 2.7 ± 2.7 (range: 0 – 17.5, 95% used 6 or fewer drinks) caffeinated drinks; 0.9 ± 0.9 (range: 0 – 5) chocolate bars; and 0.2 ± 0.7 (range: 0 – 4) chocolate-containing drinks. Demographics are summarized in Table 2.
Table 2.
Participant demographics
| Variable | Mean ± SD or Percent |
|---|---|
| Gender | 36M (43%), 48F (57%) |
| Age (years) | 23.3 ± 3.53 |
| Height (inches) | 67 ± 3.58 |
| Weight (kg) | 64.8 ± 8.2 |
| BMI | 22.3 ± 1.68 |
| Education (years) | 15.5 ± 1.38 |
| Ethnicity | 81 (96%) Caucasian 3 (4%) Hispanic |
| Race | 100% White |
Genotyping results
Genotype data were missing from four individuals (insufficient DNA for genotyping). Thirty-five participants were heterozygous CG for rs4822492 (ADORA2A) while 23 were homozygous GG and 20 homozygous CC. Forty participants were heterozygous for rs5751876 (ADORA2A) with 26 CC homozygous and 15 TT homozygous. All single nucleotide polymorphisms (SNPs) were in Hardy-Weinberg equilibrium in our sample.
Mood
As summarized in Table 3, caffeine, but not theobromine, produced stimulant-like mood effects. Caffeine significantly increased participant ratings of Feel, High, Like, and Want items of the DEQ; the A, BG, and MBG scales of the ARCI; and the anxiety and arousal scales of the POMS. Theobromine increased scores on the “Want” item of the DEQ at 250 mg, and had very few effects on the ARCI and POMS. The highest dose of theobromine increased ratings of Feel on the DEQ and produced dysphoric effects on the ARCI. Theobromine appeared to dose-dependently increase ratings of Dislike (i.e., increases at 500 and 1000 mg). Figure 1 shows the mean (and SEM) maximum change from baseline for each condition on the DEQ. There were no significant main or interaction effects of genotype on ratings of anxiety and depression (POMS).
Table 3.
Effects of Study Conditions on Peak Change in Physiological and Mood Measures
| Placebo | Caffeine | TH250 | TH500 | TH1000 | F test | |
|---|---|---|---|---|---|---|
| Heart Rate (bpm) | 0.51 ± 2.03 | 1.43 ± 3.59 | 1.42 ± 2.83 | 2.49 ± 4.82* | 3.27 ± 5.25**† | F(4,328)=7.198 |
| Systolic (mmHg) | 3.45 ± 5.44 | 8.01 ± 8.75** | 4.04 ± 5.70 | 5.22 ± 7.08 | 5.59 ± 7.17 | F(4,328)=5.947 |
| Diastolic (mmHg) | 3.11 ± 4.22 | 7.27 ± 7.36** | 3.37 ± 5.34 | 3.84 ± 5.64 | 3.47 ± 4.92‡ | F(4,328)=10.007 |
| DEQ (0–100) | ||||||
