Abstract
Background
Correlation of intraoperative frozen section diagnosis with final diagnosis can be an important component of an institution’s quality assurance process.
Methods
We performed a quality assurance review of 1207 frozen section diagnoses from 812 surgical cases performed in the Hamilton Regional Laboratory Medicine Programme during a 6-month period in 2007. We reviewed the frozen section and permanent slides from all potentially discordant cases using a multiheaded microscope to arrive at a consensus pertaining to the type and reason for error. We reviewed the clinical record to determine whether there had been a potential adverse impact on immediate clinical management.
Results
Frozen sections were most commonly requested for head and neck, nervous system and female genital tract specimens. Twenty-eight frozen sections (3%) were deferred. We identified 24 discordant diagnoses involving 3% of cases and 2% of specimens. The organ systems showing the greatest frequency of discordance relative to the total number from that system were the nervous system, head and neck, and the lungs. Of the errors identified, most occurred owing to diagnostic misinterpretation, followed by problems related to tissue sampling. There was a potential adverse impact on immediate clinical management in 14 cases.
Conclusion
Our results add to the Canadian data on the correlation between frozen sections and permanent sections; we note comparability to the concordance rates reported in the literature.
Abstract
Contexte
La corrélation entre le diagnostic fondé sur une analyse peropératoire des coupes congelées et le diagnostic final pourrait être un élément important du processus d’assurance qualité dans les établissements de santé.
Méthodes
À des fins d’examen de l’assurance qualité, le Programme régional de médecine de laboratoire d’Hamilton a procédé à une revue de 1207 diagnostics fondés sur l’analyse de coupes congelées prélevées lors de 812 interventions chirurgicales au cours d’une période de 6 mois en 2007. Nous avons analysé les coupes congelées et les spécimens fixés pour tous les cas potentiellement discordants à l’aide d’un microscope multitête, dans la recherche d’un consensus quant au type d’erreur et à la raison de celleci. Nous avons passé en revue les dossiers cliniques pour mesurer, le cas échéant, un quelconque impact négatif sur la prise en charge clinique immédiate.
Résultats
Les coupes congelées ont le plus souvent été demandées pour des spécimens de tissu de la tête et du cou, du système nerveux et des voies génitales féminines. Vingthuit coupes congelées (3 %) ont été écartées. Nous avons relevé 24 diagnostics discordants concernant 3 % des cas et 2 % des spécimens. Les systèmes et organes pour lesquels la fréquence de la discordance a été la plus élevée par rapport au nombre total de spécimens du même type, ont été le système nerveux, la tête et le cou et les poumons. Parmi les erreurs relevées, la plupart ont été attribuables à une mauvaise interprétation diagnostique, suivie de problèmes relatifs au prélèvement tissulaire. Dans 14 cas, l’erreur a pu exercer un impact négatif sur la prise en charge clinique immédiate.
Conclusion
Nos résultats viennent étayer les données canadiennes sur la corrélation entre les coupes congelées et les lames adhérentes; nous notons que nos taux de concordance sont comparables à ceux qui sont cités dans la littérature.
