Abstract
Metastatic tumors have been shown to establish permissive microenvironments for metastases via recruitment of bone marrow (BM)- derived cells. Here, we show that metastasis-incompetent tumors are also capable of generating such microenvironments. However, in these situations the otherwise pro-metastatic Gr1+ myeloid cells create a metastasis-refractory microenvironment via the induction of thrombospondin-1 (Tsp-1) by tumor-secreted prosaposin. (BM)-specific genetic deletion of Tsp-1 abolished the inhibition of metastasis, which was restored by BM transplant from Tsp-1+ donors. We also developed a 5-amino acid peptide from prosaposin as a pharmacological inducer of Tsp-1 in Gr1+ BM cells, which dramatically suppresses metastasis. These results provide mechanistic insights into why certain tumors are deficient in metastatic potential and implicate recruited Gr1+ myeloid cells as the main source of Tsp-1. The results underscore the plasticity of Gr1+ cells, which, depending on the context, promote or inhibit metastasis, and suggest that the peptide could be a potential therapeutic agent against metastatic cancer.
Introduction
The majority of cancer-related deaths are caused by organ failure brought about by metastatic dissemination of tumor cells. At the metastatic site, the disseminated tumor cells proliferate and induce angiogenesis to allow further tumor outgrowth to form lethal macrometastases (1-3). However, despite our increased understanding of the physiological processes involved in tumor metastasis, there are no clinically approved drugs that have shown significant efficacy at treating advanced, metastatic cancer. As early as 1889, Steven Paget set forth his “seed” and “soil” hypothesis establishing the concept that breast cancer metastasizes to specific organs which harbor a receptive microenvironment (4). Experimental support for this hypothesis has been provided by the demonstration that primary tumors release specific cytokines such as vascular endothelial growth factor (VEGF), stromal-derived factor (SDF-1), transforming growth factor (TGF-β) and tumor necrosis factor (TNF-α), which systemically initiate premetastatic niches, characterized by the accumulation of BM-derived cells such as VEGFR1+ hematopoietic cells and CD11b+ myeloid cells (5). Furthermore, these pre-metastatic niches are characterized by the selective induction of organ-specific chemoattractants, growth factors, and ECM-related proteins including fibronectin, lysyl oxidase, and S100A8 (6-9). While these studies have provided key insights into metastasis-promoting niches, the existence of niches, which may confer metastasis suppression, has not been examined.
In this study, we demonstrate that tumors that lack adequate metastatic potential are as capable of recruiting BM-derived myeloid cells to potential metastatic organs as highly metastatic tumors. However, metastasis-incompetent tumors systemically stimulate expression of the anti-tumorigenic factor Tsp-1 in the recruited CD11b+Gr1+ cells, converting these pro-metastatic cells into metastasis-inhibitory cells that are refractory to the outgrowth of metastatic tumor cells. As such, our study provides a novel insight into the exquisite functional plasticity of the Gr1+ cells previously demonstrated to enhance carcinogenesis (10-12). Moreover, we describe the development of a novel peptide that stimulates Tsp-1 in Gr1+ cells and blunts metastasis when administered systemically.
Results
Poorly metastatic tumors recruit BM-derived cells to the premetastatic microenvironment in the lungs
It has been demonstrated that metastatic tumors are able to induce the recruitment of BM-derived cells to future sites of metastasis, creating a permissive microenvironment for colonization (5). However, there has been no analysis of whether this process is impaired in metastasis-incompetent tumors or whether these tumors create metastasis-refractory microenvironments. To determine if tumors that lack robust metastatic capabilities are able to generate metastasis-suppressive niches in distant organs, we examined human prostate and breast cancer models that exhibit varying grades of metastatic potential (13, 14). These included weakly metastatic parental cells, PC3 or MDA-MB-231, and their highly metastatic variants, PC3M-LN4 (LN4) and MDA-MB-LM2 (LM2), respectively. In order to mimic the paracrine and endocrine effects of tumor-secreted factors on the pre-metastatic niche in the lung microenvironment in vivo, we administered conditioned media (CM) prepared from either the prostate or breast cancer cells to mice. To visualize the BM-derived metastatic microenvironment, we performed BM transplantation (BMT) of wild type mice with syngeneic GFP+ BM in these mice (2, 15). The CM from metastatic LN4 cells was able to efficiently increase the recruitment and formation of a GFP+ BM-derived premetastatic microenvironment, as would have been predicted (Fig. 1A-B). Strikingly, the CM from weakly metastatic PC3 tumor cells was also able to generate these microenvironments with similar efficiency (Fig. 1A-B). Further analysis revealed that the GFP+ BM cells recruited in both cases were predominantly CD11b+ myeloid cells (Fig. 1A-C, Supplementary Figs. S1A-B).
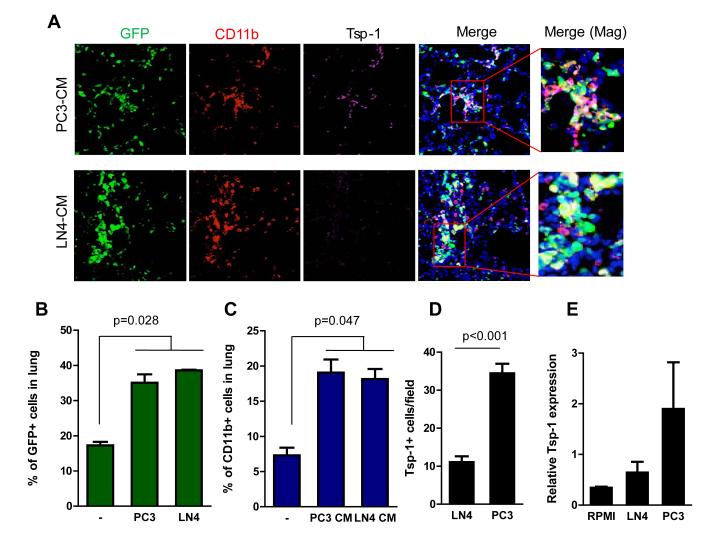
Figure 1. Conditioned media from metastasis-incompetent tumor cells induce Tsp-1 in the lung microenvironment.
A, Representative immunofluorescence images showing recruitment of GFP+ BM-derived cells in the lungs of mice treated with PC3 CM compared to LN4 CM. Elevated Tsp-1 expression is observed in a population of CD11b+ cells in the lungs of mice treated with PC3 CM. DAPI was used to label the nuclei of cells. Magnified images on the right show colocalization of signals.