| Feel | 13.1 ± 2.08 | 33.35 ± 3.28** | 17.65 ± 2.40 | 20.20 ± 2.82 | 31.22 ± 3.38** | F(4,320)=12.781 |
| High | 5.86 ± 1.52 | 19.07 ± 2.67** | 9.13 ± 1.93 | 10.39 ± 2.29 | 11.22 ± 2.02† | F(4,320)=7.611 |
| Like | 15.41 ± 2.51 | 26.40 ± 3.16** | 20.96 + 2.82 | 18.04 + 2.63 | 19.99 ± 2.85 | F(4,320)=4.007 |
| Dislike | 14.33 ± 2.61 | 21.08 ± 2.9 | 20.86 ± 2.73 | 23.02 ± 3.1* | 31.53 ± 3.57** | F(4,320)=6.788 |
| Want | 10.05 ± 1.90 | 21.71 ± 2.76** | 18.72 ± 2.59† | 14.2 ± 2.44 | 12.63 ± 2.25† | F(4,320)=6.366 |
| ARCI | ||||||
| A (0–11) | 0.69 ± 1.06 | 1.47 ± 1.90** | 1.03 ± 1.53 | 0.70 ± 1.29 | 0.88 ± 1.35 | F(4,320)=4.739 |
| BG (0–13) | 0.91 ± 1.92 | 1.73 ± 2.94** | 0.78 ± 1.52 | 0.55 ± 1.15 | 0.74 ± 1.78† | F(4,320)=5.108 |
| LSD (0–14) | 0.84 ± 1.09 | 1.46 ± 1.99 | 1.94 ± 2.11 | 0.84 ± 1.35 | 1.17 ± 1.62** | F(4,320)=6.752 |
| MBG (0–16) | 0.67 ± 1.22 | 1.54 ± 1.91* | 0.96 ± 1.84 | 0.92 ± 1.69 | 0.74 ± 1.13† | F(4,320)=2.977 |
| PCAG (0–15) | 1.96 ± 2.66 | 1.20 ± 2.22 | 2.87 ± 3.02 | 1.98 ± 2.69 | 2.30 ± 3.36‡ | F(4,320)=4.624 |
| POMS (0–4) | ||||||
| Anger | 0.04 ± 0.10 | 0.05 ± 0.12 | 0.05 ± 0.16 | 0.05 ± 0.17 | 0.05 ± 0.10 | F(4,320)=0.160 |
| Anxiety | 0.13 ± 0.21 | 0.30 ± 0.46** | 0.15 ± 0.26 | 0.15 ± 0.23 | 0.28 ± 0.44** | F(4,320)=5.540 |
| Arousal | 0.46 ± 0.78 | 0.70 ± 0.80* | 0.46 ± 0.55 | 0.43 ± 0.57 | 0.56 ± 0.89 | F(4,320)=2.490 |
| Confusion | 0.20 ± 0.31 | 0.16 ± 0.22 | 0.14 ± 0.23 | 0.15 ± 0.27 | 0.21 ± 0.29 | F(4,320)=1.144 |
| Depression | 0.04 ± 0.10 | 0.04 ± 0.15 | 0.02 ± 0.07 | 0.04 ± 0.11 | 0.07 ± 0.16 | F(4,320)=1.802 |
| Elation | 0.16 ± 0.30 | 0.22 ± 0.35 | 0.20 ± 0.30 | 0.18 ± 0.28 | 0.16 ± 0.28 | F(4,320)=0.821 |
| Fatigue | 0.27 ± 0.52 | 0.12 ± 0.27 | 0.22 ± 0.37 | 0.29 ± 0.53 | 0.35 ± 0.48‡ | F(4,320)=3.843 |
| Friendliness | 0.12 ± 0.23 | 0.15 ± 0.23 | 0.15 ± 0.28 | 0.14 ± 0.25 | 0.13 ± 0.29 | F(4,320)=0.248 |
| Positive Mood | 0.18 ± 0.31 | 0.25 ± 0.39 | 0.25 ± 0.34 | 0.22 ± 0.34 | 0.19 ± 0.31 | F(4,320)=1.086 |
| Vigor | 0.18 ± 0.31 | 0.30 ± 0.45 | 0.20 ± 0.30 | 0.20 ± 0.32 | 0.24 ± 0.44 | F(4,320)=1.630 |
Means (± Stand. Dev.) and F-test statistics. P-values (corrected for multiple comparisons) are highlighted when < 0.05.
Statistically significant from placebo p<0.05.
Statistically significant from placebo p<0.001.
Statistically significant from caffeine p<0.05.
Statistically significant from caffeine p<0.001.
Figure 1.