The primary purpose of intraoperative pathologist consultation (IC) is to guide immediate surgical management;1 ICs can provide surgeons with important information that may be used to modify or even terminate a surgical procedure. Frozen sections performed during ICs can also be used to establish the nature and extent of a lesion, to determine the status of surgical margins and to confirm that sampling of lesional tissue is sufficient for further investigations.2
Periodic review of the correlation between frozen section diagnosis and final diagnosis is useful for several reasons. It can serve as a measure of an institution’s quality of service.3 Once errors are identified, the potential cause of the frozen section error can be investigated, and measures can be implemented to help prevent similar occurrences. Errors can occur because of diagnostic misinterpretation (i.e., the pathologist may make a false diagnosis or miss the diagnosis on the frozen section slide); the frozen sections may not be taken from lesional tissue; or technical issues, such as tissue section folds or uneven staining, may preclude proper evaluation.1,2,4,5 The impact of frozen section errors on changes to diagnoses can also be investigated, and some studies have categorized these as false-positive or false-negative frozen section diagnoses.1,2,4,5
The accuracy of frozen section diagnosis can be documented by comparing the diagnosis made on the frozen sections to the final diagnosis made on the pathology specimen after review of both the frozen and permanent sections. Given the limited amount of tissue that can be submitted or sampled during frozen section examination, and given the technical quality of frozen sections compared with permanent sections, discrepancies can be expected between frozen section diagnoses and final diagnoses. The literature reports discordance rates between frozen section diagnoses and final diagnoses ranging from 1.4% to 12.9%; about 75% of studies report a discordance rate below 5%, with an overall median of 2.9%.5–30 Whereas most studies have focused on the discordance between frozen section diagnoses and final diagnoses, 1 study reported that errors impacting patient care, defined as frozen section errors that may have affected intraoperative patient management, occurred in 0.1% of the frozen sections performed.5
We report the results of a review of ICs performed in the Hamilton Regional Laboratory Medicine Program (HRLMP) in Hamilton, Ont., and add to the Canadian data. This review was performed by a panel of staff pathologists and senior pathology residents from the HRLMP academic hospitals. We assessed the discordance rate between frozen section diagnoses and final diagnoses, the causes of frozen section errors and the impact of change in diagnosis on patient care.
Methods
We obtained a list of all ICs performed in the HRLMP from the electronic medical records system covering 6 consecutive months of the 2007 calendar year. A review of 6 months’ worth of consecutive records provided a sufficient number of nonbiased cases balanced against the logistical/time constraints of a longer period of review. We chose the 2007 calendar year because of its relatively recent timeframe and because of the reasonably lengthy period of follow-up for which data would be available. We excluded lymph nodes received for the purpose of lymphoma protocol, as these cases are not appropriate for frozen section interpretation.
In our institution, quality assurance reviews are exempt from research ethics board oversight; nonetheless, patient confidentiality and security of the medical record were adhered to strictly. The list of ICs was divided among a panel of 6 participating senior residents, under the supervision of 3 staff pathologists. Each resident reviewed the IC report, the final report and the pertinent patient clinical data for each surgical specimen. Subsequently, a panel of residents and staff pathologists reviewed all the relevant slides identified as potentially discordant using a multi-headed microscope. For each IC, with panel consensus, the following data were recorded: the organ/tissue type submitted for IC, the intraoperative and final diagnoses, whether the IC diagnosis was deferred, whether there was an intraoperative discordance, the type of error, the reason for the error and the immediate intraoperative impact. The type of error was classified as described by the Association of Directors of Anatomic and Surgical Pathology:
change in category, (i.e., from benign to malignant or vice versa) leading to false-positive or false-negative IC;
change within the same category (e.g., the histologic type of malignancy);
change in the status of the resection margin (i.e., false-positive or false-negative for malignancy); and
change in lymph node status (i.e., false-positive or false-negative for malignancy).31 The reason for the IC error was categorized as
diagnostic misinterpretation;
specimen sampling error (i.e., the tissue submitted for frozen section did not contain the pathologic lesion that was subsequently identified in the additional tissue submitted for permanent sections);
problem in block sampling (i.e., the pathologic lesion was present only in deeper permanent sections taken of the frozen section block); or
technical error (e.g., suboptimal quality of the frozen section slide, such as tissue folding).5
We determined whether the change in diagnosis would potentially have led to different intraoperative management by reviewing the operative report and clinical chart.
