Abstract
Conventional diagnostic imaging methods such as X-ray CT, MRI, and nuclear medicine are inherently monochromatic meaning that they can depict only one molecular target at a time. Optical imaging has the unique ability to be polychromatic and therefore multi-color imaging employing targeted agents conjugated to fluorophores of varying wavelength enables multiple simultaneous readouts thus providing greater multiplexed information. Numerous successful multicolor imaging techniques have recently been reported using optical imaging in vivo animal disease models, thus adding to a growing body of research supporting the clinical viability and applicability of these technologies. Herein, we review multicolor optical imaging from the basic chemistry and physics perspective and then extend this to biological and medical applications.
Keywords: Molecular imaging, Fluorescence, Multi-color, Cancer, Surgery, Endoscope, Fluorescence-guidance
1. Introduction
Over the past 20 years, imaging has become integral to clinical oncology for disease detection (e.g. screening, staging etc.), disease characterization (e.g. vascularity, receptor expression etc.), surgical guidance, and evaluation of response to medical therapy. Conventional diagnostic imaging includes x-ray radiography, ultrasonography, x-ray computed tomography, magnetic resonance imaging, and nuclear imaging [1–3]. Given that each technology has advantages and disadvantages, the combined use of different modalities has become standard practice. Fluorescence imaging also has a long history of clinical use, mostly in field of ophthalmology, but has recently been applied specifically in cancer. Fluorescent dyes such as fluorescein or indocyanine green (ICG), have been widely used as contrast agents for chorioretinal fluorescence angiography [4, 5]. However, it is increasingly recognized that fluorescence imaging has unique advantages over conventional imaging modalities. These advantages include the capability of dynamic or real-time imaging without harmful ionizing radiation, comprehensive activatable signaling switched on by target binding, and simultaneous multi-color imaging. Given these strengths, likely is expected that there will be continued growth over the coming years. Many potential clinical applications of fluorescence imaging have been proposed and early results in humans are encouraging. The depth of penetration of the fluorescence is proportionate to the wavelength of the emitted photon. Therefore, fluorophores can be strategically selected for specific clinical applications. Fluorophores within the visible portion of the spectrum have generally been used for fluorescence guidance of surgical and endoscopic procedures. Molecules in this group are generally bright organic fluorophores that can be seen with the unaided eye making them appealing for clinical translation. However, as these fluorpohores have a shorter wavelength, the depth of penetration limits their use to superficial area. Fluorescent molecules that emit light in the near infrared (NIR) portion of the spectrum are advantageous because they allow deeper imaging since NIR light has better tissue penetration and NIR fluorophores are excited at wavelengths with less autofluorescent background signal than visible spectrum fluorophores. The advent of multicolor imaging combining fluorophores across a broader light spectrum has provided an important extra dimension of information that could prove useful when developing applications for complex clinical problems.
Herein, we review advanced technologies and applications of multi-color fluorescence imaging with a focus on cancer detection. We next discuss efforts to design injectable multicolor agents which have potential for clinical application.
2. Basic optical mechanisms for obtaining multi-color imaging
2.1. Synthetic organic fluorophores
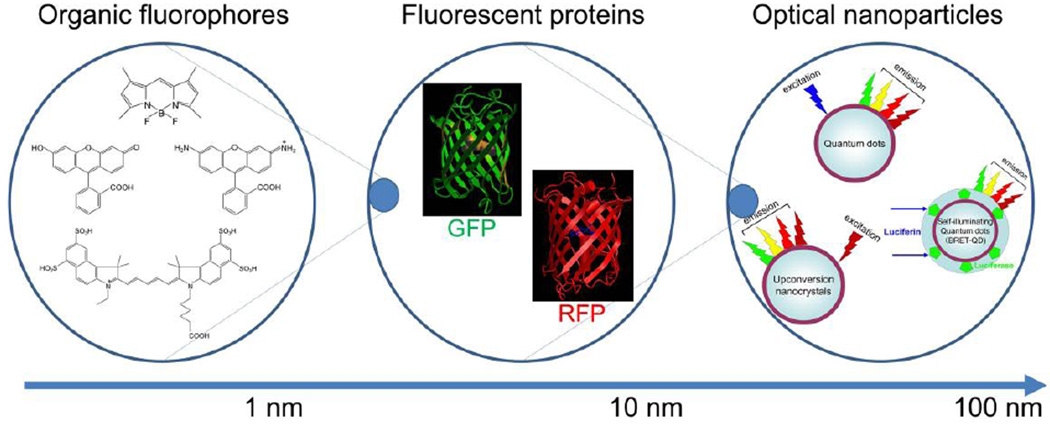
An array of low molecular weight synthetic fluorophores with various core structures including fluorescein-, boron-dipyrromethene (BODIPY)-, rhodamine-, cyanine-derivatives (Figure 1), ranging from 300 Dalton to 2,000 Dalton, are available from commercial providers and span the emission spectrum from blue to NIR. Additionally, research in dye chemistry is rapidly advancing including the ability to employ chemical approaches to control the properties and direct the position of the fluorophore as well as the emergence of strategies enabling efficient tailoring of the chemical structure to obtain probes for specific biological experiments [6, 7]. However, in general, those that are bright, small, hydrophilic and contain no net charge are better candidates for applying to in vivo imaging as these features will minimally influence the pharmacokinetics of the targeting moiety to which they will be conjugated [8]. By conjugating two or more different fluorophores with different targeting moieties against distinct molecules, injectable multicolor probes can be easily synthesized. These synthetic fluorophores usually absorb light at a lower wavelength than they emit light, a phenomenon referred to as the Stokes shift. Most synthetic fluorphores have characteristic emission spectra although some fluorophores have multiple emission spectra. Although we commonly refer to the emission spectra as occurring at a single wavelength (e.g. 690nm) in fact the emitted light has a distribution centered around the peak wavelength. Thus, there can be partial overlap between two fluorophores emission spectra. Therefore, in order to perform multi-color imaging with synthetic organic fluorophores, some technical tricks and post-processing of imaging data are required.
Figure 1.
Schema of variable groups of fluorescent and luminescent materials separated by size.
