Abstract
Objective
To determine whether cognitive behavior therapy (CBT), which we previously showed restored ovarian function in women with functional hypothalamic amenorrhea (FHA), also ameliorated hypercortisolemia and improved other neuroendocrine and metabolic concomitants of in FHA.
Design
Randomized controlled trial.
Intervention
CBT vs. observation.
Setting
Clinical research center at an academic medical university.
Patient(s)
Seventeen women with FHA were randomized either to CBT or observation.
Main Outcome Measure(s)
Circulatory concentrations of cortisol, leptin, TSH, total and free thyronine (T3), and total and free thyroxine (T4) before and immediately after completion of CBT or observation. Each woman served as her own control.
Results
CBT but not observation reduced cortisol levels in women with FHA. There were no changes in cortisol, leptin, TSH, T3, or T4 levels in women randomized to observation. Women treated with CBT showed increased levels of leptin and TSH, while levels of T3 and T4 remained unchanged.
Conclusions
CBT ameliorated hypercortisolism and improved neuroendocrine and metabolic concomitants of FHA while observation did not. We conclude that a cognitive, nonpharmacological approach aimed at alleviating problematic attitudes not only restored ovarian activity but also improved neuroendocrine and metabolic function in women with FHA.
Keywords: Functional hypothalamic amenorrhea, cognitive behavioral therapy, cortisol, stress, reproduction
Introduction
Functional hypothalamic amenorrhea (FHA) is a reversible form of anovulation. The proximate cause of FHA is reduced GnRH drive that manifests as reduced LH pulse frequency and FSH levels (1). Chronically reduced GnRH drive has been attributed to the combined effect of metabolic and psychogenic stresses (2–4). Indeed, women with FHA present with increased limbic-hypothalamic-pituitary-adrenal (LHPA) axis activation as evidenced by elevated circulating and cerebrospinal fluid levels of cortisol (1, 5–7). Importantly, cortisol was not increased in women with other causes of anovulation (8). Additionally, women with FHA who spontaneously recovered ovarian function displayed lower serum cortisol levels after recovery than women who did not recover from FHA (8). Our findings that women with FHA have increased LHPA activity, metabolic disturbances (1), and attitudes that compromise coping responses to stressors (2, 3, 9) led us to design a behavioral intervention targeted to improve problematic attitudes. As previously reported, women with FHA were randomized to a 20-week program of cognitive behavior therapy (CBT) or observation, and ovarian responses to intervention (CBT vs. observation) were gauged by determining weekly levels of estradiol and progesterone before and after intervention (10). CBT restored ovarian activity and ovulation in most subjects whereas most women with FHA randomized to observation remained anovulatory (10). To extend our initial findings, we investigated if CBT also would ameliorate other neuroendocrine and metabolic concomitants of FHA such as hypercortisolism and hypothyroidism.
Our previous research established that FHA is more than an isolated disruption of GnRH drive (1). Pharmacologic approaches include exogenous sex steroid administration if fertility is not immediately desired or ovulation induction if it is. However, neither approach corrects ongoing hypercortisolism and associated metabolic disturbances. Further, exogenous sex steroid administration may not fully prevent or reverse health consequences associated with chronic stress and FHA such as osteopenia (11) and cardiovascular disease (12). There may be both maternal and fetal consequences to pregnancy in the presence of hypercortisolism and hypothalamic hypothyroidism (13–17). If CBT not only restored ovulatory ovarian function but also ameliorated neuroendocrine and metabolic concomitants of FHA, this would buttress the rationale for utilizing CBT as a primary intervention.
The goal of the secondary analysis was to determine the extent to which CBT reversed neuroendocrine and metabolic concomitants of FHA, namely hypercortisolism, hypoleptinemia, and nonthyroidal illness. We hypothesized that CBT, but not observation, would lower cortisol levels in women with FHA, and that leptin levels and thyroid function would increase only in women with FHA treated with CBT.
Materials and Methods
Experimental subjects
The Institutional Review Board of Magee-Women’s Hospital at the University of Pittsburgh approved the study protocol. The risks and benefits of study participation and alternative treatments were described verbally by the principal investigator and in the written consent document. Participants gave written informed consent prior to study interventions. All subjects completed the study. The diagnosis of FHA was established by excluding organic and other functional causes of anovulation and amenorrhea (1–3, 7, 8, 10). Inclusion criteria included an ideal body weight between 90 and 110% and a day-awake, night-rest schedule (10). Exclusion criteria were a psychiatric diagnosis, weight loss >10 lbs within the last 5 years, and exercise >10 h/wk of any type or running more than 10 miles weekly. Exclusion of subjects with current and past psychiatric disorders, including eating disorders and drug dependence, was based upon assessment tools described previously (2, 3). Women with syndromal psychiatric conditions were referred for appropriate care and excluded from participation. We used both interviews and inventories, including the Structured Interview for the Diagnostic and Statistical Manual of Mental Disorders-IV, the Beck Depression Inventory, the Hamilton Rating Scale for Depression, the Dysfunctional Attitudes Scale, the Self-Control Scale, the Eating Disorder Inventory, and the Bulimia Test-Revised (7).