Results
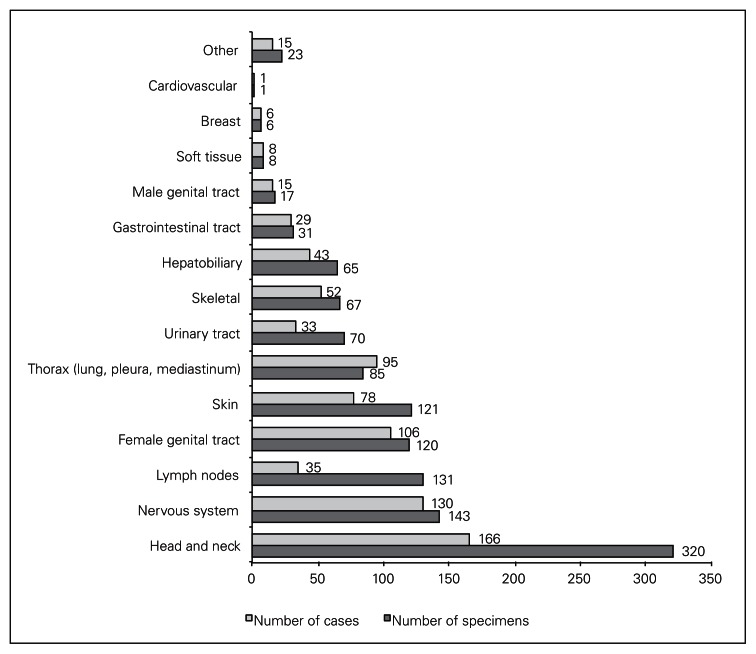
In the 6-month period of review, ICs were performed in 812 cases for 1208 specimens. Most ICs were for specimens from the head and neck, nervous system, lymph nodes, skin and female genital tract (Fig. 1).
Fig. 1.
Distribution of intraoperative consultations, by organ system.
Intraoperative diagnoses were deferred for 28 (3%) patients. Deferred diagnoses involved specimens from the head and neck (n = 6), thorax (n = 5), female genital tract (n = 5), lymph nodes (n = 5), nervous system (n = 3), skeletal system (n = 2), skin (n = 1) and soft tissue (n = 1).
Intraoperative pathologist consultation diagnoses on 24 (2%) specimens (1 from each of 24 [3%] patients) were discordant with the final diagnoses (Table 1). Frozen sections had been performed on all 24 specimens. Five of the specimens with discordant diagnoses were from the nervous system; 4 from the head and neck; 4 from the female genital tract; 3 from the thorax; 3 from the lymph nodes; 2 from the skeletal system; and 1 each from the skin, urinary tract and hepatobiliary tract.
Table 1.
Discordant cases
| Organ system (specimen) | Frozen section diagnosis | Permanent diagnosis | Reason for error | Impact on pathology report | Potential intraoperative impact |
|---|---|---|---|---|---|
| Central nervous system (sellar lesion) | Pituitary adenoma | Metastatic adenocarcinoma | Misinterpretation | Category change: false negative | No |
| Central nervous system (brain) | Reactive astrocytosis | Metastatic carcinoma | Specimen sampling | Category change: false negative | No |
| Central nervous system (brain) | Ganglioglioma | Glioblastoma | Misinterpretation | Category change: false negative | No |
| Central nervous system (brain) | Multifocal perivascular inflammation with necrosis | Diffuse B cell lymphoma | Misinterpretation | Category change: false negative | No |
| Central nervous system (brain) | Oligodendroglioma | Progressive multifocal leukencephalopathy | Misinterpretation | Category change: false positive | No |
| Head and neck (thyroid) | Adenomatous hyperplastic nodule | Microscopic papillary thyroid carcinoma | Specimen sampling | Category change: false negative | No |
| Head and neck (thyroid) | Atypical follicular adenoma | Folicular variant papillary thyroid carcinoma | Other* | Category change: false negative | Yes |
| Head and neck (parathyroid) | Follicular neoplasm | Parathyroid adenoma | Misinterpretation | Change within same category | No |
| Head and neck (tongue margin) | Squamous cell carcinoma | Negative for malignancy | Misinterpretation | Margin status change: false positive | Yes |
| Female genital tract (ovary) | Mucinous cystadenoma, possible borderline | Serous borderline tumour | Misinterpretation | Change within same category | Yes |
| Female genital tract (ovary) | Mucinous cystadenoma | Mucinous borderline tumour | Specimen sampling | Category change: false negative | Yes |
| Female genital tract (ovary) | Serous tumour, favour benign | Mucinous cystadenoma | Misinterpretation | Change within same category | No |