When two different organic dyes have relatively close but different excitation and emission spectra, both fluorophores can be excited by using single excitation light source. However, distinct emission spectra cannot be not easily distinguished by a single detector such as a CCD camera. Therefore, a series of stepwise acquisitions are obtained by systematically changing either the wavelength of light used for excitation or the wavelength of light that is captured by the camera. This is then followed by creating spectral responses for each fluorophore so that each has a characteristic signature of excitation and emission [9]. By defining the spectral signature of each fluorophore, the fluorophores can be distinguished, a process known as spectral unmixing. In contrast, when two different organic dyes have well separated excitation and emission signals, a single excitation light cannot equally excite both fluorophores. Therefore, multiple excitatios are required but in this case, there will be no overlap on the spectral images. Nonetheless, one uses the same strategy to spectrally resolve the two emission spectra. [10, 11]. When simultaneously employing two different “activatable” probes [12] that emit two distinct fluorescence signals after binding to their respective targets, spectral unmixing is not necessary for obtaining two color images. However, two different wavelengths of light are still needed for excitation [13].
Organic fluorophores with long Stokes shifts (large differences between the excitation and emission) are highly desirable for multi-color imaging. For example, a bacteriocholine-based fluorophore, which has multiple excitation wavelengths at green and NIR was able to depict both surface (using visible emission) and deep (using NIR emission) lesions of peritoneal ovarian cancer thus, satisfying both demands with one agent [14].
2.2. Fluorescent proteins
In general, optical characteristics of fluorescent proteins are similar to organic fluorescent dyes, therefore, similar optical technologies are employed to image them. Similar to organic fluorophores, fluorescent proteins with long Stokes shift have been actively developed and numerous in vivo imaging applications have been developed for use with fluorescent proteins [15]. However, fluorescent proteins must be transfected as genes into cells and then be endogenously produced by the cell over time. This requires stable transfection using viral vectors. The injectable use of fluorescent proteins is therefore, only theoretical [16–19]. Although applications are very flexible, for the medical application, in vivo gene transfection is unlikely to be permitted in humans in the near term.
2.3. Optical nano-particles
The synthesis of nano-crystals is currently a developing field in material science and nanotechnology. Numerous nano-crystals with a broad range of optical properties have recently been reported. Among them, quantum dots (Qdot) are characterized by a broad excitation range, a narrow emission spectra, resistance to photo-bleaching, and ultrahigh brightness [20, 21]. Therefore, Qdots have nearly perfect optical characteristics for performing multi-color imaging in vivo. Unfortunately their large size makes their pharmacokinetics more difficult. Nonetheless, target-specific Qdot conjugates with monoclonal antibodies or peptides have been successfully synthesized and tested [22, 23]. As a further extension of quantum dots, self-illuminating quantum dots, in which the Qdot is excited by bioluminescent light at blue or green part of spectrum produced from energy consumed during the catalysis of a substrate (luciferin or coelenterazine) by an enzyme (luciferase), have been developed. This process, known as bioluminescence resonance energy transfer (BRET) has the capability of producing multicolor imaging by selecting Qdots with different emissions [24, 25]. Note, however, that clinical translation is doubtful because of the requirement for genetic modification of the cell (the LUC gene is inserted in the genome to produce luciferase) and because of the undesirable kinetics of such a large molecule.
Another group of unique optical nano-crystals, which can be used for multi-color imaging, are upconversion nano-crystals. Typical fluorophores emit light at a longer wavelength than their excitation wavelength. Upconverting nanocrystals are unique in that they emit light at shorter wavelengths (visible and/or near infrared) after excitation in the near infrared. This unique emission characteristic dramatically reduces background autofluorescence because endogenous fluorophores are not activated by the longer excitation wavelengths. This results in high target to background ratios along with the good tissue penetration afforded by the use of NIR both for excitation and emission [26].
Another major concern for clinical translation of nano-materials is their potential toxicity. Qdots, for instance, contain heavy metals such as cadmium, selenium, in their core. These fluorescent crystals are generally larger than the renal excretion limit (<6 nm in diameter) and therefore are slowly excreted by the body [27]. Unless a nano-particle can be made especially small [28, 29] and thus is excreted via the kidney, these particles typically have delayed clearance and are mostly excreted through the liver and into the bile without significant metabolism [30]. The long exposure time of the body to heavy metals is of some concern but the absolute amounts are small. To answer this question, formal toxicity studies must be performed. For developing targeted reagents, conjugation with targeting moieties only adds to the size of the particles. Therefore, a major challenge with nanoparticle-based molecular probes is synthesizing agents that are large enough to take advantage of the unique features of nano-particles (e.g. brightness) while minimizing toxicity by having particles small enough to be excreted via the urinary tract.
3. Imaging technology for multi-color imaging
Because of their unique optical characteristics, optical nano-material-based imaging agents allow us to perform multi-color imaging in a relatively simple setting with a single excitation light source and a detector or even the human eye. However, most practical injectable imaging probes are organic fluorophores which will require individual excitation and spectral imaging to resolve the different peaks. Spectral resolved imaging is the most appropriate technology for performing in vivo multi-color imaging (Figure 2A). Now, most of the in vivo fluorescence imagers are equipped with different versions of this technology, which collect data by serially changing either the excitation peak or the band pass filter for emission spectra or both. Among them, the combination of multiple excitation and spectrally-resolved imaging technique is ideal, when performing multi-color imaging with different organic NIR fluorophores [10, 11, 31] (Figure 2B).