B, Flow cytometry-based quantitation of recruited BM-derived GFP+ cells in lungs of mice treated with control serum-free RPMI media (-), LN4 CM, or PC3 CM (n=3 per group).
C, Flow cytometry-based quantitation of recruited CD11b+ cells in lungs of mice treated with control serum-free RPMI media (-), LN4 CM, or PC3 CM. (n=3 per group).
D, Quantitation of CD11b+ cells expressing Tsp-1 in the lungs after one-week challenge with PC3 CM or LN4 CM. (n=16, 4 random fields from 4 mice per group, p<0.001).
E, Quantitative RT-PCR analysis of Tsp-1 expression in lungs of control RPMI, PC3, or LN4 CM-treated mice (n= 3 mice).
Poorly metastatic tumors induce Tsp-1 expression in the BM-derived cells in the premetastatic microenvironment
Having observed no differences in the cellular composition of premetastatic lungs generated by metastatic-competent and incompetent tumors, we postulated that the BM-derived cells recruited by the different tumor types might exhibit molecular differences that would provide insights into metastasis-resistant microenvironments. As such, we performed gene expression analysis of lungs of PC3 and LN4 CM-treated mice (Supplementary Fig. S2). Among the genes differentially regulated by PC3 CM, we focused on secreted proteins that are most likely to reprogram the local microenvironment, and exert a negative impact on metastatic outgrowth of tumor cells. Among candidate genes, thrombospondin-1 (Tsp-1) was selected for further analysis as it is a secreted extracellular matrix protein and one of the most potent inhibitors of tumor angiogenesis and growth (16, 17). Both cell count and RT-PCR analysis confirmed that Tsp-1 was upregulated in the lungs of PC3 CM-treated mice compared with controls or LN4 CM-treated mice (Figs. 1D, E).
BM-derived CD11b+Gr1+ myeloid cells are the major contributors of Tsp-1
We next performed immunostaining analysis to determine the source of Tsp-1 in the lung stroma, and observed that Tsp-1 expression was mainly confined to the BM-derived CD11b+ cells recruited in the lungs of mice treated with PC3 CM (Fig. 1A, Supplementary Fig. S1A). Conversely, we observed no discernible induction of Tsp-1 expression in the lungs of mice treated with LN4 CM (Figs. 1A). To further assess which BM cell populations expressed Tsp-1, we analyzed specific flow sorted BM cell populations from the lungs by RT-PCR (Fig. 2A), and observed that Tsp-1 expression was largely confined to Gr1+ myeloid cells and not the Gr1− myelocytes or other hematopoietic cells (Fig. 2A). To further exclude the possibility that other cell-types may also express Tsp-1, we performed Western blot analysis of different cellular components from peripheral blood harvested from wild type mice or Tsp-1 knockout (Tsp-1−/−) mice. Notably, the Gr1+ cells expressed abundant Tsp-1 compared to Gr1− cells (Fig. 2B, left panel, Supplementary Fig. S3A). As expected, there was a complete lack of Tsp-1 in all compartments examined in Tsp-1−/− mice (Fig. 2B, left panel).
Figure 2. Tsp-1 expression is mainly confined to BM-derived Gr1+ cells.
A, Quantitative RT-PCR analysis of Tsp-1 expression in different subsets of flow sorted CD45+ BM hematopoietic cells isolated from the lung. Of these, the Gr1+ cells showed elevated Tsp-1 compared to Gr1− cells or other CD45− cells. Total lung, all cells from the lung.
B, Western blot analysis of Tsp-1 levels in cellular hematopoietic compartments in the peripheral blood from WT mice and Tsp-1−/− mice show Gr1+ cells as a major source of Tsp-1. WBC, white blood cells. PRP: Platelet-rich plasma.
C, Immunofluorescence staining of lungs showing expression of Tsp-1 by Gr1+ cells. The boxed area highlights Tsp-1-expressing Gr1+ cells depicted by arrows.
D, Quantitative RT-PCR analysis of Tsp-1 expression in different subsets of sorted BM-hematopoietic cells from the lung. Both subsets of Gr1+ cells, Ly6Chigh and Ly6G+ cells express Tsp-1.
E, Western blot analysis of Tsp-1 levels in lungs of mice after BM transplantation. Transplantation of Tsp-1−/− BM to WT recipients (Tsp-1 KO BM >WT) rendered lungs devoid of Tsp-1. Conversely, transplantation of WT BM to Tsp-1−/− recipients (WT BM >Tsp-1 KO) restored Tsp-1 expression in the lung. WT BM >WT: transplantation of WT BM into WT recipients. Tsp-1 KO BM>Tsp1 KO: transplantation of Tsp-1−/− BM into Tsp-1−/− recipients.
Further analysis of the peripheral blood showed that Gr1+ cells express predominantly the higher molecular weight isoforms of Tsp-1 (160-170 kDa), whereas the platelets express a lower molecular weight isoform (135-140 kDa) (Fig. 2B, right panel). Indeed, both forms have been observed previously in platelets (18). Importantly, Gr1+ cells expressed higher levels of Tsp-1 compared to the platelets. Taken together, these data indicate that the BM-derived Gr1+ cells are the major source of Tsp-1 in the premetastatic microenvironments generated by weakly metastatic tumors.
Given that immunostaining analysis confirmed that Tsp-1 expression was confined to Gr1+ cells (Figs. 2C) and the Gr1+ population is comprised of two major subpopulations; Ly6G+ granulocytic myeloid cells, and Ly6Chigh myelomonocytic cells, we further analyzed these Gr1+ subpopulations (Supplementary Fig. S3B). Our analyses revealed that both subsets of Gr1+ cells expressed Tsp-1 (Fig. 2D). To conclusively determine that the chief source of Tsp-1 expression in the lungs was the Gr1+ BM cells and not other lung stromal cells, we performed bone marrow transplantation (BMT) experiments. We observed Tsp-1 expression in the lungs of Tsp-1−/− mice (Tsp-1 KO) that were transplanted with wild type (WT) BM but not in WT mice transplanted with Tsp-1−/− BM (Fig. 2E).
