Abstract
Objective
To estimate 12-month prevalence rate of mood, anxiety, and alcohol-use disorders among community samples of diabetic persons. We assess whether associations of specific mental disorders with diabetes are consistent across diverse countries after controlling for age and gender.
Research design and methods
Eighteen surveys of household-residing adults were conducted in two phases across 17 countries in Europe, the Americas, the Middle East, Africa, Asia, and the South Pacific (Part 1, N=85,088). Mental disorders, identified by the World Mental Health–Composite International Diagnostic Interview, included anxiety disorders (generalized anxiety disorder, panic disorder/agoraphobia, posttraumatic stress disorder, and social phobia), mood disorders (dysthymia and major depressive disorder), and alcohol abuse/dependence. Diabetes was ascertained by self-report (Part 2, N=42,697). Association was assessed by age–gender adjusted odds ratios.
Results
Risk of mood and anxiety disorders was slightly higher among persons with diabetes relative to those without: odds ratio of 1.38 for depression (95% CI=1.15–1.66) and 1.20 for anxiety disorders, (95 % CI=1.01–1.42), after adjusting for age and gender. Odds ratio estimates across countries did not differ more than chance expectation. Alcohol-use disorders were uncommon among persons with diabetes in most countries, and not associated with diabetes in pooled survey data.
Conclusions
Population sample surveys revealed mood and anxiety disorders occurred with somewhat greater frequency among persons with diabetes than those without diabetes. Prevalence of major depression among persons with diabetes was lower in the general population than suggested by prior studies of clinical samples. Strength of association did not differ significantly across disorders or countries.
Keywords: Anxiety, Co-morbidity, Depression, Diabetes, World health
Introduction
The World Health Organization estimates that diabetes will affect more than 350 million persons worldwide by 2030, with the number of persons affected more than doubling from the year 2000 [1]. Rising diabetes incidence among younger age groups worldwide is magnifying the adverse impact of this chronic illness and its complications [2,3]. The cost of diabetes extends beyond individual disability and increased mortality, to include the societal burdens of lost productivity and increased health care costs [4].
Prevalence studies in both general populations and clinical settings have shown that depression is more common among diabetes patients (both Type 1 and Type 2), with a 12-month prevalence rate estimates typically falling in the 10–15% range [5,6]. Longitudinal epidemiologic studies provide strong evidence that depression increases the risk of developing diabetes [7–9]. Depression–diabetes comorbidity is associated with adverse diabetes outcomes, functional disability, increased mortality, and increased health care costs [10–15].
Recent studies suggest that anxiety disorders may also be associated with less favorable glycemic control among adults with diabetes [16,17]. A systematic review found that elevated anxiety symptoms were present in 40% of patients with diabetes who participated in clinical studies [18]. Generalized anxiety disorder, a common anxiety disorder, has been reported to be present in as many as 14% of patients with diabetes [18].
Population-based studies differ in their estimates of the magnitude of the association of diabetes and depression, with estimates ranging from slight differences to a two-fold increase in risk [19,20]. Methodological differences across these studies included sample size, methods of case identification of depression and of diabetes, sample characteristics, and whether a prospective or cross-sectional design was employed. Chance variation in estimates of the strength of association may, of course, also be a factor contributing to differences across studies.
Using general population samples from 18 surveys participating in the World Mental Health (WMH) Surveys, we provide new information regarding the occurrence of common mental disorders among persons reporting diabetes. The objectives of this paper are (1) to estimate the prevalence of specific mood, anxiety, and alcohol use disorders among persons with diabetes in general population samples of adults from diverse countries; (2) to determine which kinds of mental disorder are most strongly associated with diabetes after controlling for age and gender; and (3) to assess whether the associations of specific mental disorders with diabetes are consistent across adult populations in diverse countries of Europe, the Americas, Asia, Middle East, Africa, and the South Pacific. This paper provides the first cross-national comparison of the occurrence of mood, anxiety, and alcohol use disorders among persons with diabetes in community samples. It provides a global perspective on the associations of mental disorders with diabetes across culturally and socioeconomically diverse countries.
Methods
Samples
From 2001 to 2004, 18 surveys were carried out in 17 countries (N=85,088) in the Americas (Colombia, Mexico, United States), Europe (Belgium, France, Germany, Italy, the Netherlands, Spain, Ukraine), the Middle East (Israel, Lebanon), Africa (Nigeria, South Africa), Asia (Japan, separate surveys in Beijing and Shanghai in the People's Republic of China), and the South Pacific (New Zealand). All surveys were based on multistage, clustered area probability household samples. All interviews were carried out face-to-face by trained lay interviewers. The six Western European surveys were carried out jointly [21]. Sample size ranged from 2372 (the Netherlands) to 12,992 (New Zealand), with a total of 85,088 participating adults. Response rates range from 45.9% (France) to 87.7% (Colombia), with a weighted average of 70.8 %.
Internal sub-sampling was used to reduce respondent burden by dividing the interview into two parts. Part 1 included the core diagnostic assessment of mental disorders. All respondents completed Part 1. All Part 1 respondents who met criteria for any mental disorder and a probability sample of other respondents were administered Part 2 (N=42,697). Part 2 included additional information relevant to a wide range of survey aims, including assessment of chronic physical conditions. Part 2 respondents were weighted by the inverse of their probability of selection for Part 2 of the interview to adjust for differential sampling. Analyses in this article were based on the weighted Part 2 sample. The samples showed appreciable cross-national differences in age structure (younger in less developed countries) and educational status (lower in less developed countries).
Training and field procedures
The central WMH staff trained bilingual supervisors in each country. Consistent interviewer training documents and procedures were used across surveys. The WHO translation protocol was used to translate instruments and training materials. Interviews were carried out in the dominant language(s) of the regions where the surveys were conducted. Standardized descriptions of the goals and procedures of the study, data uses and protection, and the rights of respondents were provided in both written and verbal form to all potentially eligible respondents before obtaining verbal informed consent for participation in the survey. Quality control protocols, described in more detail elsewhere [22], were standardized across countries to check on interviewer accuracy and to specify data cleaning and coding procedures. The institutional review board of the organization that coordinated the survey in each country approved and monitored compliance with procedures for obtaining informed consent and protecting human subjects.