Mean and SEM maximum ratings on Drug Effects Questionnaire (DEQ) after placebo (PL) caffeine (Caff) and 250, 500, and 1000 mg theobromine (TH). Asterisks indicate means that differ significantly from placebo (p<.05).
Cognitive performance
Data from the ANT task were unusable for two participant-sessions because they did not meet our accuracy criterion (one in the 500 mg theobromine conditions, the other in the caffeine condition). In addition, for the ANT Alerting measure one participant was an outlier on two sessions, using 4.5* interquartile range criteria (caffeine and 1000 mg theobromine conditions). Omitting these data led the otherwise nonsignificant Alerting measure to be significant in the caffeine condition. For the simple reaction time task, data from two participants were missing, probably because of equipment failure (no responses were recorded).
The study compounds did not affect the Digit Span test or the Hopkins Verbal Learning Test (all F ≤ 1.3, all p ≥ 0.27). Numeric results for cognitive tasks are in Supplemental Table 1.
We did not detect effects of genotype on cognitive performance. We restricted analyses of relationships between genotype effects and cognitive performance to reaction measures from the simple reaction time and ANT task in order to limit multiple testing. Analysis of the ANT Alerting index again excluded the outlier participant.
Attention Network Task
Caffeine impaired performance on the Conflict and Alerting indices of the ANT task, whereas theobromine had no effect at the lower doses and impaired Alerting performance only at the highest dose. Caffeine significantly decreased Conflict (z = 2.92, p = 0.016) compared to placebo (main effect of Condition: F(4,324) = 2.5, p = 0.04). After excluding an outlier as described above, caffeine decreased Alerting compared to placebo: z = 2.69, p=0.031; main effect of Condition: F(4,322) = 2.8, p = 0.026). Only the highest dose of theobromine decreased Alerting as compared to placebo (z = −2.69, p = 0.031). Caffeine significantly decreased overall response time (z = 3.28, p = 0.005) whereas 1000 mg theobromine increased response time (z = 3.97, p < 0.001) as compared to placebo (main effect of Condition: F(4,324) = 5.1, p < 0.001).
Simple reaction time (ANAM)
Caffeine, but not theobromine, decreased reaction times. There was a significant main effect of Condition for the mean reaction time (F(4,327) = 2.8, p = 0.025). Compared to placebo, caffeine lowered the mean response time (z = 3.00, p = 0.012).
Physiological measures
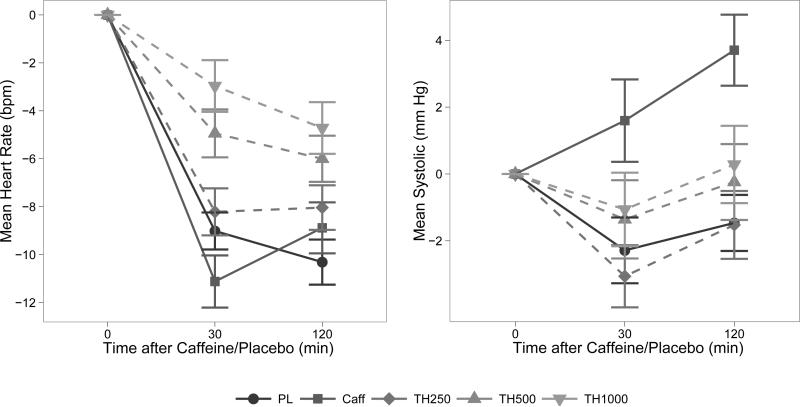
Both caffeine and theobromine had statistically significant physiological effects, as depicted in Figure 2. Mean Emax and standard deviations are given in detail in Table 3. There was a significant main effect of Condition on heart rate as well as systolic and diastolic blood pressure (Emax: all F ≥ 5.9 and all p ≤ 0.001). Theobromine dose-dependently increased heart rate (Emax: 250 mg: n.s., 500 mg: z = 3.52, p < 0.002; 1000 mg: z = 4.89, p < 0.001; see Figure 2). Caffeine had no effect on heart rate as compared to placebo. Caffeine increased both systolic and diastolic blood pressure (Emax for systolic and diastolic blood pressure: both p < 0.001). Theobromine had no effect on blood pressure at any dose as compared to placebo. Peak theobromine and caffeine effects appeared to occur at different times, with theobromine effects becoming maximal before caffeine administration.