To exclude the possibility that these findings were somehow unique to the prostate cancer model, we examined a breast cancer model as well. As described above, we used the weakly metastatic parental cells MDA-MB-231 (MDA), and their highly metastatic variants MDA-MB-LM2 (LM2). Consistent with the observations in the prostate cancer model, CM from weakly metastatic MDA breast cancer cells also induced upregulation of Tsp-1 expression in BM recruited Gr1+ cells as compared to CM from highly metastatic LM2 breast cancer cells (Supplementary Fig. S4). These results, when taken together, strongly indicate that BM-derived cells are the exclusive source of Tsp-1 induced by weakly metastatic tumors in the lungs.
Genetic deletion of Tsp-1 in the bone marrow results in increased metastasis
We next postulated that expression of Tsp-1 in recruited Gr1+ myeloid cells in the lungs could generate a metastasis-refractory environment. In order to test the significance of BM-derived Tsp-1 in modulating metastasis, we generated Tsp-1- deficiency in the BM. Specifically, we harvested BM cells from Tsp-1−/− mice and transplanted these cells into irradiated syngeneic WT mice to generate a cohort of Tsp-1−/− BMT mice. As controls, BM from WT mice was transplanted into irradiated WT mice to generate a cohort of WT BMT control mice. As expected of a successful BMT and engraftment of recipient mice with donor BM, Western blot and RT-PCR analysis of peripheral blood leukocytes from Tsp-1−/− BMT mice showed undetectable Tsp-1 protein levels and low mRNA levels compared to wild type BMT controls (Supplementary Figs. S5A-B).
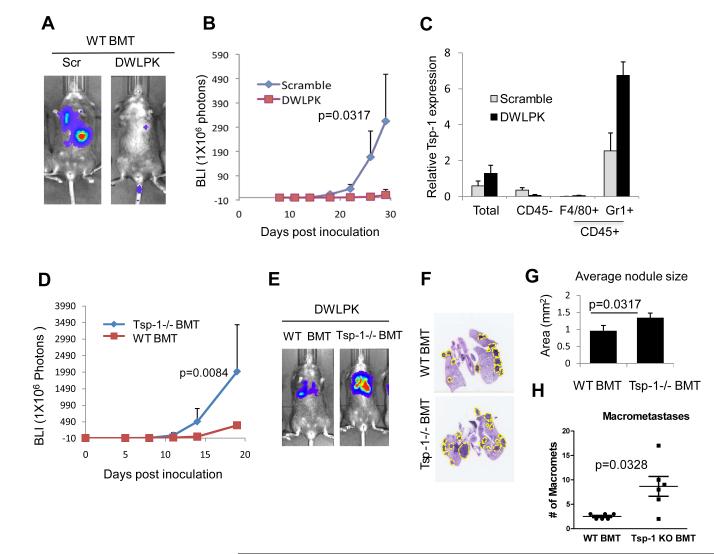
We then sought to determine the impact on metastasis of BM-derived Tsp-1 in the lung microenvironment. As such, we examined the ability of Lewis Lung Carcinoma cells (LLC) to colonize the lungs of mice that received a BM transfer from WT mice or Tsp-1−/− mice. Specifically, we injected LLC cells stably expressing the firefly luciferase gene (LLC-luc) (2, 14) via tail vein and monitored lung metastases. Strikingly, we observed accelerated metastases in Tsp-1−/− BMT mice compared to WT BMT mice (Figs. 3A-B). Western blot analysis of the total lung lysates showed complete absence of Tsp-1 in the lungs of Tsp-1−/− BMT (Fig. 3C), indicating that in a metastatic setting the BM-derived cells are the main source of Tsp-1 in the lung parenchyma. Moreover, as expected, in Tsp-1−/− BMT mice the Gr1+ cells were devoid of Tsp-1 expression (Supplementary Fig. S6). Importantly, Tsp-1 deficiency in the BM cells in Tsp-1−/− BMT mice did not perturb the recruitment of Gr1+ cells in the lung microenvironment compared to WT BMT mice (Supplementary Fig. S6), nor did the lack of Tsp-1 perturb biogenesis of other BM hematopoietic cells (Supplementary Fig. S5C). To exclude the possibility that enhanced metastasis was due to differential tumor cell seeding in the lung parenchyma of Tsp-1−/− BMT mice, we performed a seeding experiment. We found, via a time course evaluation of the lungs following administration of tumor cells, that seeding efficiency was comparable in the lungs of BMT mice regardless of the donor source (Supplementary Fig. S7).
Figure 3. BM-derived Gr1+ cells confer a metastasis-suppressive phenotype through the secretion of Tsp-1.
A, Quantitation of pulmonary metastases in WT BMT mice and Tsp-1−/− BMT mice (n=7 per group). BLI estimates were obtained at days 0,5,8,11,14,19 following tail vein injections of 1×105 LLC cells. p=0.0013 between WT BMT and Tsp-1−/− BMT groups at day 19.
B, Representative BLI images showing metastasis suppression in WT BMT mice compared to Tsp-1−/− BMT mice depicted in panel A.
C, Western blot analysis of Tsp-1 from the lungs of WT BMT mice and Tsp-1−/− BMT mice. Lung from whole body Tsp-1−/− mice was used as a control.
D, Quantitation of pulmonary metastases in Tsp-1−/− mice transplanted with WT BM and Tsp-1−/− mice transplanted with Tsp-1−/− BM (n=5 per group). BLI estimates were obtained following tail vein injections of 1×105 LLC cells. p=0.033 between the two group at day 30.
E, Representative bioluminescence images showing metastasis suppression in Tsp-1−/− mice transplanted with WT BM compared to Tsp-1−/− mice transplanted with Tsp-1−/− BM depicted in panel D.
F, Quantitation of Tsp-1 protein expression in the lungs of mice following BMT.
G, Left panel; Representative immunofluorescence images showing Ki67+ cells in the metastatic lungs of WT BMT mice and Tsp-1−/− BMT mice. Right panel; Quantitation of Ki67+ cells. Sections evaluated from 6 animals per group.
H, Left panel; Representative immunofluorescence images showing active-caspase 3+ cells in the metastatic lungs of WT BMT mice and Tsp-1−/− BMT (p=0.0903). Right panel; Quantitation of active caspase 3+ cells. Sections evaluated from 6 animals per group.