Mental disorder status
All surveys used the WMH Survey version of the WHO Composite International Diagnostic Interview [23], a fully structured diagnostic interview, to assess disorders and treatment. Disorders considered in this paper include anxiety disorders (generalized anxiety disorder, panic disorder and/or agoraphobia, posttraumatic stress disorder, and social phobia), mood disorders (dysthymia and major depressive disorder), and alcohol abuse or dependence. The analyses in this paper employed mental disorders reported present in the prior 12 months. Disorders were assessed using the definitions and criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV, SCID) [24]. CIDI medical exclusion rules were imposed in making all diagnoses. Studies of cross-national reliability and validity comparing the WMH-CIDI and SCID showed concordance for anxiety disorder and any mood disorder was high (AUC=0.88, and 0.83 respectively) [22].
Diabetes status
In a series of questions about chronic conditions adapted from the US Health Interview Survey [25], respondents were asked about the presence of selected chronic conditions. Respondents were asked whether a doctor or other health professional had ever told them they had diabetes, and any treatment received in the prior 12 months. While the validity of self-report of diabetes has not been assessed in cross-national research, an evaluation of self-report of chronic conditions in the US National Health Interview Survey found that self-report of diabetes showed very high agreement with medical records data (kappa=0.82) [25]. Data from Taiwan also showed that self-report of diabetes yielded high agreement (kappa=0.86) when compared with physical examination and glycosylated hemoglobin [26]. However, diabetes self-report consistently underestimated diabetes prevalence when compared to medical or laboratory records [25]. This underestimation is likely to be increased among developing countries with less access to medical services [27–30].
Analytic methods
This paper reports prevalence rates for specific mental disorders among persons with and without diabetes. Odds ratios and 95% confidence interval (CI) for the association of a mental disorder with diabetes (adjusted for age and gender) were estimated using logistic regression for each survey with at least 25 respondents who reported diabetes. (Nigeria had fewer than 25 respondents with diabetes.) Odds ratios were also reported as nonestimable if any of the cell values of the cross-classification table were zero. Ninety-five percent CIs for the prevalence rates and for the odds ratios were estimated using the Taylor Series method [31] with SUDAAN software [32] to adjust for clustering and weighting. With the use of meta-analytic methods to summarize results across surveys, pooled estimates of the odds ratios were developed describing the association of each mental disorder with diabetes across surveys. The pooled estimate of the odds ratio was weighted by the inverse of the variance of the estimate for each survey, to reflect the relative sample sizes of the 18 surveys. Confidence intervals of the pooled odds ratio estimates were estimated [33]. For each association of a specific mental disorder with diabetes, we assessed whether the heterogeneity of the odds ratio estimates across surveys was greater than that expected by chance. Since these tests were consistently nonsignificant, we concluded that pooled estimates of the odds ratios, and CIs for the pooled estimates, could be appropriately reported. A funnel graph (Fig. 1) plots the odds ratio estimates on a log scale (y-axis) against the precision of the estimate of each odds ratio (x-axis) [34] at varying levels of precision (i.e., the reciprocal of the standard error of the odds ratio estimate). Precision increases as the standard error of the estimate becomes smaller. The “funnel” in these graphs shows the band around that pooled estimate that would include survey odds ratios whose 95% CI included the pooled estimate, at varying levels of precision. On this graph, the less precise estimates are to the left (where the funnel is wider), and the more precise estimates are to the right (where the funnel is narrower). These graphs provide a visual summary of the association of any mood disorder and any anxiety disorders with diabetes across the participating surveys.
Fig. 1.
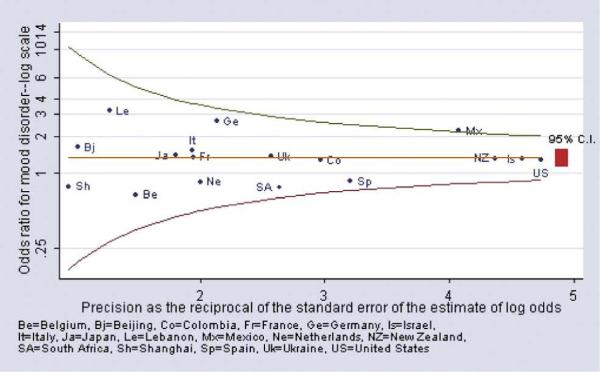
Mood disorder among persons with vs. without diabetes (age-sex adjusted odds ratio).
Results
Sample characteristics
Across the 18 surveys, with 42,697 second-stage respondents providing information on diabetes status, diabetes was reported by 2212 persons with weighted prevalence rates ranging from 0.5% in Nigeria to 8.1% in Israel (see Table 1). As expected, developed countries had populations that tended to be older and more highly educated. Among persons reporting diabetes, countries with more access to medical services had slightly higher treatment rates (94%) than countries with less access to medical services (77%).
Table 1.