A provisional diagnosis of FHA was made if secondary amenorrhea persisted for more than 6 months, a urinary pregnancy test was negative, serum levels of LH, FSH, TSH, free thyroxine, and prolactin were within normal range, the LH:FSH ratio was less than 2, and no other identifiable cause of secondary amenorrhea, including polycystic ovary syndrome was identified (1–3, 7, 8, 10). None of the women with FHA displayed phenotypic or biochemical evidence of hyperandrogenism. Specifically, levels of androstenedione, testosterone, dehydroepiandrosterone sulfate, and 17-hydroxyprogesterone were within accepted ranges (7).
Study design
Subjects were randomized to either CBT or observation using permuted block design that was administered by an independent statistician who communicated the randomization allocation to the study nurse. The physician in charge (SLB), the master’s level clinician, and the study coordinator were not blinded. The research nurses who performed the General Clinical Research Center (GCRC) visits were blinded, as were laboratory personnel who performed the hormone assays. Nine women were allocated to observation and 8 to CBT (10). A crossover design was precluded because most subjects who recovered ovarian function remained recovered after CBT was completed. Women randomized to observation were offered CBT after their observation period. CBT consisted of 16 sessions over a 20-week period (10). Sessions 1–6 of CBT focused on evaluating nutrition and exercise habits and attitudes toward nutrition and exercise (10). Sessions 7–12 identified maladaptive attitudes about stressors, exercise, nutrition, and weight and focused on stress management techniques and adopting healthy attitudes (10). Sessions 13–16 prepared subjects for termination of CBT (10). Subject recruitment began in April of 1998, enrollment began in May of 1998, and the final follow-up visit occurred in June of 2003.
Procedures
To evaluate baseline ovarian function before CBT or observation, weekly blood samples were collected for 4 weeks and serum levels of estradiol (E2) and progesterone (P4) were determined (10). To evaluate the impact of CBT or observation upon ovarian function, weekly blood samples for E2 and P4 were obtained during the final 6 weeks of intervention (10). Subjects were admitted to a GCRC twice, once after the initial 4 weeks of observation and then after 20 weeks of observation or CBT. During the GCRC visits, blood samples were collected at 15-minute intervals for 24 hours via an indwelling intravenous catheter. Levels of cortisol, TSH, total T4, free T4, total T3, free T3, and leptin were assessed in serum samples. While in the GCRC, body mass index (BMI) was determined (10). A diagnosis of FHA was confirmed if: 1) women exhibited persistent amenorrhea during the baseline 4-week period; 2) anovulation was present as evidenced by progesterone levels lower than 1 ng/mL during the baseline 4-week period; and 3) if the initial LH pulse frequency during the first GCRC visit was less than 10 pulses in 24 hours (8, 10). Criteria for partial and full ovarian recovery have been previously reported (10). If vaginal bleeding occurred, a blood sample was obtained 21 days later to determine estradiol and progesterone levels. Full ovarian recovery required return of menses and evidence of ovulation with E2 levels >100 pg/mL and P4 levels >5 ng/mL. Partial recovery was defined as E2 levels >60 pg/mL and P4 levels <5 ng/mL. Persistent anovulation was E2 <60 pg/mL and P4 <5 ng/mL (8, 10).
Hormone measurements were determined using standard techniques. Cortisol was measured in all 97 samples obtained during frequent sampling (Q 15 min x 24 h). Leptin and TSH were measured hourly for 24 h. Total T4, free T4, total T3, free T3 were measured once in a morning blood sample. Cortisol and free and total T3 and T4 were analyzed by radioimmunoassay (Coat-A-Count, Diagnostic Products Corporation, Los Angeles, CA, USA) (8). Leptin (Linco, St. Charles, MO) (18) and TSH (Nichols Institute, San Juan Capistrano, CA) (8) were measured by immunoradiometric assays.