| Female genital tract (ovary) | Favour endometrioid adenocarcinoma of ovary | Seromucinous borderline tumour with microinvasion | Misinterpretation | Category change: false positive | No |
| Thorax (bronchial margin) | Negative for malignancy | Microscopic focus of carcinoma | Misinterpretation | Margin status change: false negative | Yes |
| Thorax (bronchial margin) | Severe squamous dysplasia | Mild squamous dysplasia | Misinterpretation | Change within same category | No |
| Thorax (lung) | Benign hyalinized calcified necrotic tissue with bony metaplasia | Nodular parenchymal amyloid | Other | Change within same category | No |
| Lymph node (mediastinal) | Negative for malignancy | Microscopic focus non- small cell carcinoma | Misinterpretation and technical error | Lymph node status change: false negative | Yes |
| Lymph node (neck) | Negative for malignancy | Squamous cell carcinoma | Block sampling | Lymph node status change: false negative | Yes |
| Lymph node (omental) | Negative for malignancy | Mantle cell lymphoma | Misinterpretation | Lymph node status change: false negative | No |
| Skeletal (femur) | Cluster of atypical cells, suspicious of malignancy | Benign | Misinterpretation | Category change: false positive | No |
| Skeletal (humerus) | Atypical infiltrate, suggestive of malignancy | Negative for malignancy | Misinterpretation | Category change: false positive | No |
| Skin (margin) | Negative for malignancy | Squamous cell carcinoma | Specimen sampling | Margin status change: false negative | Yes |
| Urinary tract (ureteric margin) | Low grade dysplasia | No dysplasia | Misinterpretation | Margin status change: false positive | No |
| Hepatobiliary (pancreatic margin, Whipple procedure) | Adenocarcinoma | Negative for malignancy | Misinterpretation | Margin status change: false positive | Yes |
The reason for error in this case was classified as “other” since the lesions “atypical follicular adenoma” and “follicular variant of papillary carcinoma” can demonstrate overlapping histological features such that only exceedingly subtle differences may be perceivable by frozen section assessment; it was inappropriate in this case, therefore, to classify the discrepancy as a “misinterpretation.”
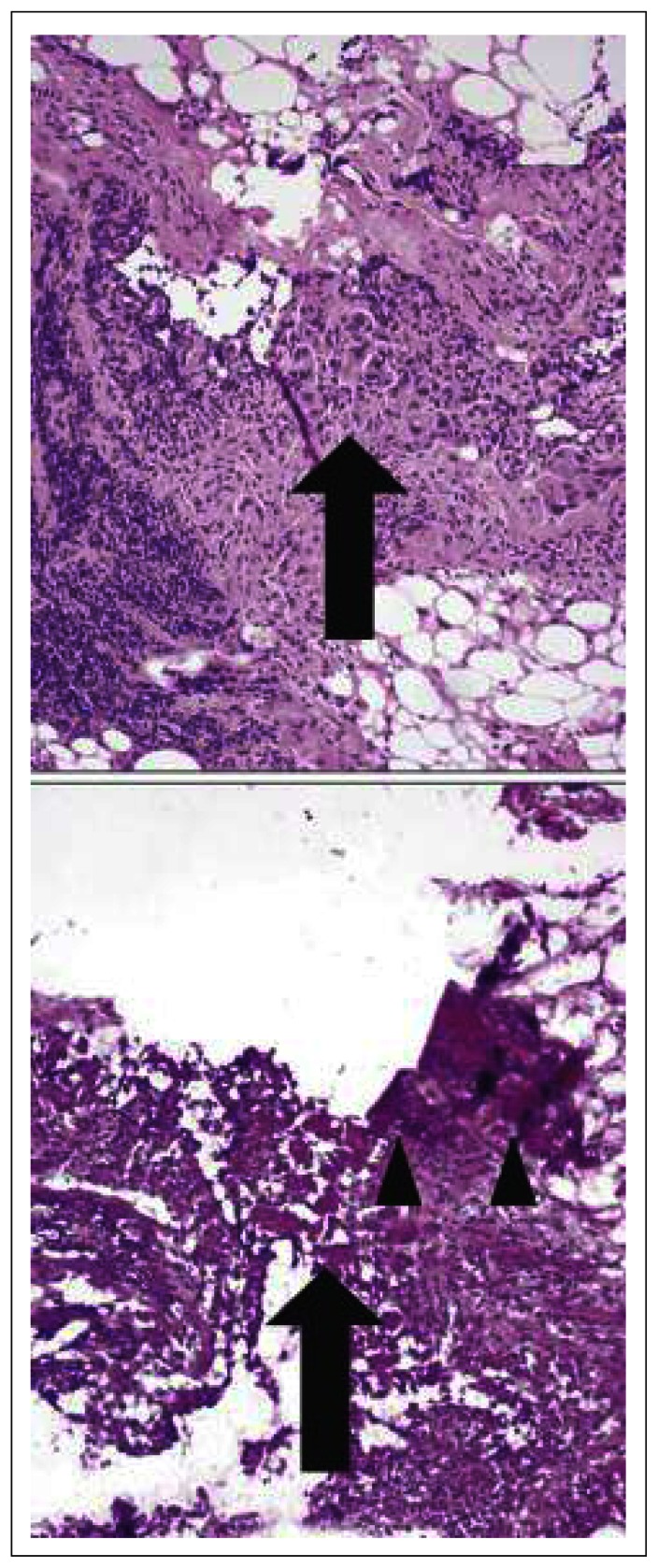
The most common cause of error was misinterpretation of the frozen section, and this involved 17 specimens. In 4 specimens, the diagnostic area was not present in the block submitted for frozen section, in 1 specimen the diagnostic area was present only on deeper sections of the frozen section block, and in 1 specimen technical issues of slide preparation contributed to diagnostic misinterpretation (Fig. 2).
Fig. 2.
Example of an exceptional discordant case in which 2 discrepancies were noted. The large arrow indicates corresponding frozen section and permanent section area involved by metastatic carcinoma (misinterpreted as negative on frozen section assessment). The arrowheads indicate “folding” resulting from technical error.