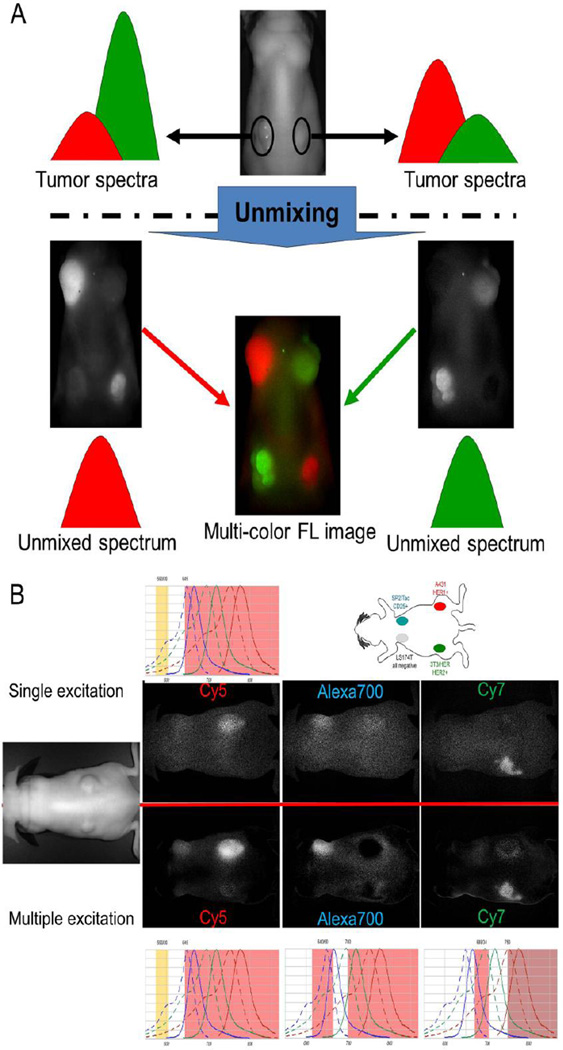
Figure 2.
A. Basis of spectrally resolved multi-color imaging. B. An practical comparison of single excitation versus multiple excitation spectrally resolved multi-color NIR imaging technologies using Cy5-, Alexa Flore 700-, and Cy7-conjugated monoclonal antibodies. Multiple excitation spectrally resolved multi-color NIR images (lower images) showed higher signal-to-noise ratios in all three spectral images than single excitation spectrally resolved multi-color NIR images.
4. Practical examples for in vivo multi-color imaging with medical application: Multicolor Imaging strategies
4.1. Separate injections; different timings, different locations of injection
One approach to multicolor imaging is to inject different probes at different times and in different places to avoid cross contamination of signal. Recently, numerous successful preclinical in vivo multicolor imaging technologies have been reported using this approach. For example, optical imaging has been used for mapping the lymphatic drainage system as part of cancer cell migration studies. A multicolor approach enables simultaneous imaging of cancer cell migration (one color) and lymphatic drainage in vivo (multiple colors depending on how many lymphatic basins are imaged) and may shed insight into the relationship between cell migration and lymphatic-drainage patterns [31].
In addition to the use of exogenous fluorescent probes, transfected fluorescent proteins provide a powerful method for creating endogenous fluorescence for research purposes. This technology is useful for gaining understanding of important processes such as gene expression and cancer biology. When considering multicolored approaches in this setting, however, there is an inherent limitation in that each transfected gene expresses only a single color fluorescent protein. This limitation has recently been overcome using a dehalogenase-based protein-Tag (HaloTag) system in which HaloTag receptors are artificially expressed in cancer cells that can subsequently bind a range of externally injected fluorophore-conjugated dehalogenase-reactive linkers. The halogenated fluorophore probe covalently binds the HaloTag receptor [32]. When this technique was used in vivo, tumors were successfully targeted with four different fluorophore-conjugated HaloTag-ligands, each emitting light at different wavelengths. These fluorophores could be alternated on serial imaging sessions permitting assessment of interval growth. This approach enables in vivo imaging without requiring modification of the underlying cell line [32] (Figure 3).
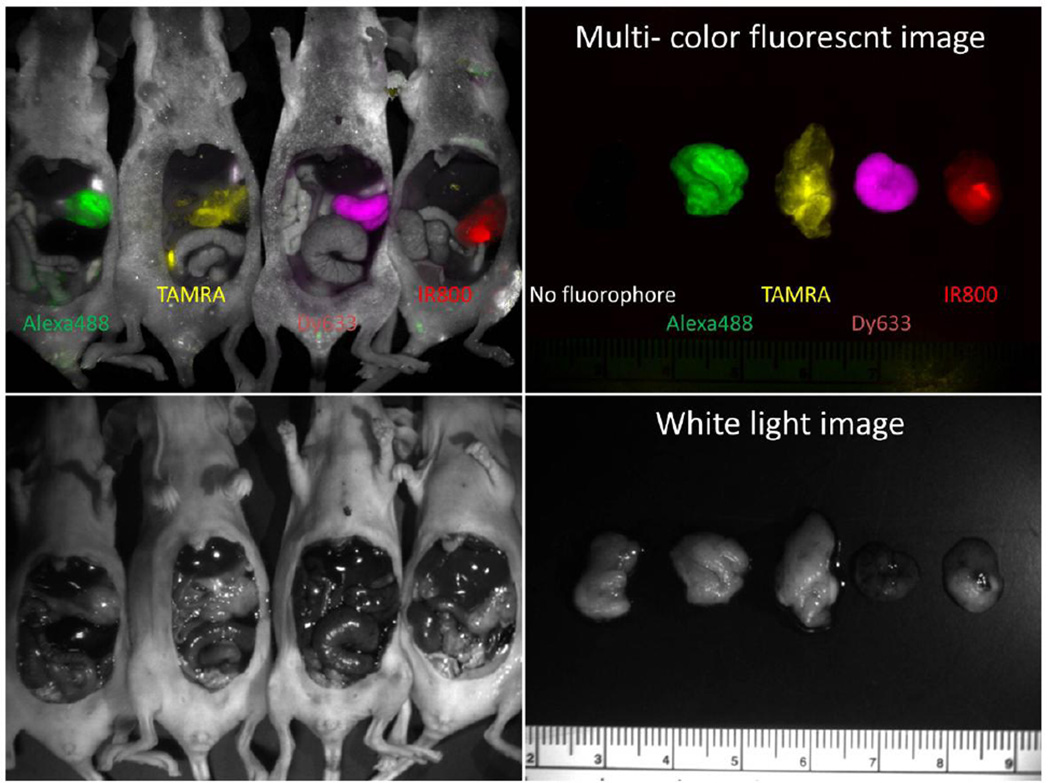
Figure 3.