Statistical Analyses
Data are reported as mean ± standard error of the mean (SEM). To determine sample size, we constructed a power table using % ovarian recovery as the primary outcome variable. An interim analysis was planned and conducted when 8 subjects in each arm completed the study. Because the interim analysis revealed that CBT was more efficacious than observation in restoring ovarian function, we stopped further enrollment and allowed an enrolled (last) subject to finish. Results related to ovarian recovery were previously reported in 16 women with FHA (10). Data from one additional subject is included in the present analysis. This was a pilot study with a small sample size intended to evaluate proof of principle. We planned to analyze hormonal variables other than E2 and P4, but we powered the study based on % ovarian recovery. We constructed a power table using between-group differences in effect size following treatment for ovarian recovery ranging from 20% to 80%. We recognized that the study was likely to be underpowered to detect differences in other hormonal levels and patterns. To avoid a Type II (beta) error, in the secondary analyses presented herein, we deemed p ≤ 0.10 significant. Post-hoc analyses were conducted when necessary. The planned analyses other than % ovarian recovery are reported here. Analysis of variance with repeated measures (RM-ANOVA) was used to detect differences in hormone levels. Each subject served as her own control. Main effects for treatment (CBT vs. observation) and time (pre-treatment vs. post-treatment) were computed. Between-group differences in responses were indicated by the interaction factor of the RM-ANOVA. The interaction term was p ≤ 0.10 for cortisol, leptin, and TSH. To then isolate the treatment effects by group (CBT vs. observation), t-tests were done for cortisol, leptin, and TSH data. Non-parametric Fisher’s exact analysis was used to assess categorical variables. All analyses were done using SPSS version 19. The main secondary outcome variable was cortisol concentration. Cortisol concentrations were measured in blood samples taken at 15-min intervals across 24 h and the mean level per 8 h segment was calculated. The 8-h segments were 0800–1545 h, 1600–2345 h, 2400–0745 h. Percent change from baseline cortisol level also was calculated. The 24-h pattern of circulatory cortisol is diurnal with higher levels during late evening and overnight. We previously established that during the daytime nadir, cortisol levels did not differ between eumenorrheic women and those with FHA (1). To delineate response to intervention, we focused our analysis of cortisol levels during the active secretory phase rather than the quiescent phase. Other outcome variables analyzed by RM-ANOVA were TSH, total and free T4, total and free T3, leptin, and body mass index (BMI).
Results
Baseline characteristics did not differ between women randomized to observation and CBT (Table 1). As previously reported, of the 8 women who underwent CBT, six resumed ovulating, one exhibited partial ovarian recovery, and one showed no ovarian recovery. Of the 9 women who underwent observation, three exhibited partial ovarian recovery and six remained anovulatory. Women randomized to CBT exhibited a higher rate of ovarian recovery (87.5%) than those randomized to observation (33.3%) (Fisher’s Exact p = 0.05). The odds ratio for ovarian recovery with CBT compared to observation was 14, with a 95% confidence interval of 1.14 to 172.6 (p = 0.01).
Table 1.
Mean ± SEM values for baseline parameters before randomization of subjects to observation versus CBT (cognitive behavior therapy).
| Parameter | Observation (n = 9) | CBT (n = 8) | Student’s t- test P value |
|---|---|---|---|
| Age (y) | 25.1 ± 1.8 | 25.3 ± 0.6 | 0.96 |
| BMI (kg/m2) | 21.5 ± 1.9 | 22.2 ± 0.9 | 0.50 |
| LH (IU/L) | 2.6 ± 0.6 | 2.7 ± 0.7 | 0.93 |
| FSH (IU/L) | 4.8 ± 0.6 | 5.3 ± 0.8 | 0.67 |
| LH pulse number (per 24 h) | 7.6 ± 1.3 | 6.5 ± 0.9 | 0.53 |
| E2 (pg/mL) | 29.9 ± 4.2 | 25.1 ± 5.3 | 0.49 |
| P4 (ng/mL) | 1.6 ± 0.4 | 2.0 ± 0.6 | 0.66 |
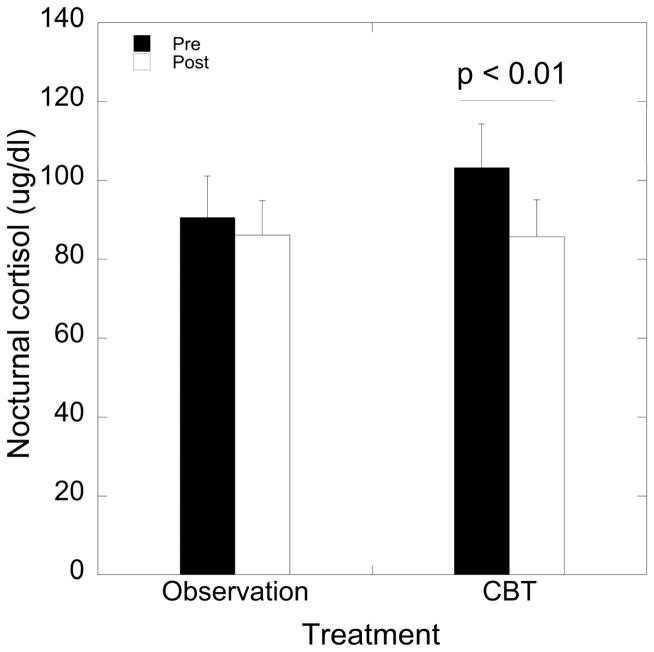
A significant effect of time on nocturnal cortisol levels was detected (F 1, 15 = 9.45, p = 0.008), with decreased cortisol levels following intervention compared to baseline (95.9 ± 7.6 vs. 85.9 ± 6.4, respectively). However, this main effect of time interacted with treatment (F 1, 15 = 3.32, p = 0.09, partial η2 = 0.18). Cortisol levels were reduced after CBT (p = 0.006; Figure 1) but not following observation (p = 0.43); Figure 1). The 95% confidence intervals for pre-CBT cortisol levels and post-CBT cortisol levels were (79.6, 126.9) and (65.8, 105.6), respectively. In the CBT arm, 87.5% exhibited a decrease in cortisol levels (defined as a decrease of ≥ 6 ug/dL) and 33.3% in the observation arm (Fisher’s Exact p = 0.05). A representative schematic of 24 h cortisol levels in 15 min intervals for a subject before and after CBT is shown in Figure 2.