To provide further evidence that the observed anti-metastatic effect is due to Tsp-1 production in Gr1+ cells, we performed a reverse BMT experiment. Specifically, in a reverse experiment, we transplanted BM from WT mice into Tsp-1−/− mice. As a control, we transplanted BM from Tsp-1−/− donors into Tsp-1−/− mice to account for any possible effects of the transplantation procedure. As expected, Tsp-1 expression was restored in the Gr1+ cells in Tsp-1−/− mice transplanted with WT BM (Supplementary Fig. S8). Strikingly, transplantation of WT BM into Tsp1−/− mice resulted in complete suppression of metastasis (Fig. 3D-E). This phenomenon was most likely due to the dramatically increased expression of Tsp-1 in the lungs of these mice compared to mice transplanted with WT BM (Fig. 3F). Consistent with previous observations, Tsp-1 expression was restored in the Gr1+ cells in Tsp-1−/− mice transplanted with WT BM (Supplementary Fig. S8). These findings, support the hypothesis that supraphysiologic induction of Tsp-1 in Gr1+ cells can blunt metastasis as well as the possibility that a therapeutic agent with such activity could be effective at treating advanced metastatic cancer
BM-derived Tsp-1 inhibits metastasis outgrowth through inhibition of tumor cell proliferation
The antitumor activity of Tsp-1 has been shown to be involved in tumor growth blockade through diverse mechanisms; angiogenesis inhibition, blockade of cell proliferation, and induction of apoptosis both in tumor cells and endothelial cells (19, 20). Consistent with these functions, the metastatic lesions in the lungs of Tsp-1−/− BMT mice showed elevated proliferation of tumor cells compared to control WT BMT mice (p=0.002) (Fig. 3G, Supplementary Fig. S9), while the tumor cell apoptotic index remained unperturbed in the lungs of Tsp-1−/− BMT mice compared to control WT BMT mice, (p= 0.09, Fig. 3H). Taken together, these results demonstrate that Tsp-1 in recruited BM-derived Gr1+ cells did not affect metastatic seeding of tumor cells, or their ability to stimulate angiogenesis, but impaired tumor outgrowth at the metastatic site by inhibiting tumor cell proliferation.
Stimulation of Tsp-1 in the lung microenvironment as a potential antimetastatic approach
We next speculated whether administration of Tsp-1 would constitute a potential anti-metastatic strategy, as it has been postulated that Tsp-1 could be a potent clinical inhibitor of tumor progression and metastasis (21-23). However, the translational relevance of this approach has been minimized by the fact that Tsp-1 is an extremely large protein whose use as a therapeutic agent is unfeasible (24). Indeed, a mimetic nonapeptide derived from the type I repeat of Tsp-1, that contains the antiangiogenic activity of the endogenous protein has been developed. However, in clinical trials this drug showed little efficacy due in part to the fact that it was unable to fully replicate the activity of the full-length protein (25, 26). We reasoned that in order to effectively utilize Tsp-1 as a therapeutic agent it would be necessary to induce the expression of full-length Tsp-1, containing all of its inhibitory activity, in the premetastatic lung particularly in the BM cells. We therefore turned our attention to prosaposin (psap), a precursor of sphingolipid activator proteins, which was previously shown to stimulate the expression of Tsp-1 in human lung fibroblasts in vitro (14). Indeed, breast cancer CM from MDA cells stimulated Tsp-1 in the Gr1+ cells in the lungs, as expected. However, CM from MDA cells expressing psap-shRNA failed to induce Tsp-1 expression in the Gr1+ cells (Supplementary Fig. S10A-B). We also examined the prostate cancer model, and as expected, Western blot analysis showed elevated Tsp-1 levels in the lungs of PC3 CM-treated mice compared with LN4 CM treatment (Supplementary Fig. S10C). However, CM from isogenic PC3 cells, in which psap expression was silenced by shRNA (PC3-shpsap), failed to enhance Tsp-1 levels in the lungs (Supplementary Fig. S10C).
We also examined whether overexpression of psap in metastatic prostate cells from the orthotopic site would stimulate Tsp-1 in the lungs. Orthotopic prostate tumors were generated by injecting either metastatic LN4 cells or LN4 cells overexpressing psap (LN4-psap) in the prostate gland of mice. As expected, LN4-psap tumors induced robust Tsp-1 expression in the premetastatic lungs compared to LN4 tumors (Supplementary Fig. S10D). Taken together, these results suggest that non-metastatic tumor cells, by virtue of expressing psap, systemically elevate Tsp-1 in the recruited BM-derived Gr1+ cells in an endocrine fashion.
We next postulated that psap might directly enhance expression of Tsp-1 in recruited Gr1+ myeloid cells in the lungs to generate a metastasis-refractory environment. To determine whether the ability of psap to inhibit metastasis via stimulation of Tsp-1 could be translated into a smaller, more easily deliverable agent, we sought to determine the minimal active region of the psap protein. We first generated psap truncation mutants, which contain saposin A, saposin AB, saposin ABC, and saposin BCD (Supplementary Fig. S11A) and ectopically expressed these mutants in LN4 cells. We then analyzed CM from these cells to determine which version harbored Tsp-1 stimulating activity. All truncation mutants except saposin BCD were able to stimulate Tsp-1 activity to the same level as the full-length protein (Supplementary Fig. S11A). Thus, we concluded that saposin A contains the Tsp-1 stimulating activity of psap. We next sought to determine whether there was a smaller domain within saposin A that contained the Tsp-1 stimulating activity. Accordingly, we generated seven 20-amino acid overlapping peptides spanning the 81 amino acid sequence of saposin A (Fig. 4A). We observed that a 20-amino acid peptide spanning residues 31-50 of saposin A was the only peptide capable of stimulating the expression of Tsp-1 (Fig. 4B). We then examined the crystal structure of saposin A and determined that residues 31-50 reside in the hairpin region between helices 2 and 3 (Supplementary Fig. S11B)(27). Accordingly, we tested a cyclic 13-amino acid peptide, corresponding to residues 35-47, constrained by a disulfide bond between cysteine residues at each end of the peptide. The cyclic peptide was able to stimulate Tsp-1 (Fig. 4C). We then tested a 6-amino acid peptide, CDWLPK, corresponding to the first six residues of the cyclic peptide and found that it also was able to stimulate Tsp-1 (Fig. 4D). Finally, we tested six peptides from within the 6-amino acid sequence of CDWLPK. Of these peptides, we found that both a 5-amino acid peptide (DWLPK) and a 4-amino acid peptide (DWLP) were able to stimulate Tsp-1 expression to an even greater extent than CDWLPK (Fig. 4D). We then tested the 5-amino acid peptide in the in vivo assay by administering it to mice in combination with CM from LN4 cells and observed that it was able to stimulate Tsp-1 in the lungs of these mice (Fig. 4E).