Sample characteristics and diabetes prevalencea
| Country | National sample (N) | Mean ageb | % 60 years or older | % Women | Education secondary or greater, % | Diabetes prevalencec |
|
|---|---|---|---|---|---|---|---|
| Prevalence (n) | Weighted % | ||||||
| Americas | |||||||
| Colombia | 2381 | 36.6 | 5.3 | 54.5 | 46.4 | 72 | 3.3 |
| Mexico | 2362 | 35.2 | 5.2 | 52.3 | 31.4 | 128 | 4.3 |
| United States | 5692 | 45.0 | 21.2 | 53.0 | 83.2 | 413 | 7.2 |
| Asia and South Pacific | |||||||
| Japan | 887 | 51.4 | 34.9 | 53.7 | 70.0 | 60 | 6.5 |
| PRC | |||||||
| Beijing | 914 | 39.8 | 15.6 | 47.5 | 61.4 | 55 | 4.7 |
| Shanghai | 714 | 42.9 | 18.7 | 48.1 | 46.8 | 28 | 4.1 |
| New Zealand | 7312 | 44.6 | 20.7 | 52.2 | 60.4 | 434 | 4.9 |
| Europe | |||||||
| Belgium | 1043 | 46.9 | 27.3 | 51.7 | 69.7 | 42 | 4.0 |
| France | 1436 | 46.3 | 26.5 | 52.2 | NA | 58 | 4.3 |
| Germany | 1323 | 48.2 | 30.6 | 51.7 | 96.4 | 63 | 4.7 |
| Italy | 1779 | 47.7 | 29.2 | 52.0 | 39.5 | 63 | 3.5 |
| Netherlands | 1094 | 45.0 | 22.7 | 50.9 | 69.7 | 68 | 7.0 |
| Spain | 2121 | 45.5 | 25.5 | 51.4 | 41.7 | 148 | 5.8 |
| Ukraine | 1720 | 46.1 | 27.3 | 55.1 | 79.5 | 60 | 2.8 |
| Middle East and Africa | |||||||
| Lebanon | 602 | 40.3 | 15.3 | 48.1 | 40.5 | 50 | 5.2 |
| Nigeria | 2143 | 35.8 | 9.7 | 51.0 | 35.6 | 14 | 0.5 |
| Israel | 4859 | 44.4 | 20.3 | 51.9 | 78.3 | 423 | 8.1 |
| South Africa | 4315 | 37.1 | 8.8 | 53.6 | 38.9 | 233 | 5.6 |
Sample restricted to Part II of World Mental Health Surveys.
Age range ≥18, except for Colombia, Mexico (18–65), Japan (≥20), and Israel (≥21).
Lifetime prevalence reported.
Mood disorders
Major depression was generally common among persons with diabetes (Table 2). Among persons with diabetes, prevalence estimates of major depression ranged from 1.5% in Shanghai to 19.5% in the Ukraine, with the large majority of the major depression estimates falling between 3% and 8%. The prevalence rates of dysthymia were lower. We do not report pooled estimates of mental disorder prevalence rates across surveys because these estimates varied considerably across the surveys.
Table 2.
Prevalence (%) of mood disorders among persons with vs. without diabetes* (adjusted for age and gender)
| Major depression |
Dysthymia |
|||||
|---|---|---|---|---|---|---|
| Country | No diabetes | Diabetes | OR (95% CI) | No diabetes | Diabetes | OR (CI) |
| Colombia | 6.1 | 8.1 | 1.3 (0.7, 2.5) | 1.0 | 1.8 | 1.5 (0.3, 8.0) |
| Mexico | 3.9 | 9.0 | 2.2 (1.4, 3.6)* | 0.9 | 1.6 | 1.5 (0.5, 5.1) |
| United States | 8.3 | 8.3 | 1.3 (0.8, 2.0) | 2.2 | 3.5 | 1.7 (1.0, 2.9) |
| Japan | 2.2 | 3.2 | 1.4 (0.5, 4.5) | 0.6 | 2.1 | 1.7 (0.3, 8.5) |
| PRC | ||||||
| Beijing | 2.4 | 3.1 | 1.6 (0.2, 12.3) | 0.4 | 0.5 | 0.8 (0.1, 7.4) |
| Shanghai | 1.7 | 1.5 | 0.8 (0.1, 7.7) | 0.4 | 0.0 | – (–, –) |
| New Zealand | 6.7 | 5.5 | 1.4 (0.9, 2.1) | 1.8 | 2.2 | 1.5 (0.9, 2.6) |
| Belgium | 5.7 | 2.9 | 0.6 (0.1, 3.0) | 1.3 | 0.8 | 0.4 (0.1, 3.8) |
| France | 6.1 | 6.4 | 1.8 (0.6, 5.0) | 1.6 | 1.5 | 0.9 (0.1, 5.3) |
| Germany | 3.0 | 5.4 | 3.1 (1.1, 8.8)* | 0.9 | 1.8 | 1.4 (0.2, 8.6) |
| Italy | 3.0 | 6.0 | 1.9 (0.7, 5.4) | 1.1 | 0.6 | 0.3 (0.0, 2.9) |
| Netherlands | 5.4 | 3.3 | 0.9 (0.3, 2.5) | 1.8 | 1.1 | 1.0 (0.2, 6.1) |
| Spain | 4.1 | 3.8 | 0.9 (0.4, 1.7) | 1.3 | 2.2 | 1.3 (0.6, 2.9) |
| Ukraine | 9.2 | 19.5 | 1.5 (0.7, 3.2) | 4.1 | 7.3 | 0.9 (0.4, 2.3) |
| Lebanon | 1.7 | 3.1 | 3.2 (0.6, 16.0) | 0.6 | 2.3 | 4.8 (0.6, 39.5) |
| Nigeria | 1.1 | 0.0 | – (–, –) | 0.2 | 0.0 | – (–, –) |
| Israel | 5.9 | 7.5 | 1.3 (0.9, 2.0) | 1.3 | 1.0 | 0.6 (0.2, 1.7) |
| South Africa | 4.9 | 4.3 | 0.8 (0.4, 1.6) | 0.1 | 0.0 | NE |
| Pooled odds ratio | – | – | 1.4 (1.2, 1.6)* | – | – | 1.3 (1.0, 1.7) |
Odds ratio not listed if fewer than 25 respondents have diabetes.
“–” means information unavailable.
NE means nonestimable.
Sample restricted to Part II of World Mental Health Surveys.
Comparison of the prevalence rates of major depression and dysthymia among persons with diabetes vs. without diabetes showed modest absolute differences in most countries except for the Ukraine, which had higher rates of depression among those with and without diabetes and larger absolute differences in prevalence rates of depression. Depression prevalence rates were generally higher among persons with diabetes than among persons without diabetes. Since mood disorders decrease in prevalence with age, while diabetes increases in prevalence with age, it is important to adjust for age (and gender) in assessing the association of diabetes and mood disorders.