Figure 1.
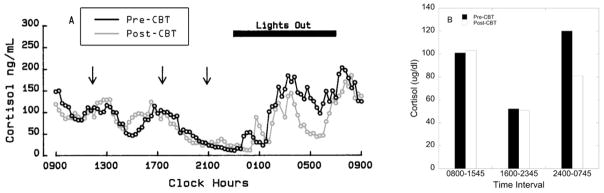
(A) Circulatory concentrations of cortisol in 15 min intervals over 24 h in a woman with FHA before (pre) and after (post) CBT. Meals are indicated by arrows. (B) Circulatory concentrations of cortisol displayed as 8-h mean in the same woman before (pre) and after (post) CBT.
Figure 2.
(A) Mean ± SEM levels of nocturnal (2400–0745 h) cortisol levels by treatment arm (observation vs. CBT) and before (pre) and after (post) treatment. CBT reduced cortisol levels (p = 0.006).
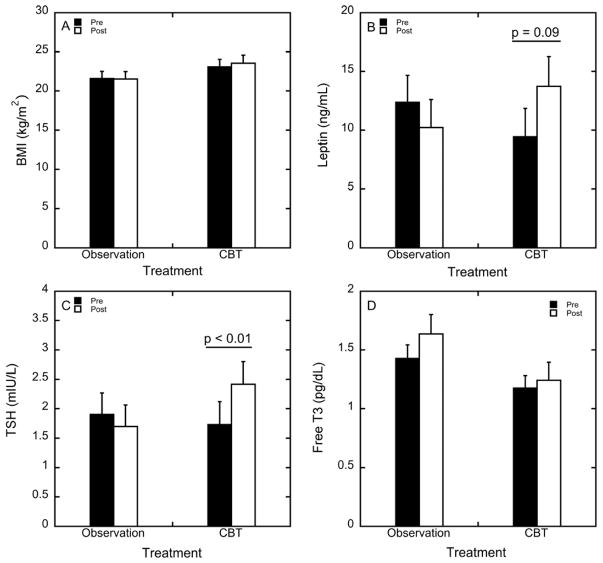
BMI was not affected by treatment or observation (F 1, 15 = 1.18, p = 0.29; Figure 3A). Nonetheless, leptin levels were significantly affected by a time by treatment interaction (F 1, 15 = 6.73, p = 0.02, partial η2 = 0.31). Leptin levels were increased in women who underwent CBT (p = 0.09), whereas leptin remained constant in women who underwent observation (Figure 3B). The 95% confidence intervals for pre-CBT leptin levels and post-CBT leptin levels were (7.51, 14.6) and (8.29, 19.1), respectively. TSH levels showed a significant treatment by time interaction (Supplemental Table 1; F 1, 15 = 5.41, p = 0.03).
Figure 3.
Mean ± SEM of (A) BMI, (B) leptin, (C) TSH, and (D) free T3 in women who underwent observation or CBT before (pre) and after (post) treatment. CBT increased leptin levels (B; p = 0.09) and increased TSH levels (C; p = 0.009).
TSH levels in women who underwent CBT increased (p = 0.009), but did not change following observation (Figure 3C). As shown in Figure 3D, there was a main effect of treatment group on levels of free T3 (p = 0.086) and total T3 (p = 0.073; Supplemental Table 1). As shown in Supplemental Table 1, levels of free T3 and total T3 were lower in women randomized to CBT than observation. Levels of free T3 (p = 0.14) and total T3 (p = 0.14) were not affected by time or time x treatment interaction (p = 0.43 and p = 0.64, respectively). Free T4 levels were not affected by time (p = 0.73), treatment (p = 0.43), or time x treatment interaction (p = 0.88). Similarly, total T4 levels were not affected by time (p = 0.55), treatment (p = 0.64), or by time x treatment interaction (p = 0.48).
Discussion
We previously reported that women with FHA treated with CBT were more likely to recover ovarian activity than those who were observed (10). We now report that cortisol levels were reduced only in women randomized to CBT. Only the group treated with CBT displayed increased leptin and TSH levels. BMI, T3, free T3, T4, and free T4 were unchanged in both groups. Increased TSH in the presence of unchanged levels of T3 and T4 indicates partial recovery of the hypothalamic-pituitary-thyroidal (HP-thyroidal) axis in the CBT treated group. Taken together, our data suggest that CBT ameliorates neuroendocrine and metabolic concomitants of FHA. Our findings substantiate the role of CBT in the treatment of FHA and highlight a role for behavioral approaches for correcting neuroendocrine aberrations in conditions associated with activation of the LHPA axis.