Figure 2.
Mean and SEM change in heart rate (beats per minute; left) and systolic blood pressure (mm/Hg; right) after placebo (PL) or caffeine (Caff) and theobromine (250, 500 and 1000 mg). Theobromine or placebo was given 150 minutes earlier so all effects would occur at the same times.
Relationship between physiological effects and genotype
Participants homozygous for the CC genotype at rs4822492 had greater physiological response to treatment conditions. Specifically, they showed elevated heart rate in all active conditions and elevated systolic blood pressure response to theobromine (Figure 3).
Figure 3.

Mean and SEM heart rate (beats per minute) and systolic blood pressure (mm/Hg) in the three rs4822492 genotypic groups (CC, CG and GG) after placebo (PL), caffeine (Caff) and theobromine (250, 500 and 1000 mg). Asterisks show significant differences between genotypes. Sample sizes are 20 CC, 35 CG, and 23 GG.
Higher peak heart rate in those with the CC genotype compared to other participants (CC vs. GC: t = −3.580, p < 0.001; CC vs. GG: t = −2.679, p = 0.001) was detected in a model that showed main effects of condition and genotype (condition: F(4,304) = 6.054, p< 0.001; genotype: F(2,75) = 4.698, p = 0.012). Genotype also modified the effects of theobromine on systolic blood pressure. There were main effects of condition and a significant interaction between condition and genotype (F(4,296) = 5.63, p< 0.001 and F(8,296)=2.98, p = 0.003). Posthoc comparisons indicated that participants the CC genotype showed greater response to 500 mg theobromine than either other genotype (CC vs. GC: t = −3.484, p < 0.001; CC vs. GG: t = −2.126706, p = 0.034) and greater response to 1000 mg than GG (t = −2.508029, p = 0.0127) with a trend for a difference from GC (p = 0.06). There were no detected significant effects of the rs5751876 polymorphism of ADORA2A.
Tolerability of study compounds
Participants generally reported mild effects from both caffeine and theobromine. The most commonly reported adverse effect was headache (n=13), of which nine were in the 1000 mg theobromine condition. The second most commonly reported adverse effect was nausea (n=8), of which six were in the 1000 mg theobromine condition. One participant vomited and another experienced intense nausea without vomiting, were in the 1000 mg condition. The remaining two reports of nausea occurred in the caffeine condition. There were no instances in which symptoms led to a participant withdrawing from the study. All six participants who withdrew from the study reported scheduling conflicts as a primary motivation for withdrawing.
Discussion
We administered three dose levels of theobromine to eighty-four healthy individuals selected for light use of caffeine and other methylxanthines. This represents the largest sample size with the widest range and highest dose of theobromine administered in a controlled study. Compared to placebo, theobromine showed differential effects according to dose: at the lowest dose limited positive effects were noted, but at higher intakes effects become negative. Physiological effects were dose dependent. Theobromine at normal intake ranges may therefore contribute to the positive effects of chocolate, but at higher intakes effects become negative.
We used 200 mg caffeine as a positive control. This confirmed both the sensitivity of our measurements and the presence of typical caffeine effects in our study sample. The typical caffeine effects were observed even in these participants who reported very light use of caffeine and related methylxanthines. Caffeine produces its typical mostly pleasurable, stimulant-like effects on the self-report measures, including ratings of Feel, High, Like, and Want on the DEQ; the A, BG, and MBG scales of the ARCI; and the anxiety and arousal scales of the POMS.