Figure 4. Development of a psap-mimetic peptide as a pharmacological approach to upregulate Tsp-1 expression in the lungs.
A, Amino acid sequence of human saposin A. The stem and hairpin regions are depicted above the sequence. The six conserved cysteines are underlined. The boxed region represents the 20-amino acid peptide spanning residues 31-50 of saposin A, capable of stimulating the expression of Tsp-1.
B, Western blot analysis of Tsp-1 expression in WI-38 fibroblasts treated with 7 overlapping 20-amino acid peptides (1-20, 10-30, 21-40, 31-50, 41-60, 51-70 and 61-81) spanning the length of saposin A.
C, Western blot analysis of Tsp-1 in WI-38 fibroblasts which were untreated (-) or treated with a cyclic 13-amino acid peptide corresponding to residues 35-47 of saposin A.
D, Western blot analysis of Tsp-1 expression in WI-38 fibroblasts which were untreated (-) or treated with 6 peptides derived from amino acids 35-40 of saposin A ranging from 3-6 amino acids in length (1=CDWLPK, 2=DWLPK, 3=DWLP, 4=DWL, 5=WLPK, 6=LPK).
E, Western blot showing Tsp-1 levels in mouse lung tissue following administration of media alone (-), LN4 CM (CM), or LN4 CM with 30mg/kg/day psap peptide (DWLPK).
F, Representative immunofluorescence images of lungs showing Tsp-1 expression by Gr1+ cells in mice treated with DWLPK compared to a scrambled peptide (Scr). Representative images were generated from lungs of 3 mice per group (4 sections per mice, 7-12 fields per section). Arrows indicate colocalization of Gr1 and Tsp-1. Right panel; Quantitation showing Tsp-1 levels in the lungs of mice treated with either Scr or DWLPK peptide shown in the left. Identical results obtained in multiple repeat experiments.
Having determined that the 5-amino acid DWLPK peptide derived from psap retains the Tsp-1 stimulating activity of the full-length psap both in vitro and in vivo, we sought to determine whether it also targeted BM-derived cells. As such, we treated mice with CM from LN4 cells alone, to simulate a systemic tumor-induced recruitment of BM-derived stromal cells in the lungs, or CM in combination with DWLPK peptide (psap peptide). As expected, the recruitment of Gr1+ cells was identical regardless of the treatment (Supplementary Fig. S12A). However, administration of the DWLPK peptide stimulated Tsp-1 expression in the Gr1+ cells by more than 2-fold, while a peptide comprised of the same amino acids in a scrambled sequence failed to stimulate Tsp-1 (Fig. 4F, Supplementary Fig. 12B). The induction of Tsp-1 occurred systemically, as demonstrated by Western blot analysis of peripheral leukocytes from peptide-treated mice (Supplementary Fig. 12C).
These results suggest that the Tsp-1-enhancing activity of psap is confined to a 5- amino acid peptide, and that administration of this peptide could create a Tsp-1- mediated metastasis-suppressive microenvironment.
Psap mimetic peptide (DWLPK) inhibits metastases through stimulation of Tsp-1 in the Gr1+ cells
To determine if psap peptide-mediated stimulation of Tsp-1 by BM Gr1+ cells in the lung parenchyma confers a metastasis-resistant niche, we administered LLC-luc cells via tail vein, into wild-type mice treated with either DWLPK peptide or scrambled control. Strikingly, administration of the DWLPK peptide dramatically reduced lung metastases compared to the scrambled peptide, as measured by bioluminescence imaging (BLI) (Figs. 5A-B). Notably, metastatic lungs from mice (day 30) treated with DWLPK peptide showed persistent Tsp-1 upregulation in the Gr1+ myeloid compartment compared to treatment with scrambled peptide (Figs. 5C, Supplementary Fig. S12B). In agreement with previous observations, immunostaining and RT-PCR analysis showed that the Tsp-1 expression was confined to the Gr1+ cells and not to the Gr1− stromal cells, which included F4/80+ macrophages (Fig. 5C, Supplementary Fig. S13).
Figure 5. DWLPK peptide-mediated stimulation of Tsp-1 in the Gr1+ cells results in suppression of lung metastases.
A, Representative bioluminescence images of animals (n=5 per group) showing suppression of lung metastases following tail vein injection of LLC cells in mice treated with DWLPK as compared to a scrambled peptide (Scr). Identical results were obtained in multiple repeat experiments.
B, Quantitation of pulmonary metastases by bioluminescence influx (BLI). The BLI measurements were obtained at days 11,14,18,22, 26 and 29 after inoculation of LLC cells. The relative BLI values show total photon count over time, n=10. (n=5 per group). p=0.0317 between Scramble and DWLPK peptide-treated groups at day 29. Identical results were obtained in repeat experiments.
C, Quantitative RT-PCR showing Tsp-1 levels in total lungs and flow cytometry sorted CD45− cells and CD45+ cells (F4/80+ macrophages and Gr1+ myeloid cells), from LLC metastases-bearing mice treated with DWLPK compared to scrambled peptide (Scramble). (n=3 per group).
D, Quantitation of pulmonary metastases in DWLPK peptide-treated WT BMT mice and Tsp-1−/− BMT mice (n=7 per group). BLI estimates were obtained at days 0,5,8,14 and19 following tail vein injections of 1×105 LLC cells. p-values between WT BMT and Tsp-1−/− BMT groups are p=0.0049 and p=0.0084 at days 14 and 19, respectively.
E, Representative bioluminescence images showing DWLPK peptide-induced metastases suppression in WT BMT mice compared to Tsp-1−/− BMT mice depicted in panel D.
F, Representative H and E stained lungs from mice in panel E showing metastases.
G, Quantitation of metastatic area in the lungs of WT BMT and Tsp-1−/− BMT mice (n=5, each group)
H, Number of macrometastases (>1 mm diameter) in the lungs of WT BMT and Tsp-1−/− BMT mice (n=6, each group).