CBT has been used to treat somatic disorders such as insomnia, migraine, chronic fatigue, rheumatoid arthritis, and depression, typically in combination with pharmacotherapy. To the best of our knowledge, this is the first demonstration that CBT ameliorated hypercortisolemia in any context other than depression (19). Our study design accurately assessed cortisol secretory patterns before and after interventions in the same individuals and therefore allowed us to detect the impact induced by active intervention versus observation. Because prior study designs used single or infrequent time points to characterize cortisol patterns, were cross-sectional in nature, and determined cortisol levels with less sensitive methods in salivary or urinary samples, the current study had greater power to detect individual and group responses to interventions. CBT has been shown to reduce cortisol responses to the Trier Social Stress Test in healthy male volunteers (20) and in patients with rheumatoid arthritis (21). However, to the best of our knowledge, our study is the first to utilize CBT to induce recovery from a psychosomatic condition.
The reduction in cortisol levels following CBT coincided with resumption of ovarian activity (10). This tight temporal link supports the notion that hypercortisolism (1, 5, 7) and activation of the LHPA axis characteristic of women with FHA (22–24) are causally related to the reduced GnRH drive that results in amenorrhea, anovulation, and infertility. A direct role of stress hormones in the etiology of FHA is suggested by pharmacological studies in humans that show that exogenous administration of hydrocortisone reduced LH pulse frequency during the follicular phase in normal women (25). While some studies indicated that short term administration of hydrocortisone in men and mixed cohorts of men and women did not suppress basal levels of LH and FSH (26–28), differences in outcomes might be due to the sex of subject, sample size, duration of exposure, and other aspects of the experimental paradigms. Importantly, data from animal studies clearly support the notion that stress, and stress hormones, directly affect reproductive physiology in females. In premenopausal monkeys, psychological stress due to low social status was associated with hypercortisolemia, osteopenia, and functional ovarian impairment that manifested as increased cycle length and reduced estradiol and progesterone secretion (29). A pharmacological elevation of cortisol suppressed LH levels in female rhesus monkeys (30). Cortisol also reduced GnRH and LH pulse frequency in ewes (31) via activation of type II glucocorticoid receptors (32). The decrease in cortisol initiated by CBT in women with FHA buttresses the notion that recovery of ovarian activity is linked to amelioration of LHPA axis activation (8).
Women with FHA report concomitant psychogenic challenges combined with behaviors that induce intermittent energy deficits and metabolic stress (2, 3, 8, 33). In a monkey model of FHA, the combination of energetic challenge and social stress disrupted ovarian function more often than either stressor alone (4). In addition to hypercortisolemia, metabolic adaptations described in FHA include hypothalamic hypothyroidism (1, 34) and, variably, hypoleptinemia (33). CBT did not alter BMI, but did increase leptin levels. While we did not provide a caloric target, suggest specific nutrient intake, or limit exercise habits, our data suggest that targeting problematic attitudes via CBT may have altered appetite and exercise behaviors. Reducing the physiological and behavioral concomitants of stress could reverse the relative energy deficit described in women with FHA (3, 35, 36). While with exogenous leptin administration restored ovarian, gonadal, thyroidal, and adrenal function in women with amenorrhea (35, 37), our findings also suggest that restoration of LHPA activation increased leptin levels without significant weight gain. While body composition may have changed with CBT, we did not assess it, and thus, based on our current experimental paradigm, low leptin levels likely reflect rather than cause FHA.
The increase in TSH levels following CBT treatment indicates that CBT altered metabolism. Glucocorticoids blunt the TSH response to thyroid-releasing hormone (TRH) and alter the thyroidal axis feedback set point. The glucocorticoid-induced inhibition of the thyroidal axis reflects an adaptation that conserves energy (38, 39). Women with FHA display hypothalamic hypothyroidism characterized by low TSH in the presence of low T3 and low T4 (1, 8). Our current data indicate that women who underwent CBT exhibited only partial recovery of thyroidal function by the time of the second assessment, as levels of T3 and T4 remained low in women who underwent CBT despite an increase in TSH. Similarly, in our prior study comparing women with eumenorrhea, polycystic ovary syndrome, and FHA, TSH levels were higher in women with FHA who spontaneously showed partial return of ovarian activity than in unrecovered FHA, PCOS, and eumenorrhea (8). Likewise, cortisol levels were lower in women recovering from FHA than in those with persistent FHA, PCOS, and eumenorrhea (8). In our current randomized trial, the concomitant decrease in cortisol and rise in TSH after CBT suggests that a change in hypothalamic feedback sensitivity precedes full thyroidal recovery. We posit that during recovery, the reduction in cortisol changes feedback sensitivity so that the hypothalamus now interprets the levels of T3 and T4 as too low. This detection presumably increases hypothalamic TRH drive to increase TSH production. The thyroidal axis is the last axis to recover following stress (40–42). For example, in men who underwent surgery, T3 and T4 levels were not recovered despite recovery of the LHPA and HPG axes (40, 41). Thus, less than complete recovery of the thyroidal axis at the conclusion of CBT was expected. The partial recovery of the thyroidal axis reflects our study design in that subjects were studied immediately following completion of CBT or observation. Greater recovery of the thyroidal axis might have been seen with a longer intervention or a longer period of follow-up.