As shown in Table 2, age and gender adjusted odds ratios measuring the association of major depression with diabetes were significantly greater than 1 (indicating a positive association greater than expected by chance) for Mexico and Germany among the 18 surveys for which odds ratios were estimated. The association of diabetes and dysthymia was not significant for any of these 18 surveys. We assessed whether the variability in the odds ratio estimates across the surveys was greater than expected by chance. The test of heterogeneity was nonsignificant for both major depression (P=.54) and for dysthymia (P=.85), which suggests that variation in odds ratio estimates across surveys is attributable to random variation. Since the estimates are not heterogeneous, it is appropriate to report a pooled estimate. After adjusting for age and gender, the pooled estimate of the odds of major depression was 1.4 (95% CI=1.2–1.6) among persons with diabetes vs. without diabetes. In contrast to the majority of the survey-specific estimates, the CI of the pooled odds ratio for the major depression–diabetes association did not include one, reaching statistical significance due to the greater precision of the pooled estimate. The odds ratio for the association of dysthymia with diabetes was slightly lower (odds ratio=1.3, 95% CI=1.0–1.7), which was not significant at the P=.05 level.
Fig. 1 shows a funnel graph of the age–gender adjusted odds ratios for any mood disorder (major depression and dysthymia combined) for the 18 surveys for which odds ratios could be estimated. In this graph, the odds ratio was plotted on a log scale as a function of the precision of the estimate of the odds ratio. The funnel lines show the band within which the 95% CI of each survey odds ratio estimate includes the pooled estimate given the precision of the survey estimate. Most of the odds ratio estimates clustered in proximity to the pooled estimate of 1.34. The 95% CIs of all but one of the estimates included the pooled odds ratio estimate. These results support a conclusion that persons with self-reported diabetes are more likely to experience mood disorders than otherwise comparable persons who do not report diabetes.
Anxiety disorders
Across the surveys, the specific anxiety disorders (generalized anxiety disorder, panic/agoraphobia, social phobia, and posttraumatic stress disorder or PTSD) were generally less prevalent than major depression (Table 3A and B). Because the specific anxiety disorders were relatively uncommon, it was often not possible to estimate odds ratios for their association with diabetes. The heterogeneity tests for the odds ratios were nonsignificant for generalized anxiety disorder (P=.41), agoraphobia/panic (P=.39), social phobia (P=.67), and for PTSD (P=.82). Given the limited number of cases available in each survey, the pooled estimate of the odds ratio provides a more precise estimate of the association of each of the anxiety disorders with diabetes. The pooled odds ratio estimates for the anxiety disorders ranged from 1.3 to 1.6, and all were significantly greater than 1.0 (see CIs of the pooled odds ratio estimates in Table 3A and B). These results indicate that the strength of the association of specific anxiety disorders with diabetes is similar to that of mood disorders. When we examined all four anxiety disorders in combination (Fig. 2), the pooled estimate of the odds ratio was 1.26 (95% CI=1.1–1.5). While most of the survey estimates clustered around the pooled estimate, there was considerable variability in the odds ratio estimates, particularly for surveys with less precise estimates (i.e., those to the left side of Fig. 2). However, the variation in these odds ratio estimates did not exceed that expected due to chance (P=.67).
Table 3.
Prevalence (%) of anxiety disorders among persons* with vs. without diabetes (adjusted for age and gender)
| A | ||||||
|---|---|---|---|---|---|---|
| Generalized anxiety |
Agoraphobia or panic disorder |
|||||
| Country | No diabetes | Diabetes | OR (95% CI) | No diabetes | Diabetes | OR (CI) |
| Colombia | 1.0 | 2.2 | 2.3 (0.5, 10.4) | 2.2 | 1.1 | 0.4 (0.1, 2.2) |
| Mexico | 0.5 | 1.4 | 2.7 (0.6, 11.4) | 1.3 | 1.7 | 1.2 (0.5, 3.2) |
| United States | 4.0 | 4.6 | 1.3 (0.8, 2.2) | 3.5 | 5.4 | 2.0 (1.3, 3.1)* |
| Japan | 1.5 | 2.9 | 1.3 (0.3, 5.1) | 0.7 | 0.5 | 1.0 (0.1, 9.2) |