In vivo four color imaging of a single peritoneally disseminated ovarian tumor (Halo-SHIN3). By using four different fluorophore-conjugated Halo-tag ligands (Alexa Flore 488, TAMRA, Dynamic 633, and IRDye800), tumors produced from a single cell line can display distinct colors.
One recently developed technique involves the use of both endogenous and exogenous fluorescence. To conduct such a study, a plasmid containing the gene encoding red fluorescent protein (RFP) was transfected into cell lines previously developed to either express or not express specific cell surface receptors. Various antibody-based or ligand-based optical-contrast agents, with organic green fluorophores were developed to concurrently target cancer cells and validate their positive and negative controls. Multicolor fluorescence imaging detected distinct fluorescence from the exogenous green fluorophore and from the endogenous RFP which served as a validation tool as a true positive result. By comparing the "staining" pattern and the "counter-staining" (RFP) pattern, the sensitivity and specificity of target-specific optical contrast agents in several distinct animal models of cancer was determined [33] (Figure 4).
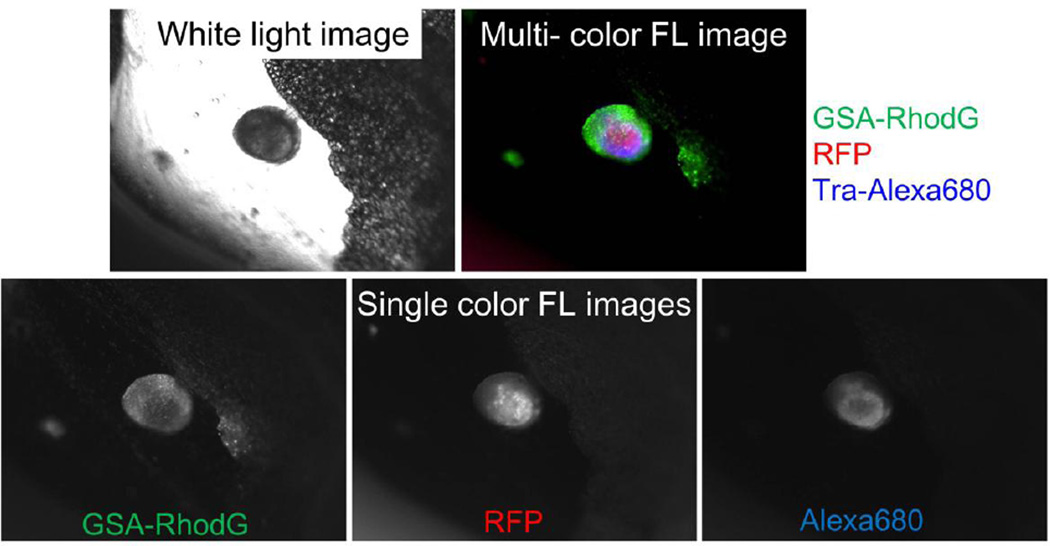
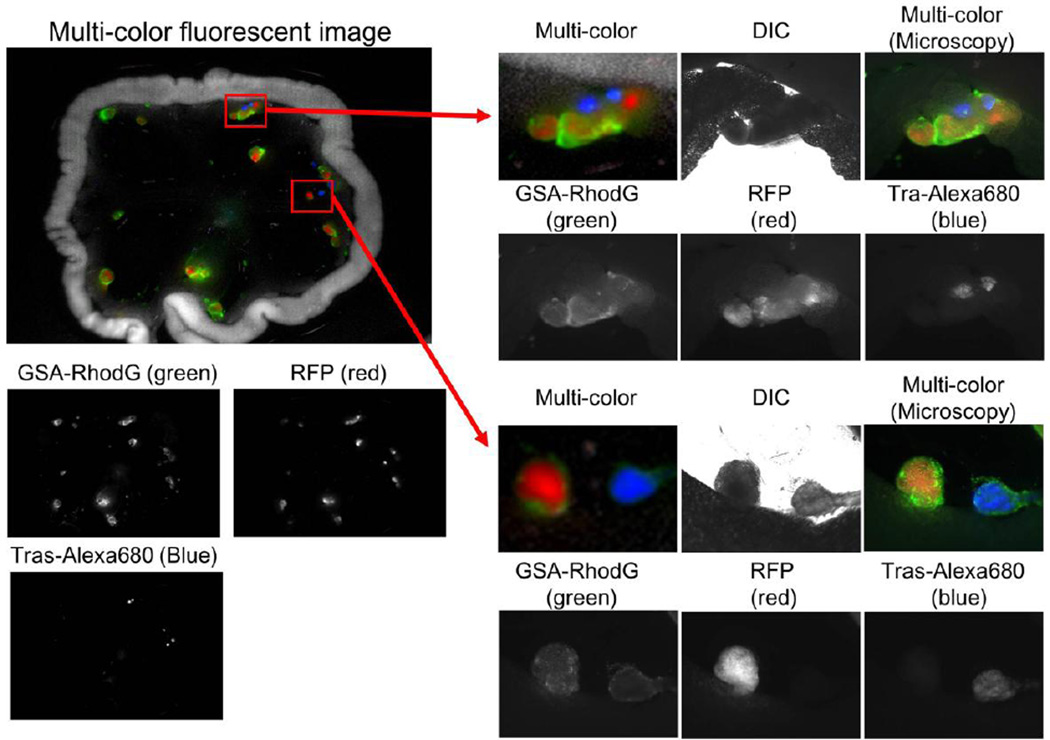
Figure 4.
Multi-color image of a peritoneal ovarian cancer micro-nodule. Intraperitoneally injected GSA-rhodamine Green probe shows mostly the surface tumor nodules. In contrast, intravenously injected trastuzumab-Alexa Fluor 680 only shows HER2+ part of viable cancer cells, which are expressing RFP.