To confirm that the DWLPK peptide-induced Tsp-1 expression in the BM cells was directly responsible for the anti-metastatic phenotype, we performed genetic deletion of Tsp-1 in the BM by generating Tsp-1−/− BMT mice and used WT BMT mice as controls. Strikingly, the DWLPK peptide failed to inhibit metastases in Tsp-1−/− BMT mice, whereas it potently inhibited lung metastases in WT BMT mice (Fig. 5D-E). Histological examination of the lungs showed increased size of nodules (p=0.0317), as well as higher percentage of macrometastases (>1mm of diameter) in Tsp-1−/− BMT mice compared to control wild type BMT mice treated with the psap peptide (Fig. 5F-H).
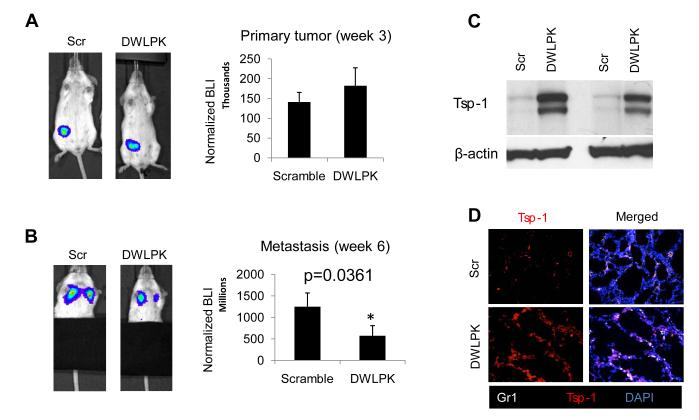
Finally, we sought to determine whether our observations with the experimental metastasis models could be recapitulated with tumors generated in the orthotopic site. As such, we tested the ability of the DWLPK peptide to inhibit metastasis in an orthotopic, clinically relevant model of breast cancer metastasis to the lung. Metastatic MDA-MB-LM2 breast cancer cells expressing luciferase were injected orthotopically in the mammary glands of SCID mice. After three weeks of growth (Fig. 6A), primary tumors were surgically resected, and one cohort of mice was treated daily with DWLPK peptide and another cohort with a scrambled peptide. Lung metastases were assessed 3 weeks after the primary tumor removal (week 6 after primary tumor injection). In DWLPK-treated mice, the metastatic burden in the lungs was significantly reduced by 50% compared to scrambled peptide-treated mice (Fig. 6B). Consistent with the mechanism of action of the peptide, Tsp-1 levels were elevated after systemic DWLPK administration, as shown by Western blot and immunofluorescence in lung extracts of these mice (Figs. 6C-D). Taken together, these results indicate that the 5-amino acid DWLPK psap peptide could have significant efficacy in treating metastatic cancer via induction of Tsp-1 in BM-derived cells in the lung microenvironment.
Figure 6. DWLPK peptide-mediated stimulation of Tsp-1 in the Gr1+ cells results in suppression of lung metastases in an orthotopic breast cancer model.
A, Left panel, Representative BLI images of animals showing orthotopic primary breast tumors derived from MDA-LM2 cells in the mammary gland of SCID mice (day 21). Right panel, Quantitation of BLI reads from mice shown in the left panel (n= 6).
B, Left panel, Representative BLI images of lungs in mice from panel A treated with either a Scramble peptide or DWLPK peptide. (Images taken 3 weeks after surgical removal of primary tumors). Right panel, Quantitation of BLI reads from mice shown in the left panel. (n= 6) (p=0.037, Mann-Whitney U test).
C, Western blot showing induction of Tsp-1 in the premetastatic lungs of LM2 tumor-bearing mice (3 weeks post primary tumor injection) treated with Scramble (Scr), or DWLPK peptide. Two representative mice per group shown, experiment was repeated and identical results obtained.
D, Representative images of premetastatic lungs immunostained for Gr1 and Tsp-1 showing upregulation of Tsp-1 in lungs from MDA-MB-LM2 tumor-bearing mice treated with DWLPK peptide compared to animals treated with scramble (Scr) peptide.
Discussion
In this study, we have demonstrated that metastasis can be acutely impacted by the tumor-induced systemic reprogramming of the BM-derived cells in the lung microenvironment. Strikingly, we observed that the very same BM-derived myeloid cells that have previously been shown to promote metastasis can also be induced to inhibit metastasis, by the paracrine activity of metastasis-incompetent primary tumors Research in metastasis suppression has been generally focused on cancer cell autonomous properties, and suppressors of metastatic tumor outgrowth, such as KISS1, MKK4, p38, and MKK7, have been identified (28, 29). Here, we have identified a novel mechanism, whereby primary tumors systemically modulate the microenvironment in distal target organs to inhibit metastasis (Fig. 7A). Significantly, we demonstrate that tumors that lack adequate metastatic potential are as capable of recruiting BM-derived CD11b+Gr1+ hematopoietic cells to potential metastatic organs as highly metastatic tumors. However, metastasis-incompetent tumors systemically stimulate expression of the anti-tumorigenic factor Tsp-1 in the recruited BM cells, converting these cells, which would otherwise serve to stimulate metastasis, into metastasis-inhibitory cells. The Tsp-1-expressing BM-derived cells thus establish a microenvironment in the potential metastatic site that is refractory to the outgrowth of metastatic tumor cells. Of significant interest is the fact that metastasis suppression was abrogated in Tsp-1 knockout BMT mice. The requirement of Tsp-1 expression in BM-derived cells suggests a novel mechanism of action in which recruited BM-derived Gr1+ cells are the chief source of Tsp-1 in the metastatic lung.
Figure 7. Prosaposin expression positively correlates with increased overall survival in prostate cancer.
A, A model showing how metastasis-incompetent primary tumors, by expressing prosaposin, can systemically generate a metastasis-suppressive microenvironment in the lungs by inducing Tsp-1 expression in the BM-derived CD11b+Gr1+ myeloid cells.
B, Immunohistochemical analysis of prosaposin protein expression in a tissue microarray showing time to biochemical failure in prostate cancer patients following radical prostatectomy.
C, Immunohistochemical analysis of prosaposin protein expression in a tissue microarray showing cancer-specific survival in prostate cancer patients following radical prostatectomy. p-values indicate significant difference for both end points; time to biochemical failure, and overall survival, p=0.027 and p=0.036, respectively.