| PRC | ||||||
| Beijing | 1.2 | 0.3 | 0.2 (0.0, 1.7) | 0.4 | 0.0 | NE |
| Shanghai | 0.8 | 0.0 | NE | 0.0 | 3.0 | NE |
| New Zealand | 3.0 | 3.9 | 1.8 (1.1, 2.9)* | 2.2 | 2.9 | 2.2 (1.2, 3.8)* |
| Belgium | 1.0 | 1.3 | 1.6 (0.1, 17.6) | 1.6 | 0.0 | NE |
| France | 2.1 | 0.0 | NE | 1.4 | 0.2 | 0.3 (0.0, 2.5) |
| Germany | 0.5 | 0.0 | NE | 1.1 | 0.0 | NE |
| Italy | 0.5 | 0.0 | NE | 1.0 | 1.1 | 1.2 (0.2, 7.0) |
| Netherlands | 1.1 | 0.3 | 0.3 (0.1, 2.3) | 1.6 | 2.4 | 2.1 (0.5, 9.7) |
| Spain | 1.0 | 0.9 | 0.8 (0.3, 2.3) | 0.8 | 0.8 | 0.9 (0.3, 3.0) |
| Ukraine | 2.2 | 5.8 | 1.7 (0.8, 3.5) | 1.8 | 3.1 | 1.2 (0.3, 4.4) |
| Lebanon | 0.2 | 0.2 | 0.7 (0.1, 6.6) | 0.2 | 0.0 | NE |
| Nigeria | 0.0 | 0.0 | – (–, –) | 0.3 | 0.0 | – (–, –) |
| Israel | 2.4 | 4.9 | 2.1 (1.2, 3.6)* | 0.9 | 1.0 | 0.8 (0.3, 2.3) |
| South Africa | 1.8 | 4.9 | 2.0 (1.0, 4.1)* | 5.5 | 6.8 | 1.2 (0.7, 2.0) |
| Pooled odds ratio | – | – | 1.6 (1.3, 2.0)* | – | – | 1.5 (1.1, 1.9)* |
| B | ||||||
|---|---|---|---|---|---|---|
| Social phobia |
PTSD |
|||||
| Country | No diabetes | Diabetes | OR (95% CI) | No diabetes | Diabetes | OR (CI) |
| Colombia | 2.8 | 4.1 | 1.5 (0.5, 4.7) | 0.6 | 0.6 | 0.9 (0.3, 3.4) |
| Mexico | 2.0 | 2.8 | 1.7 (0.6, 4.8) | 0.6 | 0.1 | 0.3 (0.0, 2.5) |
| United States | 6.9 | 6.2 | 1.2 (0.8, 1.8) | 3.5 | 4.3 | 1.5 (1.0, 2.3) |
| Japan | 0.6 | 0.9 | 1.5 (0.1, 15.0) | 0.4 | 0.3 | 1.8 (0.2, 19.0) |
| PRC | ||||||
| Beijing | 0.3 | 0.0 | NE | 0.3 | 0.0 | NE |
| Shanghai | 0.0 | 0.0 | NE | 0.1 | 0.0 | NE |
| New Zealand | 5.1 | 4.9 | 1.5 (1.0, 2.3) | 3.0 | 2.7 | 1.1 (0.6, 1.9) |
| Belgium | 1.1 | 0.0 | NE | 0.6 | 2.5 | 5.3 (0.9, 30.2) |
| France | 2.6 | 4.0 | 2.4 (0.3, 17.2) | 2.3 | 1.5 | 1.0 (0.2, 5.8) |
| Germany | 1.8 | 0.0 | NE | 0.7 | 0.5 | 1.9 (0.2, 20.4) |
| Italy | 1.0 | 2.7 | 3.9 (0.4, 40.0) | 0.7 | 0.0 | NE |
| Netherlands | 1.3 | 0.5 | 0.7 (0.1, 7.3) | 2.7 | 0.0 | NE |
| Spain | 0.7 | 0.1 | 0.2 (0.0, 1.4) | 0.5 | 0.9 | 1.8 (0.5, 5.6) |
| Ukraine | 2.1 | 0.0 | NE | 2.7 | 6.0 | 1.3 (0.3, 5.2) |
| Lebanon | 0.6 | 0.0 | NE | 1.7 | 1.3 | 1.8 (0.4, 7.7) |
| Nigeria | 0.3 | 0.0 | – (–, –) | 0.0 | 0.0 | – (–, –) |
| Israel | – | – | NE | 0.6 | 0.3 | 0.5 (0.1, 3.3) |
| South Africa | 1.9 | 1.8 | 1.0 (0.5, 1.9) | 0.6 | 1.0 | 1.5 (0.4, 6.3) |
| Pooled odds ratio | – | – | 1.3 (1.0, 1.6)* | – | – | 1.3 (1.0, 1.8)* |
Odds ratio not listed if fewer than 25 respondents have diabetes.
“–” means information unavailable.
NE means non-estimable.
Sample restricted to Part II of World Mental Health Surveys.
Fig. 2.
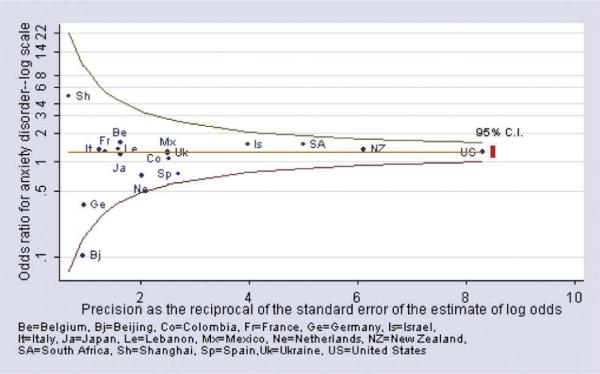
Anxiety disorder among persons with vs. without diabetes (age-sex adjusted odds ratio).
Alcohol use disorders
In two-thirds of the surveys, the prevalence of alcohol abuse or dependence was 1.0% or less among persons with diabetes (Table 4). Only Colombia, Beijing, and the Ukraine had a prevalence of alcohol abuse/dependence that exceeded 4% among persons with diabetes. The odds ratio estimates for the association of diabetes and alcohol abuse/dependence were not found to be heterogeneous across surveys (P=.22) for the nine surveys for which it was possible to estimate an odds ratio. Only one (the Ukraine) of these nine surveys found a significant association of diabetes and alcohol abuse/dependence. The pooled estimate of 1.1 was not significantly greater than 1.0 (95% CI=0.7–1.6).
Table 4.
Prevalence (%) of alcohol use disorders among persons with vs. without diabetes* (adjusted for age and gender)
| Alcohol abuse dependence |
|||
|---|---|---|---|
| Country | No diabetes | Diabetes | OR (95% CI) |
| Colombia | 2.5 | 4.9 | 2.7 (0.5, 15.9) |
| Mexico | 2.3 | 0.0 | NE |
| United States | 3.3 | 1.0 | 0.6 (0.3, 1.3) |
| Japan | 1.1 | 1.9 | 1.6 (0.2, 14.3) |
| PRC | |||
| Beijing | 2.4 | 4.4 | 4.1 (0.5, 30.8) |
| Shanghai | 0.5 | 0.0 | NE |
| New Zealand | 2.9 | 0.9 | 0.9 (0.4, 2.0) |
| Belgium | 1.4 | 0.0 | NE |
| France | 0.8 | 0.0 | NE |
| Germany | 1.2 | 1.0 | 4.6 (0.2, 133.0) |
| Italy | 0.1 | 0.0 | NE |
| Netherlands | 1.8 | 0.9 | 1.1 (0.1, 9.9) |
| Spain | 0.3 | 0.0 | NE |
| Ukraine | 6.2 | 4.7 | 4.8 (1.0, 21.7)* |
| Lebanon | 1.2 | 0.0 | NE |
| Nigeria | 0.7 | 2.6 | – (–, –) |
| Israel | 1.2 | 0.0 | NE |
| South Africa | 5.0 | 3.0 | 0.7 (0.3, 2.0) |
| Pooled odds ratio | – | – | 1.1 (0.7, 1.6) |
Odds ratio not listed if fewer than 25 respondents have diabetes.