Many new materials are being investigated for multicolor applications. For example, upconversion fluoride nanocrystals can be selected specifically for emission spectra that do not overlap with host tissue autofluorescence and thus will have less background noise. It was recently demonstrated that by employing upconversion fluoride nanocrystals, multiple biological targets and organs could be imaged with high target to background ratios. [31]. Quantum dots have size-dependent optical emission properties that make them uniquely advantageous for in vivo multi-color fluorescence imaging, traceable delivery, and targeted therapy (Figure 5). Efforts are ongoing to reduce potential QD toxicity by protecting from heavy metal deposition. One group demonstrated that a properly encapsulated biocompatible silicone QDs can be used in multiple cancer-related in vivo applications, including tumor vasculature targeting, sentinel lymph node mapping, and multicolor NIR imaging in live mice [34]. Given the continued active research in nanomaterials, science will likely lead to many interesting optical agents in the near future.
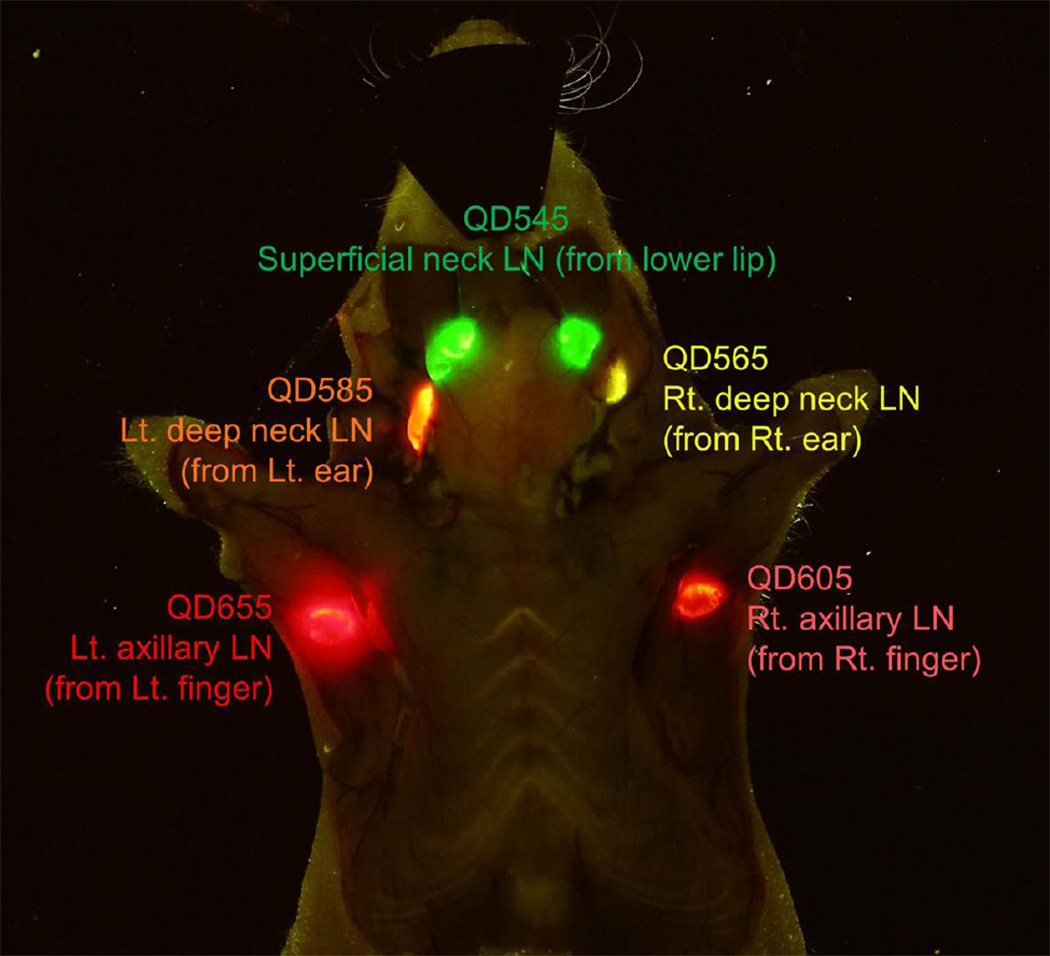
Figure 5.
Visible five color lymphatic drainage imaging by using five different visible Quantum dots (540, 560, 580, 600, 650). The image was taken by a conventional digital camera through a long pass filter, therefore, similar image can be seen by our own eyes.
4.2. The Multicolor Cocktail
Multicolor optical imaging can also be achieved using a cocktail of differently colored probes, each potentially homing to a unique target. Optical imaging using a cocktail of unique probes enables a tissue “signature” to be derived from a single imaging session. This clearly provides more data than simply a “yes or no” imaging method. To allow differentiation of multiple optical signals in vivo, each probe should have a different near-infrared emission. This technique is well suited for clinical applications such as, typing specific receptors or molecules. Tumors express a multitude of cell surface receptors which can help define their aggressiveness and prognosis as well as guide targeted therapy. Multicolor imaging offers the ability to gain insight into multiple dimensions of the molecular milieu whereas conventional monochromatic imaging can only characterize a single molecular profile per imaging session. Using pectrally resolved optical imaging with different fluorescently labeled antibodies it is possible to differentiate between different subtypes of the EGFR receptor in vivo by simultaneously imaging multiple cell surface receptors [11] (Figure 6).