Taken together, our data indicate that CBT restored ovarian activity (10), ameliorated LHPA axis activation, and elicited partial recovery of the thyroidal axis in women with FHA without concomitant weight gain (10). Our findings should be considered preliminary as the study was limited by a small sample size. Given the absence of proven psychopharmacological treatment options for women with FHA and the difficulty of blinding the subjects undergoing CBT, after careful vetting with study section and our institutional review committee, an observational control arm was selected. Regardless of these potential limitations, the ability of CBT to alter neuroendocrine feedback sensitivity demonstrates that behavioral approaches designed to address problematic attitudes that activate the LHPA axis are potent tools for treating stress syndromes including FHA. The effect size of cognitive interventions accrues with time, possibly because they foster better coping skills and thereby reduce allostatic load (43–45). We suspect that women who have undergone CBT are more likely to be more stress resilient when experiencing future challenges (46, 47). Further, mitigating hypercortisolism and hypoestrogenism (10) fosters better cardiovascular, skeletal, and neurological health (3, 11, 12, 15, 16, 48, 49).
Women with FHA are commonly offered sex hormone therapy if they are not seeking pregnancy or ovulation induction if they are (48, 50, 51). While exogenous hormone use corrects sex steroid deficiency due to anovulation, it does not target the underlying etiology of infertility or other psychoneuroendocrinologic concomitants of stressful environments. Without addressing attitudes and behaviors that sustain LHPA activation, neuroendocrine and metabolic concomitants persist and likely exact adverse acute and chronic health consequences. Ovulation induction in amenorrheic women is linked to a higher risk of preterm labor as well as decreased body mass in offspring (13). Indeed, hypothyroidism in pregnant rats altered fetal neuronal migration and development of the hippocampus and cortex (52, 53). Additionally, ongoing stress may impair parenting skills and adversely affect the psychosocial, physiological, and intellectual development of offspring (54, 55). These and other observations highlight the importance of addressing underlying attitudinal and behavioral factors linked to FHA rather than resorting to assisted reproduction (14). Given that persistent LHPA activation and concomitant hypothalamic hypothyroidism likely adversely impact future generations by multiple mechanisms, we suggest that stress management provides a more holistic approach. Psychological interventions such as CBT provide emotional support, teach stress management techniques, and foster lifestyle alterations (48). CBT is the only intervention shown to address the full constellation of neuroendocrine concomitants of FHA.
Supplementary Material
Acknowledgments
The study was supported by NIH grants R01MH50748 (SLB), RR00056 (University of Pittsburgh), TL1 RR025010 (VM), and in part by PHS Grant UL1 RR025008 from the Atlanta Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources, and the Department of Gynecology and Obstetrics at Emory University. The study was conducted with the invaluable expert technical assistance of Kathleen M. Laychak, R.N., the staff of the Magee-Women’s Satellite Clinical Research Center and the staff of the General Clinical Research Center at the University of Pittsburgh. We would also like to acknowledge the graphical assistance of Erin Barthold.
Footnotes
Disclosure summary: Authors VM, FM, TLL, and SLB do not have any conflicts to disclose.
Clinical Trial Registration Number: NCT01674426
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berga SL, Mortola JF, Girton L, Suh B, Laughlin G, Pham P, et al. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1989;68:301–8. doi: 10.1210/jcem-68-2-301. [DOI] [PubMed] [Google Scholar]
- 2.Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic a enorrhea: a controlled comparison. Fertil Steril. 1993;60:486–92. [PubMed] [Google Scholar]
- 3.Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril. 2001;76:310–6. doi: 10.1016/s0015-0282(01)01921-5. [DOI] [PubMed] [Google Scholar]
- 4.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:E270–6. doi: 10.1152/ajpendo.00108.2007. [DOI] [PubMed] [Google Scholar]
- 5.Suh BY, Liu JH, Berga SL, Quigley ME, Laughlin GA, Yen SS. Hypercortisolism in patients with functional hypothalamic-amenorrhea. J Clin Endocrinol Metab. 1988;66:733–9. doi: 10.1210/jcem-66-4-733. [DOI] [PubMed] [Google Scholar]
- 6.Berga SL, Loucks-Daniels TL, Adler LJ, Chrousos GP, Cameron JL, Matthews KA, et al. Cerebrospinal fluid levels of corticotropin-releasing hormone in women with functional hypothalamic amenorrhea. Am J Obstet Gynecol. 2000;182:776–81. doi: 10.1016/s0002-9378(00)70326-7. discussion 81–4. [DOI] [PubMed] [Google Scholar]