Fourteen discordant cases required a change in diagnostic category as a result of the IC discrepancy, 4 resulted in changes in margin status, and 1 resulted in a change in lymph node status. Twelve specimens were reported as benign at frozen section diagnosis, but were identified as malignant or borderline on final diagnosis.
It was the opinion of the review panel that for 14 patients, change in the IC diagnosis would not have resulted in different intraoperative management. However, it was felt that immediate management could have been affected in the remaining 10 patients, resulting in potential adverse clinical impact in 1.2% of patients.
Discussion
Periodic quality reviews examining discordance rates of ICs relative to final diagnoses are helpful for a variety of reasons. Crude discordance rates may assist regulatory bodies in making decisions pertaining to accreditation.31 Anatomic site–specific discordance rates may inform individual pathologists of cases requiring more detailed examination or consultation from subspecialist pathologists when available.17 Analysis of common errors may reveal specific areas of diagnostic pathology in which further training is required.31 Workload and degree of surgical complexity can also be revealed in great detail.31
The discordance rate observed in the present study is comparable to reported data.5–30 In our study, frozen sections for surgical cases from the head and neck, nervous system and female genital tract were most common and were also among the most likely to demonstrate discordance with the final diagnosis. Although fewer frozen sections were performed for thoracic and musculoskeletal specimens, these cases showed a similarly high relative discordance rate. Similar to the results of most published reports, most IC discrepancies in the present study were attributed to misinterpretation and resulted in a change in diagnostic category.5–30
We estimated that in a little more than half of the patients with discrepant IC diagnoses, the correct diagnosis would not have changed the immediate intraoperative management. However, this estimate is based on a retrospective review of the clinical notes and the judgment of the panel of reviewers. It remains feasible that surgery could have been adversely affected in a larger number of patients. However, this also raises the possibility that in at least some cases, the request for IC was unnecessary and inappropriate in that the result was not requested with the goal of guiding immediate surgical decisions.
Conclusion
It would be useful to prospectively document the reasons for intraoperative consultations, with the assistance of our surgical colleagues, and document how discordant final diagnoses might affect patient management. Furthermore, this cooperative, prospective approach might allow for a more definite assessment of the discordance rates for intraoperative consultations performed by subspecialty pathologists in comparison with those performed by general surgical pathologists.
Footnotes
A poster summary of this study was presented at the Canadian Association of Pathologists 2011 Annual Meeting in Vancouver, BC, June 6–7, 2011, and the Department of Pathology & Molecular Medicine Residents’ Research Day, May 20, 2010.