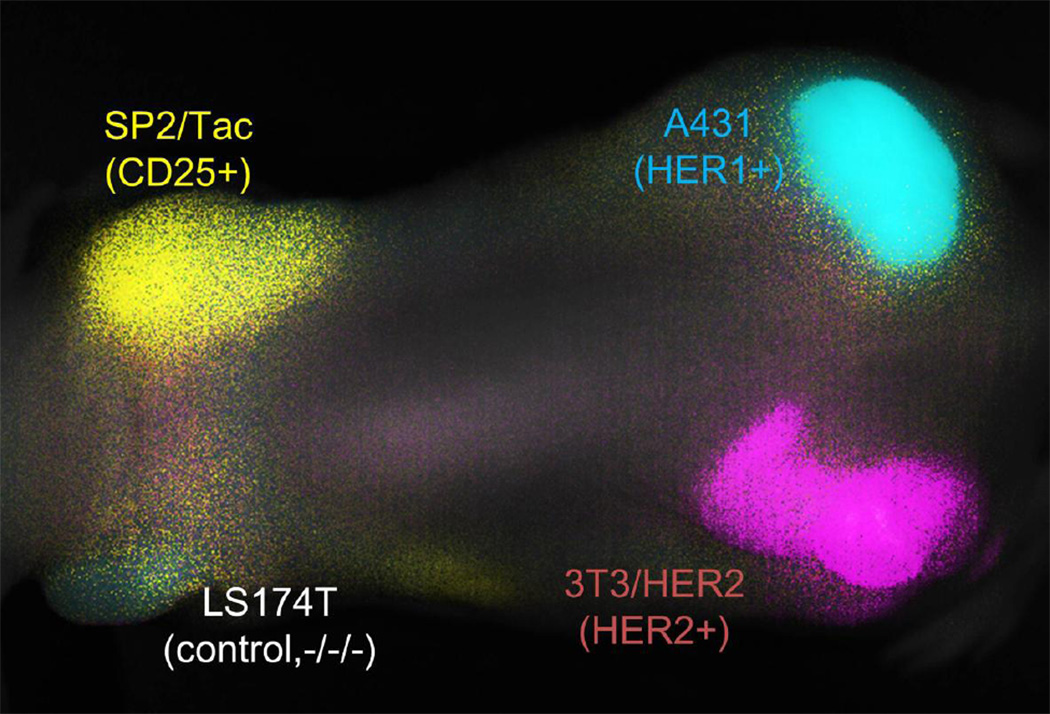
Figure 6.
A “cocktail” of three antibody-NIR fluorophore conjugates can simultaneously diagnose tumors expressing distinct receptors. A431 (EGFR+), 3T3/HER2 (HER2+), SP2/Tac (CD25+) and LS174T (triple negative control) were subcutaneously implanted. The ‘cocktail’ of daclizumab-Alexa700 (anti-CD25), trastuzumab-Cy7 (anti-HER2) and cetuximab-Cy5.5 (anti-HER1) was injected 24 hours prior to imaging. The respective tumors with their distinct receptors could be simultaneously distinguished by spectral unmixing: A431 (HER1) in cyan; 3T3/HER2+ (HER2) in magenta; SP2/Tac (CD25) in yellow; LS174T is seen as colorless.
In addition to using different colored fluorophores multiple modalities can also be employed to achieve multiparametric imaging. As an example, a single nanoparticle is loaded with multiple payloads that can be imaged with optical, MRI or radionuclide cameras. Such multimodality probes can compensate for the limitations of any single imaging modality. Using a polyamidoamine dendrimer platform linked to both radionuclides and optical probes, nanoprobes with multimodal and multicolor potential, were created and permitted dual-modality scintigraphic and five-color near-infrared optical lymphatic imaging using a multiple-excitation spectrally resolved fluorescence imaging technique [35].
Multicolor imaging is also potentially useful in drug development. When using antibody-based therapies in cancer, uniform antibody microdistribution throughout the tumor is important for achieving optimal therapeutic effects. By optically labeling antibodies, microdistribution studies can be performed to aid in therapy optimization. For example, by optically labeling trastuzumab and trastuzumab-Fab fragments with differently colored fluorophores, the antibody structure and dose that yielded optimal tumor microdistribution could be determined in a murine model of ovarian cancer. Additionally, the microdistribution effects of different dosing regimens, varying in time and drug concentration, could be studied since multicolor imaging permitted multiple parameters of data to be evaluated [36].
5. Real-time applications for surgery or endoscopy
Optical imaging has great potential for intraoperative or endoscopic settings as there is no risk of ionizing radiation exposure to the practitioner or patient and it is a relatively affordable technology. Optical imaging can serve as an intraoperative guide for identifying tumor nodules or as a tool for identifying key anatomic structures to help prevent accidental damage during invasive procedures. Multicolor imaging in this setting provides a powerful method for characterizing tissue based on its molecular signature, for instance, helping in differentiating inflammatory changes from cancer, as well as simultaneously imaging important anatomic structures and diseased tissue. The viability of intraoperative multicolored imaging was demonstrated in a novel 'coincident' ovarian cancer mouse model that simultaneously harbored tumors with two distinct molecular phenotypes. The HER2 receptor of one tumor cell line was targeted with a trastuzumab-rhodamine green conjugate to create green tumor implants, whereas an RFP plasmid was used to identify a separate phenotype. Real-time multicolor image-guided surgery was then performed in vivo to demonstrate that this technology could be used intraoperatively to differentiate the two tumor types based on their multicolor fluorescence [37] (Figure 7).
Figure 7.
Multi-color fluorescence-guided simulation surgery of HER2+ and HER2− peritoneally disseminated ovarian cancers. SHIN3-RFP (HER2−/RFP+) and SKOV3 (HER2+/RFP−) tumors were grown in the abdomen of a mouse. GSA-rhodamine Green probe detects both tumors shown in green. Trastuzumab-Alexa Fluor 680 probe shows only HER2+/RFP− tumors shown in blue.
A vexing problem in medicine is that the margin of a cancer is often not clear yet, success of a surgical removal depends highly on whether the margin is positive or not. Additionally, if the cancer is affecting critical structures such as the brain, it is critical to treat only the tumor and not remove any healthy tissue. Given these conflicting goals, one that encourages generous margins and the other that encourages strict margins, optical imaging is an active area of research. One approach relies on oral 5-aminolevulinic acid (5-ALA) as contrast agent for brain tumor resection. 5-ALA is a prodrug that is metabolized to form the fluorescent protoporphyrin IX. This metabolite demonstrates accumulation in tumor cells and other tissues. Following excitation, the compound emits a red-violet light of 635 nm, and the surgeon is then able to selectively resect the red-violet tumor tissue [38]. A similar approach can be used for real time multicolor optical imaging of malignant tumors of the skin, breast, head and neck region, and urinary bladder [39]. Traditional optical imaging with single color is gaining considerable traction as an intraoperative aid in head and neck cancer surgery as well as in the management of breast cancer [40–46]. Given this, multicolor strategies could be an important next step in furthering these clinical advances.