D, Examples of high and low prosaposin staining patterns.
This is consistent with published reports showing Tsp1 expression in monocyte/macrophage populations (30, 31).
Interestingly, we have previously shown that metastatic tumors induce metastasis-promoting potential in Gr1+ cells recruited to the premetastatic lungs (32). However, depending on the nature of the tumor-derived systemic factor, the same cells can be modulated to exhibit metastasis-suppressive potential as shown in this study. It is thereby of great importance to decipher these pathways in the tumor milieu in order to further refine current therapies. Here, we show an example of an important anti-metastatic effect of Gr1+ cells. Previously, we identified prosaposin as a tumor-secreted factor with anti-metastatic activity. Based on these findings, a therapeutic strategy such as the 5 amino acid DWLPK peptide, which targets Gr1+ BM-derived cells in the microenvironment, could have significant efficacy in treating advanced metastatic cancer and ultimately in reducing the lethality of the disease.
From the perspective of targeting metastases, it has been emphasized that therapy should be targeted not only against tumor cells but also against the host microenvironment, which contributes to, and supports, the progressive growth and survival of metastatic cancer cells (33). Given that the progression of human cancer to metastatic disease is the major contributing factor to its lethality, elevating Tsp-1 levels in the metastatic organ microenvironment to suppress metastasis may have clinical value. The prosaposin-Tsp-1 axis thus appears to be relevant in vivo. Significantly, examination of a tumor tissue microarray (TMA) compiled from 103 prostate cancer patients with long and complete follow up, revealed that prosaposin expression positively correlated with increased overall survival. Specifically, patients with higher psap levels in their primary tumors had significantly greater 15-year survival rates and reduced incidence of biochemical failure (Figure 7B-D). We thus intend to further optimize the psap-derived peptide as a potential therapeutic agent for advanced metastatic cancer (Figure 7A).
METHODS
Mice and cell lines
All animal work was conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee. Wild type C57BL/6J, and GFP transgenic C57BL/6-Tg (ACTB-EGFP) 1Osb/J were obtained from The Jackson Laboratory (Bar Harbor, Maine). CB-17 SCID mice were obtained from Charles River (Wilmington, MA). Tsp1 knockout mice in the C57BL/6J background, a kind gift from Dr. Shahin Rafii (Weill Cornell Medical College, NY) were bred in the lab and genotyped according to standard protocols.
The cell lines PC3 and PC3M-LN4 are previously described (14). Human breast cancer cell lines MDA-MB-231 and MDA-MB-LM2 are described previously (Ryu et al. PLoS one, 6, 2011). The murine Lewis lung carcinoma cell line LLCs/D122 (provided by Lea Eisenbach, Wiesmann Institute of Science, Rehovot, Israel) stably expressing RFP and firefly luciferase (1-3), was cultured in DMEM supplemented with 10% fetal bovine serum. PC3, LN4, and MDA-MB-231 cell lines were obtained from ATCC. No authentication of the cell lines was conducted by the authors.
Bone marrow isolation and transplantation
BM harvest and transplantation was performed using our published methods (2, 15). BM transplantation was performed by injecting 5×106 total BM cells via tail vein into lethally irradiated (950 rads) 7-week old C57BL/6 female mice. BM cells were harvested by flushing femurs and tibias of donor animals including C57BL/6 Tg (ACTB-EGFP) 1Osb/J, C57BL/6 Wild-type, or C57BL/6 Tsp-1 knockout mice. After 4 weeks of BM engraftment, reconstitution efficiency was monitored by Western blot analysis of peripheral blood for absence of Tsp-1 protein.
Peptide generation and administration
Prosaposin truncation mutants were created by cloning the regions of prosaposin generated by PCR amplification using the following strategy: Saposin A, Saposin AB, and Saposin ABC were all created using the same 5′ primer: ggcggcgtcgacATGTACGCCCTCTTCCTCC and the following 3′ primers: saposin A: ggcgcctctagaAGAGACTCGCAGAGGTTGAG; saposin AB: ggcgcctctagaACCTCATCACAGAACCC; saposin ABC: ggcgcctctagaGCCAGAGCAGAGGTGCAGC. The PCR products were then cleaved with Xba1 and Sal1 and cloned into pCMVneo.
The psap truncation constructs were then used to transfect PC3M-LN4 using the Fugene6 transfection reagent and protocol (Roche). CM of transfected cells was harvested 48 hours after transfection and used to treat WI-38 lung fibroblasts for 16 hours. After CM treatment, cells were harvested, lysed, and protein expression analyzed by Western blot as previously described (Kang et al, 2009).
The 7 20-amino acid peptides spanning the length of saposin A were comprised of the following sequences: 1-20- SLPCDICKDVVTAAGDMLKD; 11-30- VTAAGDMLKDNATEEEILVY; 21-40- NATEEEILVYLEKTCDWLPK; 31-50- LEKTCDWLPKPNMSASCKEI; 41-60- PNMSASCKEIVDSYLPVILD; 51-70- VDSYLPVILDIIKGEMSRPG; 61-81- IIKGEMSRPGEVCSALNLCES. All peptides were synthesized by Anaspec, Inc (Fremont, CA). The 13 amino acid cyclic peptide was comprised of the following sequence: CDWLPKPNMSASC.
For in vitro analysis of peptide activity WI-38 lung fibroblasts were treated with peptide for 16 hours at a concentration of 5μg/mL. The cells were then harvested and lysed for Western blot analysis as previously described (Kang et al, 2009).
The in vivo analysis of peptide activity was performed by treating 8-week old C57/Bl6 mice with either 300μL of PC3M-LN4 CM alone or in combination with peptide, diluted in PBS, at a dose of 30mg/kg/day via intraperitoneal injection for 4 days. After 4 days the mice were euthanized by cervical dislocation under isofluorane anaesthetic. The lungs were harvested, lysed and analyzed for protein expression as previously described (Kang et al, 2009).
For metastasis experiments, the psap peptide DWLPK and a scrambled peptide LPKDW were synthesized by AnaSpec (Fremont, CA). Peptide diluted in saline was administered to mice (30 mg/kg) via intraperitoneal injection on a daily basis for six days before tumor cell injection and until the end of the metastasis assays.