“–” means information unavailable.
NE means nonestimable.
Sample restricted to Part II of World Mental Health Surveys.
Discussion
This report provides the first large-scale population-based assessment of the frequency and association of a wide range of common mental disorders with diabetes. Since the surveys covered countries that differ in culture, language, and level of socioeconomic development, the generally consistent findings across surveys suggest generalizability across diverse populations. Key findings are that the risk of both mood and anxiety disorders are moderately higher among persons with diabetes, as compared to the persons without diabetes. Different mood and anxiety disorders showed similar levels of association with diabetes. However, the estimates of the 12-month prevalence rates of major depression were generally lower than suggested by many prior reports which have largely been based on clinical samples (i.e., a 10–15% prevalence of major depression in typical prior studies) [6], compared to prevalence rate estimates typically in the 3–8% range in the WMH Surveys. The pooled estimate of the odds ratio found in this study is similar to those reported in a series of prospective, community-based studies assessing the risk of onset of diabetes among persons with vs. without major depression [9]. Cross-sectional analyses reported in this paper do not shed light on causation. The results for alcohol abuse and dependence showed that alcohol abuse was no more common among persons with diabetes than among those without, and the rates of alcohol abuse among persons with diabetes were low in most of the countries surveyed.
Longitudinal studies report that adults with depression have a 37% increased risk of developing Type 2 diabetes (i.e., a relative risk of about 1.4) compared to those without depression [7,9,35]. Prevalence studies conducted in medical settings typically report a higher association between depression and diabetes (~2 times). The elevated odds ratios found in clinical studies of patients with diabetes and depression may reflect a sampling bias of studying persons who use health care services. Patients with depression and anxiety disorders use medical services more frequently than those without, which may explain the higher rates of depression in clinical populations than observed in these general population samples. Overall, the pooled estimate of the odds ratio for major depression from the WMH Surveys is generally consistent with prior prospective studies of the increased risk of onset of diabetes among persons with vs. without depressive illness [7,9].
Ascertainment of diabetes based on self-report and combining patients with Type 1 and Type 2 diabetes are limitations of this study. Since the WMH Surveys were multifaceted and conducted in large populations worldwide, it was not feasible to abstract medical records or to conduct a standardized medical assessment to determine whether diabetes (whether Type 1 or Type 2) was present or absent. The overall prevalence of diabetes in the WMH surveys conformed to expected epidemiological patterns (higher prevalence among older adults and persons with less education). Prior research in developed and developing countries suggests that self-report of diabetes has acceptable validity [25]. Recent epidemiologic studies of diabetes, diagnosed by abnormal blood glucose levels, showed that the diabetes prevalence rates among countries with less access to health care services (e.g., Mexico, Colombia, Nigeria, and South Africa) are higher than prevalence rates reported in this study [27–30]. Additional analysis of WMH surveys to address this limitation showed that the treatment rate for persons reporting diabetes was lower among countries with less access to health care relative to countries with more access to health care (77% vs. 94%). Although the under-ascertainment of diabetes in developing countries is a potential source of bias, there were no systematic differences observed in the association of diabetes with mood and anxiety disorders between the developed and developing countries.
Since mood and anxiety disorders are associated with many different chronic physical conditions, mechanisms contributing to the association of diabetes and psychological illness that are shared with other chronic conditions deserve further research (e.g., smoking status, obesity, physical activity). Strengths of the WMH Surveys include the use of standardized and well-validated methods to diagnose mental disorders, the size and diversity of the surveys, and the evaluation of a population sample. Population-based prevalence estimates were developed for an unprecedented range of mental disorders among community-dwelling adults reporting diabetes.
Had the WMH Surveys been reported individually, rather than as a group, most of the single surveys would not have reported a significant association of mood and anxiety disorders with diabetes. For example, for surveys with estimates of the association of major depression and diabetes, only Mexico and Germany had odds ratios that were significantly greater than 1. In contrast, the pooled estimate was significantly greater than 1. However, when the survey results are considered as a group, the results are more similar than different. For both the mood and anxiety disorder groups, the pooled estimates of the odds of the mental disorder being present were about 1.3 to 1 for persons with vs. without diabetes. The estimates from the individual surveys consistently fell within the 95% CIs of the pooled estimates. This points to the importance of having an adequate number of cases of diabetes and mood or anxiety disorder available for analysis when assessing their association. For this reason, and in light of the similar level of association of anxiety disorders with diabetes, it may be useful to examine results for anxiety disorders as a class rather than to assess specific anxiety disorders that occur with low frequency, particularly in sample surveys that are not large.
Positive associations between mood, anxiety disorders, and diabetes, presently confirmed by the World Health Survey results, have major clinical implications. Recently, screening and treatment for depression were added to the American Diabetes Association guidelines [36]. Interrelations between depression and diabetes are evident across the spectrum of diabetes disease burden from self-care to mortality to health care costs. Cross-sectional studies consistently demonstrate a link between depression and diabetes across the entire spectrum of the illness, ranging from symptom amplification, poorer self-care (e.g., exercise, monitoring blood glucose), lower medication adherence, more diabetes complications, greater work disability, higher cost, and increased mortality among depressed diabetes patients when compared to diabetes patients with no depression [11–14,37–39]. Although an early study among patients with high baseline HbA1c showed that improved depression care also resulted in better glycemic control [40], two large randomized trials that improved depression care and outcomes among primary care diabetes patients with depression did not find corresponding improvements in glycemic control [41]. While it is clearly possible to improve depression outcomes among depressed patients with diabetes, how to integrate depression and diabetes management so that both depression and diabetes outcomes are improved is an issue for future research.