6. Summary
Multicolor optical imaging is set to make important strides in the shift to using optical guidance for interventions from uniparametric to multiparametric. The rich and multidimensional nature of this data offers many advantages to solving complex clinical problems wherein simultaneous imaging of spatially and anatomically similar structures is often needed. Additionally, as medicine continues to become molecularly focused, multicolor optical imaging has the potential to play an important role intraoperative molecular characterization of disease and treatment.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frangioni JV. New technologies for human cancer imaging. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4012–4021. doi: 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi H, Longmire MR, Ogawa M, Choyke PL. Rational chemical design of the next generation of molecular imaging probes based on physics and biology: mixing modalities, colors and signals. Chemical Society reviews. 2011;40:4626–4648. doi: 10.1039/c1cs15077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi H, Longmire MR, Ogawa M, Choyke PL, Kawamoto S. Multiplexed imaging in cancer diagnosis: applications and future advances. The lancet oncology. 2010;11:589–595. doi: 10.1016/S1470-2045(10)70009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brancato R, Trabucchi G. Fluorescein and indocyanine green angiography in vascular chorioretinal diseases. Seminars in ophthalmology. 1998;13:189–198. doi: 10.3109/08820539809056052. [DOI] [PubMed] [Google Scholar]
- 5.Flower RW, Hochheimer BF. Indocyanine green dye fluorescence and infrared absorption choroidal angiography performed simultaneously with fluorescein angiography. The Johns Hopkins medical journal. 1976;138:33–42. [PubMed] [Google Scholar]
- 6.Wysocki LM, Lavis LD. Advances in the chemistry of small molecule fluorescent probes. Current opinion in chemical biology. 2011;15:752–759. doi: 10.1016/j.cbpa.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Boens N, Leen V, Dehaen W. Fluorescent indicators based on BODIPY. Chemical Society reviews. 2012;41:1130–1172. doi: 10.1039/c1cs15132k. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chemical reviews. 2010;110:2620–2640. doi: 10.1021/cr900263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levenson RM, Lynch DT, Kobayashi H, Backer JM, Backer MV. Multiplexing with multispectral imaging: from mice to microscopy, ILAR journal / National Research Council. Institute of Laboratory Animal Resources. 2008;49:78–88. doi: 10.1093/ilar.49.1.78. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Koyama Y, Barrett T, Hama Y, Regino CA, Shin IS, Jang BS, Le N, Paik CH, Choyke PL, Urano Y. Multimodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. ACS nano. 2007;1:258–264. doi: 10.1021/nn700062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyama Y, Barrett T, Hama Y, Ravizzini G, Choyke PL, Kobayashi H. In vivo molecular imaging to diagnose and subtype tumors through receptor-targeted optically labeled monoclonal antibodies. Neoplasia. 2007;9:1021–1029. doi: 10.1593/neo.07787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi H, Choyke PL. Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications. Accounts of chemical research. 2011;44:83–90. doi: 10.1021/ar1000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sano K, Mitsunaga M, Nakajima T, Choyke PL, Kobayashi H. In vivo breast cancer characterization imaging using two monoclonal antibodies activatably labeled with near infrared fluorophores. Breast cancer research : BCR. 2012;14:R61. doi: 10.1186/bcr3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander VM, Sano K, Yu Z, Nakajima T, Choyke PL, Ptaszek M, Kobayashi H. Galactosyl Human Serum Albumin-NMP1 Conjugate: A Near Infrared (NIR)-Activatable Fluorescence Imaging Agent to Detect Peritoneal Ovarian Cancer Metastases. Bioconjugate chemistry. 2012 doi: 10.1021/bc3002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 16.Bouvet M, Hoffman RM. Glowing tumors make for better detection and resection. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3003375. 110fs110. [DOI] [PubMed] [Google Scholar]
- 17.Kishimoto H, Urata Y, Tanaka N, Fujiwara T, Hoffman RM. Selective metastatic tumor labeling with green fluorescent protein and killing by systemic administration of telomerase-dependent adenoviruses. Molecular cancer therapeutics. 2009;8:3001–3008. doi: 10.1158/1535-7163.MCT-09-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishimoto H, Zhao M, Hayashi K, Urata Y, Tanaka N, Fujiwara T, Penman S, Hoffman RM. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14514–14517. doi: 10.1073/pnas.0906388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsunaga M, Kosaka N, Kines RC, Roberts JN, Lowy DR, Schiller JT, Ishihara Y, Hasegawa A, Choyke PL, Kobayashi H. In vivo longitudinal imaging of experimental human papillomavirus infection in mice with a multicolor fluorescence mini-endoscopy system. Cancer Prev Res (Phila) 2011;4:767–773. doi: 10.1158/1940-6207.CAPR-10-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alivisatos AP. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science. 1996;271:933–937. [Google Scholar]
- 21.Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and Properties of Nanocrystals of Different Shapes. Chem Rev. 2005;105:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 22.Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotech. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 23.Giepmans BNG, Deerinck TJ, Smarr BL, Jones YZ, Ellisman MH. Correlated light and electron microscopic imaging of multiple endogenous proteins using Quantum dots. Nat Meth. 2005;2:743–749. doi: 10.1038/nmeth791. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Yan, So M-k, Loening AM, Yao H, Gambhir SS, Rao J. HaloTag Protein-Mediated Site-Specific Conjugation of Bioluminescent Proteins to Quantum Dots13. Angew Chem Int Ed Engl. 2006;45:4936–4940. doi: 10.1002/anie.200601197. [DOI] [PubMed] [Google Scholar]