In contrast, theobromine produced differential self-report effects according to dose. Theobromine dose-dependently increased ratings of Dislike, as well as ratings of Feel on the DEQ and dysphoric effects on the ARCI. Theobromine produced a significant increase in Want more of the treatment, detected after 250 mg. Although this may indicate that low doses of theobromine have modest pleasurable effects, this effect was not accompanied by other measures of mood or arousal, and did not resemble the effects of caffeine.
The limited psychoactive effects from theobromine can probably not be attributed to inadequate dose. Theobromine dose-dependently increased heart rate in our study. Moreover, the dose is well above those that would be typically ingested from dietary sources. In the U.S., individuals in the 90th percentile of theobromine intake are estimated to consume only about 150 mg theobromine/day (Theocorp Holding Company LLC 2010). To achieve our highest theobromine dose (1000 mg) from chocolate, an individual would need to consume three to five 40g bars of dark chocolate (Bruinsma and Taren 1999; The Hershey Company 2012; UK Joint Food Safety and Standards Group 1998) Our highest dose level is, to our knowledge, the highest amount of theobromine to be used in a controlled clinical study. Previously, Mitchell et al. (2011) administered 700 mg theobromine to twenty-four female participants and found it decreased self-report calmness, increased ratings of how interesting participants found study tasks, and lowered blood pressure. It is not clear why they detected some subtle effects of the drug whereas we did not, though it may be due to our use of a study population self-selected for low methylxanthine intake with differential sensitivity. However, they concluded, as we do, that theobromine does not have caffeine-like stimulating properties and that it may primarily affect peripheral physiology.
Modest effects were detected on measures of attention. The highest dose of theobromine decreased alertness and increased response times on the ANT task. Inconsistent with previous reports (Brunyé et al. 2010a; Brunyé et al. 2010b; Wesensten et al. 2005) but expected based on the caffeine effects found with other measures of attention, caffeine decreased response times and improved the conflict resolution index in the ANT. Unexpectedly, caffeine also decreased, rather than increased, the alertness index. This contrasts with two previous studies, in which 200 or 400 mg caffeine improved alertness in non-habitual users (Brunyé et al. 2010b) or habitual users (Brunyé et al. 2010a).
We tested two polymorphisms of the gene encoding the ADORA2A receptor, which has previously been associated with caffeine effects. Two studies have found that 150 mg caffeine increases anxiety in individuals carrying the TT genotype of the ADORA2A SNP rs5751876, but not in the CT and CC genotype groups (Alsene et al. 2003; Childs et al. 2008). Rogers et al. (2010) more recently confirmed these findings using a slightly higher dose of caffeine (250 mg, given as 100 and 150 mg separated by 90 min). These effects were not detected in our sample, perhaps because of the relatively small sample or because of characteristics of the subjects.
We did detect significant relationships between another SNP in ADORA2A (rs4822492) and theobromine effects on physiological measures. Possession of the G allele of rs4822492 appeared to attenuate or prevent theobromine-induced increases in systolic blood pressure. This appears consistent with the known role of the adenosine A2A receptor, which is abundant in vasculature, in regulating blood pressure (Schindler et al. 2009).
Our findings raise questions about the psychoactivity of theobromine in the psychological effects of chocolate. One possibility is that the combined effects of caffeine and theobromine, typically found in chocolate, have interactive effects. Indeed, Smit & Blackburn 2005 found a role for the combination of caffeine and theobromine in liking for chocolate, suggesting that there may be unique pharmacological effects when the two compounds are taken in combination. Another possibility is that most consumers of chocolate use more caffeine than the subjects we tested, and that the effects of theobromine may be especially evident in regular caffeine users. Little is known about sometimes reported “chocolate cravings”. It is possible that these have a neurobiological basis or, they may be related to appetites for hedonically pleasurable tastes, or other psychological states. Further studies varying the levels of theobromine in highly palatable forms of chocolate may answer these questions.