Competing interests: None declared.
Contributors: All authors contributed to the study design, data acquisition and analysis, article writing and review, and each author approved publication.
References
- 1.Rosai J. Introduction. In: Rosai J, editor. Rosai & Ackerman’s surgical pathology. 9th ed. Vol. 1. Philadelphia (PA): Mosby/Elsevier; 2004. pp. 1–23. [Google Scholar]
- 2.Raab SS, Tworek JA, Souers R, et al. The value of monitoring frozen section-permanent section correlation data over time. Arch Pathol Lab Med. 2006;130:337–42. doi: 10.5858/2006-130-337-TVOMFS. [DOI] [PubMed] [Google Scholar]
- 3.The Path2Quality Executive. A proposal for laboratory physicians in Ontario. Toronto (ON): Ontario Medical Association and the Ontario Association of Pathologists; 2011. [accessed 2012 Mar. 30]. Standards2Quality: Guidelines for quality management in surgical pathology professional practices. Available: www.ontariopathologists.org/resources/quality/2011-03-31_S2Q_Proposal.pdf. [Google Scholar]
- 4.du Boulay C. Error trapping and error avoidance in histopathology. In: Lowe DG, Underwood JCE, editors. Recent advances in histopathology. London (UK): RSM Press; 2003. pp. 103–114. [Google Scholar]
- 5.Ferreiro JA, Myers JL, Bostwick DG. Accuracy of frozen section diagnosis in surgical pathology: review of a 1-year experience with 24,880 cases at mayo clinic rochester. Mayo Clin Proc. 1995;70:1137–41. doi: 10.4065/70.12.1137. [DOI] [PubMed] [Google Scholar]
- 6.Zarbo RJ, Hoffman GG, Howanitz PJ. Interinstitutional comparison of frozen-section consultation. A College of American Pathologists Q-Probe study of 79,647 consultations in 297 North American institutions. Arch Pathol Lab Med. 1991;115:1187–94. [PubMed] [Google Scholar]
- 7.Ahmad Z, Barakzai MA, Idrees R, et al. Correlation of intra-operative frozen section consultation with the final diagnosis at a referral center in Karachi, Pakistan. Indian J Pathol Microbiol. 2008;51:469–73. doi: 10.4103/0377-4929.43733. [DOI] [PubMed] [Google Scholar]
- 8.Aijaz F, Muzaffar S, Hussainy AS, et al. Intraoperative frozen section consultation: an analysis of accuracy in a teaching hospital. J Pak Med Assoc. 1993;43:253–5. [PubMed] [Google Scholar]
- 9.Altaf FJ. Audit of breast frozen sections. Ann Saudi Med. 2004;24:141–4. doi: 10.5144/0256-4947.2004.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerski CT, Lopes MF, Kliemann LM, et al. Transoperative anatomopathologic examinations: quality control. Rev Assoc Med Bras. 1994;40:243–6. [PubMed] [Google Scholar]
- 11.Collina G, Caprara G, Di Tommaso L. Quality control in pathological anatomy: 10 years’ experience. Pathologica. 2003;95:171–8. [PubMed] [Google Scholar]
- 12.Donisi PM. Problems and limitations of frozen section diagnosis. A review of 470 consecutive cases. Pathologica. 1991;83:467–75. [PubMed] [Google Scholar]
- 13.Gandour-Edwards RF, Donald PJ, Lie JT. Clinical utility of intraoperative frozen section diagnosis in head and neck surgery: a quality assurance perspective. Head Neck. 1993;15:373–6. doi: 10.1002/hed.2880150502. [DOI] [PubMed] [Google Scholar]
- 14.Gandour-Edwards RF, Donald PJ, Lie JT. Clinical utility of intraoperative frozen section diagnosis in head and neck surgery: a quality assurance perspective. Head Neck. 1993;15:373–6. doi: 10.1002/hed.2880150502. [DOI] [PubMed] [Google Scholar]