- 7.Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 2006;91:1561–5. doi: 10.1210/jc.2005-2422. [DOI] [PubMed] [Google Scholar]
- 8.Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril. 1997;67:1024–30. doi: 10.1016/s0015-0282(97)81434-3. [DOI] [PubMed] [Google Scholar]
- 9.Berga SL, Girton LG. The psychoneuroendocrinology of functional hypothalamic amenorrhea. Psychiatr Clin North Am. 1989;12:105–16. [PubMed] [Google Scholar]
- 10.Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril. 2003;80:976–81. doi: 10.1016/s0015-0282(03)01124-5. [DOI] [PubMed] [Google Scholar]
- 11.Vescovi JD, Jamal SA, De Souza MJ. Strategies to reverse bone loss in women with functional hypothalamic amenorrhea: a systematic review of the literature. Osteoporos Int. 2008;19:465–78. doi: 10.1007/s00198-007-0518-6. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell E, Goodman JM, Harvey PJ. Clinical review: Cardiovascular consequences of ovarian disruption: a focus on functional hypothalamic amenorrhea in physically active women. J Clin Endocrinol Metab. 2011;96:3638–48. doi: 10.1210/jc.2011-1223. [DOI] [PubMed] [Google Scholar]
- 13.van der Spuy ZM, Steer PJ, McCusker M, Steele SJ, Jacobs HS. Outcome of pregnancy in underweight women after spontaneous and induced ovulation. Br Med J (Clin Res Ed) 1988;296:962–5. doi: 10.1136/bmj.296.6627.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–56. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- 15.Hansen D, Lou HC, Olsen J. Serious life events and congenital malformations: a national study with complete follow-up. Lancet. 2000;356:875–80. doi: 10.1016/S0140-6736(00)02676-3. [DOI] [PubMed] [Google Scholar]
- 16.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975–87. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- 17.Glover V, Hill J. Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: An evolutionary perspective. Physiol Behav. 2012;106:736–40. doi: 10.1016/j.physbeh.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Mancini F, Loucks TL, Cameron JL, Berga SL. Sex steroid milieu does not alter the impact of fasting on leptin levels in women. Fertil Steril. 2005;84:1768–71. doi: 10.1016/j.fertnstert.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 19.Yang TT, Hsiao FH, Wang KC, Ng SM, Ho RT, Chan CL, et al. The effect of psychotherapy added to pharmacotherapy on cortisol responses in outpatients with major depressive disorder. J Nerv Ment Dis. 2009;197:401–6. doi: 10.1097/NMD.0b013e3181a61594. [DOI] [PubMed] [Google Scholar]
- 20.Gaab J, Blattler N, Menzi T, Pabst B, Stoyer S, Ehlert U. Randomized controlled evaluation of the effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrinology. 2003;28:767–79. doi: 10.1016/s0306-4530(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 21.de Brouwer SJ, Kraaimaat FW, Sweep FC, Donders RT, Eijsbouts A, van Koulil S, et al. Psychophysiological responses to stress after stress management training in patients with rheumatoid arthritis. PLoS One. 2011;6:e27432. doi: 10.1371/journal.pone.0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindahl MS, Olovsson M, Nyberg S, Thorsen K, Olsson T, Sundstrom Poromaa I. Increased cortisol responsivity to adrenocorticotropic hormone and low plasma levels of interleukin-1 receptor antagonist in women with functional hypothalamic amenorrhea. Fertil Steril. 2007;87:136–42. doi: 10.1016/j.fertnstert.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Genazzani AD, Bersi C, Luisi S, Fruzzetti F, Malavasi B, Luisi M, et al. Increased adrenal steroid secretion in response to CRF in women with hypothalamic amenorrhea. J Steroid Biochem Mol Biol. 2001;78:247–52. doi: 10.1016/s0960-0760(01)00094-2. [DOI] [PubMed] [Google Scholar]
- 24.Meczekalski B, Tonetti A, Monteleone P, Bernardi F, Luisi S, Stomati M, et al. Hypothalamic amenorrhea with normal body weight: ACTH, allopregnanolone and cortisol responses to corticotropin-releasing hormone test. European Journal of Endocrinology. 2000;142:280–5. doi: 10.1530/eje.0.1420280. [DOI] [PubMed] [Google Scholar]
- 25.Saketos M, Sharma N, Santoro NF. Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biol Reprod. 1993;49:1270–6. doi: 10.1095/biolreprod49.6.1270. [DOI] [PubMed] [Google Scholar]
- 26.Samuels MH, Luther M, Henry P, Ridgway EC. Effects of hydrocortisone on pulsatile pituitary glycoprotein secretion. J Clin Endocrinol Metab. 1994;78:211–5. doi: 10.1210/jcem.78.1.8288706. [DOI] [PubMed] [Google Scholar]
- 27.Cumming DC, Quigley ME, Yen SS. Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab. 1983;57:671–3. doi: 10.1210/jcem-57-3-671. [DOI] [PubMed] [Google Scholar]