The WMH Surveys showed that, in population-based samples, mood and anxiety disorders occurred among persons with diabetes at modestly higher rates than among persons of comparable age and gender without diabetes. This association was observed across diverse countries differing in culture, language, and level of socioeconomic development. The level of association of depression and diabetes was comparable to prior prospective studies, but lower than most clinic-based studies. The prevalence rate estimates of major depression were also generally lower than suggested by prior studies in clinical samples. The association of mood and anxiety disorder with diabetes appeared similar across specific mood and anxiety disorders. Alcohol abuse/dependence was not a prominent problem among persons with diabetes. Given prior research showing an association of mood and anxiety disorder with multiple indicators of the severity of diabetes, these results suggest that clinicians should be aware of the increased occurrence of mood and anxiety disorders among patients with diabetes.
Acknowledgments
The surveys included in this report were carried out in conjunction with the World Health Organization World Mental Health (WMH) Survey Initiative. We thank the WMH staff for assistance with instrumentation, fieldwork, and data analysis. These activities were supported by the United States National Institute of Mental Health (R01MH070884); the John D. and Catherine T. MacArthur Foundation; the Pfizer Foundation; the US Public Health Service (R13-MH066849, R01-MH069864, and R01 DA016558); the Fogarty International Center (FIRCA R01-TW006481); the Pan American Health Organization; Eli Lilly and Company; Ortho-McNeil Pharmaceutical, Inc.; GlaxoSmithKline; and Bristol-Myers Squibb. A complete list of WMH publications can be found at http://www.hcp.med.harvard.edu/wmh/. The Mexican National Comorbidity Survey (MNCS) is supported by The National Institute of Psychiatry Ramon de la Fuente (INPRFMDIES 4280) and by the National Council on Science and Technology (CONACyT-G30544- H), with supplemental support from the PanAmerican Health Organization (PAHO). The Lebanese survey is supported by the Lebanese Ministry of Public Health, the WHO (Lebanon) and unrestricted grants from Janssen Cilag, Eli Lilly, GlaxoSmithKline, Roche, Novartis, and anonymous donations. The ESEMeD project was funded by the European Commission (Contracts QLG5-1999-01042; SANCO 2004123); the Piedmont Region (Italy); Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spain (FIS 00/0028); Ministerio de Ciencia y Tecnología, Spain (SAF 2000-158-CE); Departament de Salut, Generalitat de Catalunya, Spain; and other local agencies and by an unrestricted educational grant from GlaxoSmithKline. The Chinese World Mental Health Survey Initiative is supported by the Pfizer Foundation. The Colombian National Study of Mental Health (NSMH) is supported by the Ministry of Social Protection, with supplemental support from the Saldarriaga Concha Foundation. The Israel National Health Survey is funded by the Ministry of Health with support from the Israel National Institute for Health Policy and Health Services Research and the National Insurance Institute of Israel. The World Mental Health Japan (WMHJ) Survey is supported by the Grant for Research on Psychiatric and Neurological Diseases and Mental Health (H13-SHOGAI-023, H14-TOKUBETSU-026, H16-KOKORO-013) from the Japan Ministry of Health, Labour and Welfare. The New Zealand Mental Health Survey (NZMHS) is supported by the New Zealand Ministry of Health, Alcohol Advisory Council, and the Health Research Council. The Nigerian Survey of Mental Health and Wellbeing (NSMHW) is supported by the WHO (Geneva), the WHO (Nigeria), and the Federal Ministry of Health, Abuja, Nigeria. The South Africa Stress and Health Study (SASH) is supported by the US National Institute of Mental Health (R01-MH059575) and National Institute of Drug Abuse with supplemental funding from the South African Department of Health and the University of Michigan. The Ukraine Comorbid Mental Disorders during Periods of Social Disruption (CMDPSD) study is funded by the US National Institute of Mental Health (RO1-MH61905). The US National Comorbidity Survey Replication (NCS-R) is supported by the National Institute of Mental Health (NIMH; U01-MH60220) with supplemental support from the National Institute of Drug Abuse (NIDA), the Substance Abuse and Mental Health Services Administration (SAMHSA), the Robert Wood Johnson Foundation (RWJF; Grant 044708), and the John W. Alden Trust.
References
- [1].Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- [2].Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- [3].Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116:473–80. doi: 10.1542/peds.2004-2536. [DOI] [PubMed] [Google Scholar]
- [4].Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–32. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- [5].Eaton WW. Epidemiologic evidence on the comorbidity of depression and diabetes. J Psychosom Res. 2002;53:903–6. doi: 10.1016/s0022-3999(02)00302-1. [DOI] [PubMed] [Google Scholar]
- [6].Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- [7].Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care. 1996;19:1097–102. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- [8].Engum A. The role of depression and anxiety in onset of diabetes in a large population-based study. J Psychosom Res. 2007;62:31–8. doi: 10.1016/j.jpsychores.2006.07.009. [DOI] [PubMed] [Google Scholar]
- [9].Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–45. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- [10].Simon G, Ormel J, VonKorff M, Barlow W. Health care costs associated with depressive and anxiety disorders in primary care. Am J Psychiatry. 1995;152:352–7. doi: 10.1176/ajp.152.3.352. [DOI] [PubMed] [Google Scholar]