The study had both strengths and limitations. The pharmacology of theobromine was tested in an adequate number of subjects, using a double-blind, placebo-controlled design. The inclusion of several doses of theobromine and the inclusion of caffeine as a positive control were both major strengths. Finally, we carefully screened the participants to ensure a homogeneous group with respect to age, other drug use, light caffeine use, and physical and psychiatric health. This careful selection of participants improved the sensitivity of the measures. On the other hand, for a candidate gene study, it could be argued that the number of subjects was relatively small. Because we performed multiple tests of the effects of two SNPs on several dependent measures, multiple comparisons necessitate a more stringent criteria for statistical significance. In such cases replication of these association is critical; we have not yet replicated our findings, so they should be considered preliminary. We limited the participants to light caffeine users to reduce variability due to tolerance, and it may be that these light users differ in sensitivity compared to non-or high-methylxanthine consumers. Further, it is possible that the effects of theobromine may be more pronounced among more frequent consumers of chocolate, who might respond in a unique manner to theobromine. Finally, it may be that co-administration of caffeine and theobromine alters the pharmacokinetics or pharmacodynamics of caffeine, a possibility that our study was not designed to evaluate. Taken together, however, the findings show that theobromine has differential effects on mood and behavior in a population of healthy young adults according to dose.
In conclusion, we found that theobromine generally lacked caffeine-like self-reported effects despite our use of a broad range of theobromine doses and a relatively large sample size of individuals. Instead, theobromine showed differential effects depending on dose: at 250 mg it showed limited positive effects on mood that became negative at higher doses. It also dose-dependently increased heart rate. Together, this suggests that theobromine at normal intake levels, as can be found in a standard 40 g bar of dark chocolate, may contribute to the positive effects of chocolate.
Supplementary Material
Acknowledgments
This research was supported by Unilever R and D. Additional support from T32 MH020065 (MJB), DA02812 (HdW), T32 DA007255 (ABH), and DA021336 (AAP).
References
- Alsene K, Deckert J, Sand P, de Wit H. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2003;28:1694–702. doi: 10.1038/sj.npp.1300232. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Memory Test: Development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991:125–142. [Google Scholar]
- Brice CF, Smith AP. Effects of caffeine on mood and performance: a study of realistic consumption. Psychopharmacology. 2002;164:188–192. doi: 10.1007/s00213-002-1175-2. [DOI] [PubMed] [Google Scholar]
- Bruinsma K, Taren DL. Chocolate: food or drug? Journal of the American Dietetic Association. 1999:1249–56. doi: 10.1016/S0002-8223(99)00307-7. [DOI] [PubMed] [Google Scholar]
- Brunk SF, Ferguson RK, Toubes DB, Leaverton PE, Nordschow CD, Wilson WR. A teaching format in clinical pharmacology. Comparison of two xanthines and a placebo. The Journal of Clinical Pharmacology. 1973;13:121–126. doi: 10.1002/j.1552-4604.1973.tb00073.x. [DOI] [PubMed] [Google Scholar]
- Brunyé TT, Mahoney CR, Lieberman HR, Giles GE, Taylor HA. Acute caffeine consumption enhances the executive control of visual attention in habitual consumers. Brain and Cognition. 2010a:186–192. doi: 10.1016/j.bandc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Brunyé TT, Mahoney CR, Lieberman HR, Taylor HA. Caffeine modulates attention network function. Brain and Cognition. 2010b;72:181–188. doi: 10.1016/j.bandc.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Carney JM. Effects of caffeine, theophylline and theobromine on scheduled controlled responding in rats. British Journal of Pharmacology. 1982:451–454. doi: 10.1111/j.1476-5381.1982.tb09161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JM, Holloway FA, Modrow HE. Discriminative stimulus properties of methylxanthines and their metabolites in rats. Life Science. 1985:913–920. doi: 10.1016/0024-3205(85)90386-8. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Subjective, behavioral and physiological effects of acute caffeine in light, nondependent caffeine users. Psychopharmacology (Berl) 2006;185.4:514–23. doi: 10.1007/s00213-006-0341-3. [DOI] [PubMed] [Google Scholar]