- 28.Doerr P, Pirke KM. Cortisol-induced suppression of plasma testosterone in normal adult males. J Clin Endocrinol Metab. 1976;43:622–9. doi: 10.1210/jcem-43-3-622. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan JR, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, et al. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum Reprod. 2010;25:3083–94. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol Reprod. 2009;81:1154–63. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology. 2009;150:341–9. doi: 10.1210/en.2008-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breen KM, Oakley AE, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Karsch FJ. Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress? Endocrinology. 2007;148:1882–90. doi: 10.1210/en.2006-0973. [DOI] [PubMed] [Google Scholar]
- 33.Warren MP, Voussoughian F, Geer EB, Hyle EP, Adberg CL, Ramos RH. Functional hypothalamic amenorrhea: hypoleptinemia and disordered eating. J Clin Endocrinol Metab. 1999;84:873–7. doi: 10.1210/jcem.84.3.5551. [DOI] [PubMed] [Google Scholar]
- 34.Loucks AB, Laughlin GA, Mortola JF, Girton L, Nelson JC, Yen SS. Hypothalamic- pituitary-thyroidal function in eumenorrheic and amenorrheic athletes. J Clin Endocrinol Metab. 1992;75:514–8. doi: 10.1210/jcem.75.2.1639953. [DOI] [PubMed] [Google Scholar]
- 35.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–97. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 36.Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1998;83:25–32. doi: 10.1210/jcem.83.1.4502. [DOI] [PubMed] [Google Scholar]
- 37.Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108:6585–90. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuels MH, Kramer P, Wilson D, Sexton G. Effects of naloxone infusions on pulsatile thyrotropin secretion. J Clin Endocrinol Metab. 1994;78:1249–52. doi: 10.1210/jcem.78.5.8175985. [DOI] [PubMed] [Google Scholar]
- 39.Samuels MH, McDaniel PA. Thyrotropin levels during hydrocortisone infusions that mimic fasting-induced cortisol elevations: a clinical research center study. J Clin Endocrinol Metab. 1997;82:3700–4. doi: 10.1210/jcem.82.11.4376. [DOI] [PubMed] [Google Scholar]
- 40.Spratt DI, Kramer RS, Morton JR, Lucas FL, Becker K, Longcope C. Characterization of a prospective human model for study of the reproductive hormone responses to major illness. Am J Physiol Endocrinol Metab. 2008;295:E63–9. doi: 10.1152/ajpendo.00472.2007. [DOI] [PubMed] [Google Scholar]
- 41.Barton RN. The neuroendocrinology of physical injury. Baillieres Clin Endocrinol Metab. 1987;1:355–74. doi: 10.1016/s0950-351x(87)80067-8. [DOI] [PubMed] [Google Scholar]
- 42.Spratt DI, Cox P, Orav J, Moloney J, Bigos T. Reproductive axis suppression in acute illness is related to disease severity. J Clin Endocrinol Metab. 1993;76:1548–54. doi: 10.1210/jcem.76.6.8501163. [DOI] [PubMed] [Google Scholar]
- 43.Bondolfi G, Jermann F, der Linden MV, Gex-Fabry M, Bizzini L, Rouget BW, et al. Depression relapse prophylaxis with Mindfulness-Based Cognitive Therapy: replication and extension in the Swiss health care system. J Affect Disord. 2010;122:224–31. doi: 10.1016/j.jad.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fjorback LO, Arendt M, Ornbol E, Fink P, Walach H. Mindfulness-based stress reduction and mindfulness-based cognitive therapy: a systematic review of randomized controlled trials. Acta Psychiatr Scand. 2011;124:102–19. doi: 10.1111/j.1600-0447.2011.01704.x. [DOI] [PubMed] [Google Scholar]
- 45.Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2007:CD003388. doi: 10.1002/14651858.CD003388.pub3. [DOI] [PubMed] [Google Scholar]
- 46.Griffin MG, Resick PA, Galovski TE. Does physiologic response to loud tones change following cognitive-behavioral treatment for posttraumatic stress disorder? J Trauma Stress. 2012;25:25–32. doi: 10.1002/jts.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40. doi: 10.1186/1471-2296-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berga SL, Loucks TL. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Ann N Y Acad Sci. 2006;1092:114–29. doi: 10.1196/annals.1365.010. [DOI] [PubMed] [Google Scholar]
- 49.Berga SL, Loucks TL. The diagnosis and treatment of stress-induced anovulation. Minerva Ginecol. 2005;57:45–54. [PubMed] [Google Scholar]
- 50.Hurley DM, Brian R, Outch K, Stockdale J, Fry A, Hackman C, et al. Induction of ovulation and fertility in amenorrheic women by pulsatile low-dose gonadotropin-releasing hormone. N Engl J Med. 1984;310:1069–74. doi: 10.1056/NEJM198404263101702. [DOI] [PubMed] [Google Scholar]
- 51.Miller DS, Reid RR, Cetel NS, Rebar RW, Yen SS. Pulsatile administration of low-dose gonadotropin-releasing hormone. Ovulation and pregnancy in women with hypothalamic amenorrhea. Jama. 1983;250:2937–41. [PubMed] [Google Scholar]
- 52.Lavado-Autric R, Auso E, Garcia-Velasco JV, Arufe Mdel C, Escobar del Rey F, Berbel P, et al. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest. 2003;111:1073–82. doi: 10.1172/JCI16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145:4037–47. doi: 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- 54.Pike KM, Rodin J. Mothers, daughters, and disordered eating. J Abnorm Psychol. 1991;100:198–204. doi: 10.1037//0021-843x.100.2.198. [DOI] [PubMed] [Google Scholar]
- 55.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.