- [11].Lin EHB, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, Ciechanowski P, Ludman E, Bush T, Young B. Relationship of depression and diabetes self-care, medication adherence and preventive care. Diabetes Care. 2004;27:2154–60. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- [12].Katon WJ, Rutter C, Simon G, Lin EH, Ludman E, Ciechanowski P, Kinder L, Young B, Von Korff M. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28:2668–72. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- [13].de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- [14].Von Korff M, Katon W, Lin EH, Simon G, Ciechanowski P, Ludman E, Oliver M, Rutter C, Young B. Work disability among individuals with diabetes. Diabetes Care. 2005;28:1326–32. doi: 10.2337/diacare.28.6.1326. [DOI] [PubMed] [Google Scholar]
- [15].Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25:464–70. doi: 10.2337/diacare.25.3.464. [DOI] [PubMed] [Google Scholar]
- [16].Anderson RJ, Grigsby AB, Freedland KE, de Groot M, McGill JB, Clouse RE, Lustman PJ. Anxiety and poor glycemic control: a meta-analytic review of the literature. Int J Psychiatry Med. 2002;32:235–47. doi: 10.2190/KLGD-4H8D-4RYL-TWQ8. [DOI] [PubMed] [Google Scholar]
- [17].Lloyd CE, Dyer PH, Barnett AH. Prevalence of symptoms of depression and anxiety in a diabetes clinic population. Diabet Med. 2000;17:198–202. doi: 10.1046/j.1464-5491.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- [18].Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res. 2002;53:1053–60. doi: 10.1016/s0022-3999(02)00417-8. [DOI] [PubMed] [Google Scholar]
- [19].Zhang J, Markides KS, Lee DJ. Health status of diabetic Mexican Americans: results from the Hispanic HANES. Ethn Dis. 1991;1:273–9. [PubMed] [Google Scholar]
- [20].Wells KB, Golding JM, Burnam MA. Chronic medical conditions in a sample of the general population with anxiety, affective, and substance use disorders. Am J Psychiatry. 1989;146:1440–6. doi: 10.1176/ajp.146.11.1440. [DOI] [PubMed] [Google Scholar]
- [21].Alonso J, Ferrer M, Romera B, Vilagut G, Angermeyer M, Bernert S, Brugha TS, Taub N, McColgen Z, de Girolamo G, et al. The European Study of the Epidemiology of Mental Disorders (ESEMeD/MHEDEA 2000) project: rationale and methods. Int J Methods Psychiatr Res. 2002;11:55–67. doi: 10.1002/mpr.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Haro JM, Arbabzadeh-Bouchez S, Brugha TS, de Girolamo G, Guyer ME, Jin R, Lepine JP, Mazzi F, Reneses B, Vilagut G, et al. Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health surveys. Int J Methods Psychiatr Res. 2006;15:167–80. doi: 10.1002/mpr.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington (DC): 1994. [Google Scholar]
- [25].National Center for Health Statistics . Vital Health Stat 2. 1994. Evaluation of National Health Interview Survey diagnostic reporting; pp. 1–116. [PubMed] [Google Scholar]
- [26].Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. J Clin Epidemiol. 2003;56:148–54. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- [27].Aguilar-Salinas CA, Velazquez Monroy O, Gomez-Perez FJ, Gonzalez Chavez A, Esqueda AL, Molina Cuevas V, Rull-Rodrigo JA, Tapia Conyer R. Characteristics of patients with type 2 diabetes in Mexico: results from a large population-based nationwide survey. Diabetes Care. 2003;26:2021–6. doi: 10.2337/diacare.26.7.2021. [DOI] [PubMed] [Google Scholar]
- [28].Nyenwe EA, Odia OJ, Ihekwaba AE, Ojule A, Babatunde S. Type 2 diabetes in adult Nigerians: a study of its prevalence and risk factors in Port Harcourt, Nigeria. Diabetes Res Clin Pract. 2003;62:177–85. doi: 10.1016/j.diabres.2003.07.002. [DOI] [PubMed] [Google Scholar]
- [29].Alberts M, Urdal P, Steyn K, Stensvold I, Tverdal A, Nel JH, Steyn NP. Prevalence of cardiovascular diseases and associated risk factors in a rural black population of South Africa. Eur J Cardiovasc Prev Rehabil. 2005;12:347–54. doi: 10.1097/01.hjr.0000174792.24188.8e. [DOI] [PubMed] [Google Scholar]
- [30].Bautista LE, Orostegui M, Vera LM, Prada GE, Orozco LC, Herran OF. Prevalence and impact of cardiovascular risk factors in Bucaramanga, Colombia: results from the Countrywide Integrated Noncommunicable Disease Intervention Programme (CINDI/CARMEN) baseline survey. Eur J Cardiovasc Prev Rehabil. 2006;13:769–75. doi: 10.1097/01.hjr.0000219113.40662.dd. [DOI] [PubMed] [Google Scholar]
- [31].Wolter KM. Introduction to Variance Estimation. Springer-Verlag; New York (NY): 1985. [Google Scholar]
- [32].Research Triangle Institute . SUDAAN Version 8.0.1. Research Triangle Institute; Research Triangle Park (NC): 2002. [Google Scholar]
- [33].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- [34].Bird SM, Cox SD, Farewell VT, Goldstein H, Holt T, Smith PC. Performance indicators: good, bad and ugly. J R Stat Soc. 2005;Series A:1–27. [Google Scholar]
- [35].Golden SH, Lazo M, Carnethon M, Bertoni A, Shreiner P, Roux A, Lee HB, Lyketsos C. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751–9. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2004;27(Suppl 1):S15–35. doi: 10.2337/diacare.27.2007.s15. [DOI] [PubMed] [Google Scholar]
- [37].Ludman EJ, Katon W, Russo J, Von Korff M, Simon G, Ciechanowski P, Lin E, Bush T, Walker E, Young B. Depression and diabetes symptom burden. Gen Hosp Psychiatry. 2004;26:430–6. doi: 10.1016/j.genhosppsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [38].Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–85. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- [39].Simon GE, Katon W, Lin EHB, Ludman E, Von Korff M, Ciechanowski P, Young B. Diabetes complications and depression as predictors of health service costs. J Gen Hosp Psych. 2005 doi: 10.1016/j.genhosppsych.2005.04.008. In press. [DOI] [PubMed] [Google Scholar]
- [40].Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;129:613–21. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- [41].Williams JW, Katon W, Lin EHB, Noel PH, Worchel J, Cornell J, Harpole L, Fultz BA, Hunkeler E, Unutzer J. The effectiveness of depression care management on depression and diabetes-related outcomes in older patients. Ann Intern Med. 2004;140:1015–24. doi: 10.7326/0003-4819-140-12-200406150-00012. [DOI] [PubMed] [Google Scholar]