- Childs E, Hoffoff C, Deckert J, et al. Association between ADORA2A and DRD2 Polymorphisms and Caffeine-Induced Anxiety. Neuropsychopharmacology. 2008;33:2791–2800. doi: 10.1038/npp.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Griffiths RR. Testing the abuse liability of anxiolytic and hypnotic drugs in humans. Drug Alcohol Depend. 1991;28:83–111. doi: 10.1016/0376-8716(91)90054-3. [DOI] [PubMed] [Google Scholar]
- Dorfman L, Jarvik M. Comparative stimulant and diuretic actions of caffeine and theobromine in man. Clinical pharmacology and therapeutics. 1970;11:869. doi: 10.1002/cpt1970116869. [DOI] [PubMed] [Google Scholar]
- Drouillard D, Vesell E, Dvorchik B. Studies on theobromine disposition in normal subjects. Alterations induced by dietary abstention from or exposure to methylxanthines. Clinical pharmacology and therapeutics. 1978;23:296. doi: 10.1002/cpt1978233296. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. Journal of neurochemistry. 2008;105:1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105:110–113. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death and Differentiation. 2007:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological reviews. 1999;51:83–133. [PubMed] [Google Scholar]
- Freedman ML, Reich D, Penney KL, McDonald GJ, Mignault AA, Patterson N, Gabriel SB, Topol EJ, Smoller JW, Pato C, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004:388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- Haertzen CA. Development of scales based on patterns of drug effects, using the Addiction Research Center Inventory (ARCI) Psychological Reports. 1966:163–194. doi: 10.2466/pr0.1966.18.1.163. [DOI] [PubMed] [Google Scholar]
- Hart AB, de Wit H, Palmer AA. Genetic factors modulating the response to stimulant drugs in humans. Behavioral Neurogenetics. 2012;12:537–577. doi: 10.1007/7854_2011_187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim F, Ammon HPT. Caffeine and Other Methyl Xanthines. Schattauer-Verlag, Schattauer-Verlag; 1969. [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: d-amphetamine. Psychopharmacology. 1980;71:275–279. doi: 10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- Lelo A, Birkett D, Robson R, Miners J. Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man. British journal of clinical pharmacology. 1986;22:177. doi: 10.1111/j.1365-2125.1986.tb05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod JW, Lawrence MA, McConnell MM, Eskes GA, Klein RM, Shore DI. Appraising the ANT: Psychometric and theoretical considerations of the Attention Network Test. Neuropsychology. 2010;24:637. doi: 10.1037/a0019803. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of mood states (POMS) Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Mitchell ES, Slettenaar M, vd Meer N, Transler C, Jans L, Quadt F, Berry M. Differential contributions of theobromine and caffeine on mood, psychomotor performance and blood pressure. Physiol Behav. 2011;104:816–822. doi: 10.1016/j.physbeh.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Moffat A. Clarke's Isolation and Identification of Drugs. 2 edn. Pharmaceutical Press, Pharmaceutical Press; 1986. [Google Scholar]
- Mumford GK, Benowitz NL, Evans SM, Kaminski BJ, Preston KL, Sannerud CA, Silverman K, Griffiths RR. Absorption rate of methylxanthines following capsules, cola and chocolate. Eur J Clin Pharmacol. 1996;51:319–325. doi: 10.1007/s002280050205. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Evans SM, Kaminski BJ, Preston KL, Sannerud CA, Silverman K, Griffiths RR. Discriminative stimulus and subjective effects of theobromine and caffeine in humans. Psychopharmacology (Berl) 1994;115:1–8. doi: 10.1007/BF02244744. [DOI] [PubMed] [Google Scholar]