Conditioned medium experiments
PC3 or PC3M-LN4 cells were cultured in RPMI with 10% FBS. 5×106 cells were then subcultured in serum-free medium in 10cm plates for 24 hours in order to generate CM. Harvested media was centrifuged and filtered through 0.22 M pore-size filters to remove any cells or cell debris.
Wild-type BMT and Tsp-1−/− BMT C57BL/6J mice were pretreated with 200 μL serum-free CM from PC3 or LN4 cells or serum-free RPMI media daily for 6 days via intraperitoneal (i.p.) injection.
Supplementary Material
Significance.
The mechanisms of metastasis suppression are poorly understood. Here, we have identified a novel mechanism, whereby metastasis-incompetent tumors generate metastasis-suppressive microenvironments in distant organs by inducing Tsp-1 expression in the BM-derived Gr1+ myeloid cells. A 5-amino acid peptide with Tsp-1 inducing activity was identified as a therapeutic agent against metastatic cancer.
Acknowledgments
We thank Bruce Zetter (Childrens Hospital, Boston) for comments on the manuscript, and Jenny Xiang of the Genomics Resources Core Facility of Weill Cornell Medical College for the microarray experiments, and Sharrell Lee, Mary Hahn, Gerd Lillian Hallseth, and Bendik Nordanger for technical support.
This study was supported by NIH grant CA135417 to VM and RW, and by grants from the Norwegian Cancer Society and the Norwegian Research Council to LAA. This study was also partially supported by the Cornell Center on the Microenvironment and Metastasis through Award Number U54CA143876 from the NCI and Robert I. Goldman Foundation to VM, and by the Elsa U. Pardee Foundation and Children’s Hospital Boston Technology Development Grant to RW. Raúl Catena was supported by fellowships from the “Government of Navarra” and the “Camara Navarra de Comercio”, Navarra, Spain.
Abbreviations
- Psap
(Prosaposin)
- Tsp1
(Thrombospondin 1)
- CM
(Conditioned medium)
- BM
(Bone Marrow)
- BMT
(Bone Marrow Transplantation)
- WBC
(White Blood Cell)
- BLI
(Bioluminiscence Imaging)
Footnotes
Conflicts of interest : Authors do not have any.
REFERENCES
- 1.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 3.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 5.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ’pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev. 2006;25:521–9. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- 7.Carlini MJ, De Lorenzo MS, Puricelli L. Cross-talk between tumor cells and the microenvironment at the metastatic niche. Curr Pharm Biotechnol. 2011;12:1900–8. doi: 10.2174/138920111798377058. [DOI] [PubMed] [Google Scholar]
- 8.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 9.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–49. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang SY, Halvorsen OJ, Gravdal K, Bhattacharya N, Lee JM, Liu NW, et al. Prosaposin inhibits tumor metastasis via paracrine and endocrine stimulation of stromal p53 and Tsp-1. Proc Natl Acad Sci U S A. 2009;106:12115–20. doi: 10.1073/pnas.0903120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21:1546–58. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–8. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starlinger P, Moll HP, Assinger A, Nemeth C, Hoetzenecker K, Gruenberger B, et al. Thrombospondin-1: a unique marker to identify in vitro platelet activation when monitoring in vivo processes. J Thromb Haemost. 2010;8:1809–19. doi: 10.1111/j.1538-7836.2010.03908.x. [DOI] [PubMed] [Google Scholar]
- 19.Xie L, Duncan MB, Pahler J, Sugimoto H, Martino M, Lively J, et al. Counterbalancing angiogenic regulatory factors control the rate of cancer progression and survival in a stage-specific manner. Proc Natl Acad Sci U S A. 2011;108:9939–44. doi: 10.1073/pnas.1105041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sund M, Hamano Y, Sugimoto H, Sudhakar A, Soubasakos M, Yerramalla U, et al. Function of endogenous inhibitors of angiogenesis as endothelium-specific tumor suppressors. Proc Natl Acad Sci U S A. 2005;102:2934–9. doi: 10.1073/pnas.0500180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–8. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nature Medicine. 2000;6:41–8. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 23.Reiher FK, Volpert OV, Jimenez B, Crawford SE, Dinney CP, Henkin J, et al. Inhibition of tumor growth by systemic treatment with thrombospondin-1 peptide mimetics. International Journal of Cancer Journal International Du Cancer. 2002;98:682–9. doi: 10.1002/ijc.10247. [DOI] [PubMed] [Google Scholar]
- 24.Raugi GJ, Mumby SM, Abbott_Brown D, Bornstein P. Thrombospondin: synthesis and secretion by cells in culture. The Journal of Cell Biology. 1982;95:351–4. doi: 10.1083/jcb.95.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebbinghaus S, Hussain M, Tannir N, Gordon M, Desai AA, Knight RA, et al. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:6689–95. doi: 10.1158/1078-0432.CCR-07-1477. [DOI] [PubMed] [Google Scholar]
- 26.Markovic SN, Suman VJ, Rao RA, Ingle JN, Kaur JS, Erickson LA, et al. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am J Clin Oncol. 2007;30:303–9. doi: 10.1097/01.coc.0000256104.80089.35. [DOI] [PubMed] [Google Scholar]
- 27.Ahn VE, Leyko P, Alattia JR, Chen L, Privé GG. Crystal structures of saposins A and C. Protein Sci. 2006;15:1849–57. doi: 10.1110/ps.062256606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shevde LA, Welch DR. Metastasis suppressor pathways--an evolving paradigm. Cancer Lett. 2003;198:1–20. doi: 10.1016/s0304-3835(03)00304-5. [DOI] [PubMed] [Google Scholar]
- 29.Cook LM, Hurst DR, Welch DR. Metastasis suppressors and the tumor microenvironment. Semin Cancer Biol. 2011;21:113–22. doi: 10.1016/j.semcancer.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaffe EA, Ruggiero JT, Falcone DJ. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. 1985;65:79–84. [PubMed] [Google Scholar]
- 31.DiPietro LA, Polverini PJ. Angiogenic macrophages produce the angiogenic inhibitor thrombospondin 1. Am J Pathol. 1993;143:678–84. [PMC free article] [PubMed] [Google Scholar]
- 32.Gao D, Joshi N, Choi H, Ryu S, Hahn M, Catena R, et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012;72:1384–94. doi: 10.1158/0008-5472.CAN-11-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- 34.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4:648–55. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.