- 25.Yao Hequan, Zhang Y, Xiao F, Xia Z, Rao J. Quantum Dot/Bioluminescence Resonance Energy Transfer Based Highly Sensitive Detection of Proteases13. Angewandte Chemie International Edition. 2007;46:4346–4349. doi: 10.1002/anie.200700280. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Yu WW, Zhang J, Aldana J, Peng X, Xiao M. Photoluminescence upconversion in colloidal CdTe quantum dots. Phys Rev B. 2003;68:125318. [Google Scholar]
- 27.Ohlson M, Sorensson J, Haraldsson B. A gel-membrane model of glomerular charge and size selectivity in series. Am J Physiol Renal Physiol. 2001;280:F396–F405. doi: 10.1152/ajprenal.2001.280.3.F396. [DOI] [PubMed] [Google Scholar]
- 28.Zimmer JP, Kim SW, Ohnishi S, Tanaka E, Frangioni JV, Bawendi MG. Size series of small indium arsenide-zinc selenide core-shell nanocrystals and their application to in vivo imaging. Journal of the American Chemical Society. 2006;128:2526–2527. doi: 10.1021/ja0579816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SW, Zimmer JP, Ohnishi S, Tracy JB, Frangioni JV, Bawendi MG. Engineering InAs(x)P(1−x)/InP/ZnSe III–V alloyed core/shell quantum dots for the near-infrared. Journal of the American Chemical Society. 2005;127:10526–10532. doi: 10.1021/ja0434331. [DOI] [PubMed] [Google Scholar]
- 30.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomed. 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi H, Ogawa M, Kosaka N, Choyke PL, Urano Y. Multicolor imaging of lymphatic function with two nanomaterials: quantum dot-labeled cancer cells and dendrimer-based optical agents. Nanomedicine (Lond) 2009;4:411–419. doi: 10.2217/nnm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosaka N, Ogawa M, Choyke PL, Karassina N, Corona C, McDougall M, Lynch DT, Hoyt CC, Levenson RM, Los GV, Kobayashi H. In vivo stable tumor-specific painting in various colors using dehalogenase-based protein-tag fluorescent ligands. Bioconjugate chemistry. 2009;20:1367–1374. doi: 10.1021/bc9001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCann TE, Kosaka N, Choyke PL, Kobayashi H. The use of fluorescent proteins for developing cancer-specific target imaging probes. Methods Mol Biol. 2012;872:191–204. doi: 10.1007/978-1-61779-797-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erogbogbo F, Yong KT, Roy I, Hu R, Law WC, Zhao W, Ding H, Wu F, Kumar R, Swihart MT, Prasad PN. In vivo targeted cancer imaging, sentinel lymph node mapping and multi-channel imaging with biocompatible silicon nanocrystals. ACS nano. 2011;5:413–423. doi: 10.1021/nn1018945. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi H, Koyama Y, Barrett T, Hama Y, Regino CA, Shin IS, Jang BS, Le N, Paik CH, Choyke PL, Urano Y. Multimodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. ACS Nano. 2007;1:258–264. doi: 10.1021/nn700062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosaka N, Ogawa M, Paik DS, Paik CH, Choyke PL, Kobayashi H. Semiquantitative assessment of the microdistribution of fluorescence-labeled monoclonal antibody in small peritoneal disseminations of ovarian cancer. Cancer science. 2010;101:820–825. doi: 10.1111/j.1349-7006.2009.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longmire M, Kosaka N, Ogawa M, Choyke PL, Kobayashi H. Multicolor in vivo targeted imaging to guide real-time surgery of HER2-positive micrometastases in a two-tumor coincident model of ovarian cancer. Cancer science. 2009;100:1099–1104. doi: 10.1111/j.1349-7006.2009.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadjipanayis CG, Jiang H, Roberts DW, Yang L. Current and future clinical applications for optical imaging of cancer: from intraoperative surgical guidance to cancer screening. Seminars in oncology. 2011;38:109–118. doi: 10.1053/j.seminoncol.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svanberg K, Wang I, Colleen S, Idvall I, Ingvar C, Rydell R, Jocham D, Diddens H, Bown S, Gregory G, Montan S, Andersson-Engels S, Svanberg S. Clinical multi-colour fluorescence imaging of malignant tumours--initial experience. Acta Radiol. 1998;39:2–9. doi: 10.1080/02841859809172141. [DOI] [PubMed] [Google Scholar]
- 40.Helman EE, Newman JR, Dean NR, Zhang W, Zinn KR, Rosenthal EL. Optical imaging predicts tumor response to anti-EGFR therapy. Cancer biology & therapy. 2010;10:166–171. doi: 10.4161/cbt.10.2.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keereweer S, Mieog JS, Mol IM, Van Driel PB, Snoeks TJ, Baatenburg de Jong RJ, Vahrmeijer AL, Kerrebijn JD, Lowik CW. Detection of oral squamous cell carcinoma and cervical lymph node metastasis using activatable near-infrared fluorescence agents. Archives of otolaryngology--head & neck surgery. 2011;137:609–615. doi: 10.1001/archoto.2011.89. [DOI] [PubMed] [Google Scholar]
- 42.Keereweer S, Kerrebijn JD, Mol IM, Mieog JS, Van Driel PB, Baatenburg de Jong RJ, Vahrmeijer AL, Lowik CW. Optical imaging of oral squamous cell carcinoma and cervical lymph node metastasis. Head & neck. 2012;34:1002–1008. doi: 10.1002/hed.21861. [DOI] [PubMed] [Google Scholar]
- 43.Keereweer S, Hutteman M, Kerrebijn JD, van de Velde CJ, Vahrmeijer AL, Lowik CW. Translational optical imaging in diagnosis and treatment of cancer. Current pharmaceutical biotechnology. 2012;13:498–503. doi: 10.2174/138920112799436294. [DOI] [PubMed] [Google Scholar]
- 44.Melancon MP, Lu W, Zhong M, Zhou M, Liang G, Elliott AM, Hazle JD, Myers JN, Li C, Stafford RJ. Targeted multifunctional gold-based nanoshells for magnetic resonance-guided laser ablation of head and neck cancer. Biomaterials. 2011;32:7600–7608. doi: 10.1016/j.biomaterials.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel R, Khan A, Wirth D, Kamionek M, Kandil D, Quinlan R, Yaroslavsky AN. Multimodal optical imaging for detecting breast cancer. Journal of biomedical optics. 2012;17 doi: 10.1117/1.JBO.17.6.066008. 066008. [DOI] [PubMed] [Google Scholar]
- 46.Walsh A, Cook RS, Rexer B, Arteaga CL, Skala MC. Optical imaging of metabolism in HER2 overexpressing breast cancer cells. Biomedical optics express. 2012;3:75–85. doi: 10.1364/BOE.3.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]