- Posner MI, Peterson SE. The attentional system of the human brain. Annual Review of Neuroscience. 1990:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- Reeves DL, Bleiberg J, Roebuck-Spencer T, Cernich AN, Schwab K, Ivins B, Salazar AM, Harvey SC, Brown FH, Warden D. Reference values for performance on the Automated Neuropsychological Assessment Metrics V3.0 in an active duty military sample. Mil Med. 2006;171:982–994. doi: 10.7205/milmed.171.10.982. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Butcher RW, Sutherland EW. Adenyl cyclase as an adrenergic receptor. Annals of the New York Academy of Sciences. 1967:703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Hohoff C, Heatherley SV, Mullings EL, Maxfield PJ, Evershed RP, Deckert J, Nutt DJ. Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption. Neuropsychopharmacology. 2010;35:1973–1983. doi: 10.1038/npp.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruxton C. The impact of caffeine on mood, cognitive function, performance and hydration: a review of benefits and risks. Nutrition Bulletin. 2008;33:15–25. [Google Scholar]
- Schindler CW, Karcz-Kubicha M, Thorndike EB, Müller CE, Tella SR, Ferré S, Goldberg SR. Role of central and peripheral adenosine receptors in the cardiovascular responses to intraperitoneal injections of adenosine A1 and A2A subtype receptor agonists. British Journal of Pharmacology. 2009;144:642–650. doi: 10.1038/sj.bjp.0706043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Daly JW. Chronic effects of xanthines on levels of central receptors in mice. Celular Molecular Neurobiology. 1999:719–932. doi: 10.1023/A:1006901005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit HJ. Theobromine and the pharmacology of cocoa. Handbook of Experimental Pharmacology. 2011;200:201–234. doi: 10.1007/978-3-642-13443-2_7. [DOI] [PubMed] [Google Scholar]
- Snyder S, Katims J, Annau Z, Bruns R, Daly J. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Fredholm BB. The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J Neurosci. 1999;19:4011–4022. doi: 10.1523/JNEUROSCI.19-10-04011.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarka SM, Cornish HH. The toxicology of cocoa and methylxanthines: a review of the literature. CRC critical Reviews in Toxicology. 1982;9:275–312. doi: 10.3109/10408448209037495. [DOI] [PubMed] [Google Scholar]
- The Hershey Company Chocolate and caffeine. 2012 [Google Scholar]
- Theocorp Holding Company LLC GRAS exemption claim for theobromine (3,7-dimethylxanthine) summary of data concerning the safety and GRAS determination of theobromine (3,7-dimethylxanthine) for use as an ingredient in specified foods. 2010 [Google Scholar]
- UK Joint Food Safety and Standards Group . Survey of caffeine and other methylxanthines in energy drinks and other caffeine-containing products Food Surveillance Information Sheet. Joint Food Safety and Standards Group; London, UK: 1998. [Google Scholar]
- van den Bogaard B, Draijer R, Westerhof BE, van den Meiracker AH, van Montfrans GA, van den Born BJH. Effects on Peripheral and Central Blood Pressure of Cocoa With Natural or High-Dose Theobromine A Randomized, Double-Blind Crossover Trial. Hypertension. 2010;56:839–846. doi: 10.1161/HYPERTENSIONAHA.110.158139. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Measurement and Appraisal of Adult Intelligence. 4 edn. Williams & Wilkins, Williams & Wilkins; 1958. [Google Scholar]
- Wesensten NJ, Killgore WDS, Balkin TJ. Performance and alertness effects of caffeine, dextroamphetamine, and modafinil during sleep deprivation. Journal of Sleep Research. 2005;14:255–266. doi: 10.1111/j.1365-2869.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- Yang A, Childs E, Palmer AA, de Wit H. More on ADORA. Psychopharmacology (Berl) 2010;212:699–700. doi: 10.1007/s00213-010-2003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.