- 15.Gephardt GN, Zarbo RJ. Interinstitutional comparison of frozen section consultations. A College of American Pathologists Q-Probes study of 90,538 cases in 461 institutions. Arch Pathol Lab Med. 1996;120:804–9. [PubMed] [Google Scholar]
- 16.Howanitz PJ, Hoffman GG, Zarbo RJ. The accuracy of frozen-section diagnoses in 34 hospitals. Arch Pathol Lab Med. 1990;114:355–9. [PubMed] [Google Scholar]
- 17.Ismiil N, Ghorab Z, Nofech-Mozes S, et al. Intraoperative consultation in gynecologic pathology: a 6-year audit at a tertiary care medical center. Int J Gynecol Cancer. 2009;19:152–7. doi: 10.1111/IGC.0b013e318199617b. [DOI] [PubMed] [Google Scholar]
- 18.Khoo JJ. An audit of intraoperative frozen section in johor. Med J Malaysia. 2004;59:50–5. [PubMed] [Google Scholar]
- 19.Nemoto N, Sakurai I, Baba S, et al. A study of intraoperative rapid frozen section diagnosis focusing on accuracy and quality assessment. Rinsho Byori. 1992;40:1319–28. [Article in Japanese] [PubMed] [Google Scholar]
- 20.Nigrisoli E, Gardini G. Quality control of intraoperative diagnosis. annual review of 1490 frozen sections. Pathologica. 1994;86:191–5. [Article in Italian] [PubMed] [Google Scholar]
- 21.Nofech-Mozes S, Hanna WM, Cil T, et al. Intraoperative consultation for axillary sentinel lymph node biopsy: an 8-year audit. Int J Surg Pathol. 2010;18:129–37. doi: 10.1177/1066896909332114. [DOI] [PubMed] [Google Scholar]
- 22.Novis DA, Gephardt GN, Zarbo RJ College of American Pathologists. Interinstitutional comparison of frozen section consultation in small hospitals: a College of American Pathologists Q-Probes study of 18,532 frozen section consultation diagnoses in 233 small hospitals. Arch Pathol Lab Med. 1996;120:1087–93. [PubMed] [Google Scholar]
- 23.Oneson RH, Minke JA, Silverberg SG. Intraoperative pathologic consultation. an audit of 1,000 recent consecutive cases. Am J Surg Pathol. 1989;13:237–43. [PubMed] [Google Scholar]
- 24.Pirini MG, Eusebi V. Quality control of intraoperative diagnosis. Pathologica. 1996;88:29–35. [Article in Italian] [PubMed] [Google Scholar]
- 25.Plesec TP, Prayson RA. Frozen section discrepancy in the evaluation of nonneoplastic central nervous system samples. Ann Diagn Pathol. 2009;13:359–66. doi: 10.1016/j.anndiagpath.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Rao S, Rajkumar A, Ehtesham MD, et al. Challenges in neurosurgical intraoperative consultation. Neurol India. 2009;57:464–8. doi: 10.4103/0028-3886.55598. [DOI] [PubMed] [Google Scholar]
- 27.Rogers C, Klatt EC, Chandrasoma P. Accuracy of frozen-section diagnosis in a teaching hospital. Arch Pathol Lab Med. 1987;111:514–7. [PubMed] [Google Scholar]
- 28.Teichmann W, Rost W, Thieme D, et al. Intraoperative consultation as an instrument of quality management. World J Surg. 2009;33:6–11. doi: 10.1007/s00268-008-9786-3. [DOI] [PubMed] [Google Scholar]
- 29.Wendum D, Flejou JF. Quality assessment of intraoperative frozen sections in a teaching hospital: an analysis of 847 consecutive cases. Ann Pathol. 2003;23:393–9. [PubMed] [Google Scholar]
- 30.White VA, Trotter MJ. Intraoperative consultation/final diagnosis correlation: relationship to tissue type and pathologic process. Arch Pathol Lab Med. 2008;132:29–36. doi: 10.5858/2008-132-29-IFDCRT. [DOI] [PubMed] [Google Scholar]
- 31.Association of Directors of Anatomic and Surgical Pathology. Recommendations for quality assurance and improvement in surgical and autopsy pathology. Am J Surg Pathol. 2006;30:1469–71. doi: 10.1097/01.pas.0000213303.13435.27. [DOI] [PubMed] [Google Scholar]