Abstract
Purpose
Intraoperative blood loss in scoliosis surgery often requires transfusions. Autogenous blood decreases but does not eliminate risks typically associated with allogenic blood transfusion. Costs associated with transfusions are significant. Tranexamic acid (TXA) has been shown to decrease blood loss in cardiac and joint surgery. Few studies have examined its use in pediatric spine surgery, and the results are inconsistent. The aim of this study was to determine whether TXA decreases intraoperative blood loss and transfusion requirements in adolescent idiopathic scoliosis patients undergoing posterior spinal fusion by a single surgeon.
Methods
The medical records and operative reports of surgically treated patients with adolescent idiopathic scoliosis between 2000 and 2009 were retrospectively reviewed. The inclusion criteria were: (1) patients who underwent instrumented posterior spinal fusion, (2) had complete medical records, and (3) were treated by the same surgeon. Forty-nine patients who met the inclusion criteria were divided into two groups: Group A (25 patients) received TXA, while Group B (24 patients) did not receive TXA.
Results
After controlling for age at the time of surgery, gender, and number of vertebral levels fused, the mean intraoperative blood loss was significantly lower in Group A (537 ml) than in Group B (1,245 ml) (p = 0.027). The mean volume of blood transfused intraoperatively was 426 and 740 ml for Group A and Group B, respectively. The difference was not statistically significant after controlling for age, gender, and number of levels fused (p = 0.078).
Conclusion
TXA significantly decreased intraoperative blood loss in posterior spinal fusions performed for adolescent idiopathic scoliosis.
Keywords: Adolescent idiopathic scoliosis, Tranexamic acid, Transfusion, Spinal fusion
Introduction
Posterior spinal fusion surgery for adolescent idiopathic scoliosis is often associated with significant blood loss requiring transfusion due to prolonged operative times, extensive soft tissue dissection, and significant bone bleeding during instrumentation and decortications [1–4]. Allogenic blood transfusion has several inherent risks, including the transmission of blood-borne pathogens as well as hemolytic and immune-mediated transfusion reactions, such as graft versus host disease [5]. Homologous blood, when available, decreases but does not eliminate the risks associated with transfusion [1, 6–9]. Postoperative infections have been associated with the immunomodulatory effects of homologous transfusions [10–14]. In addition, the costs associated with transfusions are significant [15].
Tranexamic acid [TXA, 4-(aminomethyl)cyclohexanecarboxylic acid], a synthetic lysine analog, acts as an antifibrolytic agent by binding reversibly to plasminogen and plasmin and completely blocking the interaction of plasminogen and plasmin with lysine on the surface of fibrin [16]. Thus, TXA inhibits fibrinolysis by preventing the proteolytic action of plasmin on fibrin at the surgical wound [16]. The inhibition of fibrinolysis by TXA is not readily analyzed by a simple inhibition model, due to multiple overlapping ligand–kringle interactions or tranexamic–fibrin interactions [17]. It has been shown that TXA decreases blood loss in a variety of settings, including hepatic, ocular trauma, oral, nasal, gynecologic, and cardiac surgery [16–21]. In orthopedics, TXA has been shown to be effective in joint surgery [22–25]. Up to this point and to our knowledge, there are only a few studies evaluating the role of TXA in pediatric spine surgery, but there is a lack of agreement concerning the reduction of both blood loss and transfusion requirements [26–29]. Moreover, in the majority of these studies, the pediatric population was mixed and included both idiopathic and nonidiopathic patients, and, in all of these studies, surgeries were performed by different surgeons employing different surgical techniques and having variable surgical experience.
The purpose of this study was to determine whether using TXA may affect intraoperative blood loss and transfusion requirements in adolescent idiopathic scoliosis patients undergoing posterior spinal fusion by a single surgeon.
Materials and methods
After obtaining Institutional Review Board approval, the medical records and operative reports of surgically treated patients with adolescent idiopathic scoliosis between 2000 and 2009 were retrospectively reviewed. The inclusion criteria were: (1) patients who underwent instrumented posterior spinal fusion with either all pedicle screw or hybrid hook–screw constructs, (2) had complete medical records, and (3) were treated by the same surgeon. Patients who underwent revision spine surgery were excluded.
Clinical data for each patient, including age, gender, and number of vertebral levels fused, was collected. Intraoperative blood loss and intraoperative transfusion data were obtained from the operative reports and chart reviews.
Patients were divided into two groups: Group A consisted of patients who received TXA intraoperatively, while Group B included patients who did not receive TXA. In Group A, TXA was infused at a high rate, which consisted of an initial loading dose of 100 mg, followed by a dose of 10 mg/h throughout the entire case.
The surgical team’s indication for an intraoperative transfusion was a hemoglobin level of 7.0 g/dL or less. A cell saver was used in all patients.
Independent t-tests compared the continuous variables of age and number of levels fused between groups. A Fisher exact test compared the dichotomous variable of sex between groups. Two linear regressions were performed in order to determine the effect of the number of levels fused on intraoperative blood loss and on transfusion volume. All tests were calculated with use of the SPSS, version 21.0 (SPSS Inc., Chicago, IL) statistical package for personal computers. Statistical significance was set at p ≤ 0.05.
Results
Among 478 adolescent idiopathic scoliosis cases surgically treated during the study period, 49 met the inclusion criteria. There were 43 females and 6 males, with an average age of 14.1 ± 1.5 years (range, 11–19 years). Group A included 25 patients (21 females and 4 males), with an average age at the time of surgery of 14.7 years (range, 12–19 years). Group B included 24 patients (22 females and 2 males), with an average age at the time of surgery of 13.5 years (range, 11–16 years). Nine patients in Group A (36 %) and 11 patients in Group B (46 %) were operated during the period 2000–2004.
The mean number of levels fused was 10.7 (range, 8–14) for Group A and 12.6 (range, 6–15) for Group B. This difference was found to be statistically significant (p < 0.001). The number of levels fused showed a significantly positive linear relationship with both intraoperative blood loss (r = 0.41, p = 0.004) and transfusion volume (r = 0.37, p = 0.012).
Due to differences between groups in age and the number of levels fused, intraoperative blood loss and transfusion volume were compared between groups using stepwise linear regressions with group as the sole predictor in step 1 and group, age, sex, and number of levels fused as predictors in step 2. The adjusted values were calculated using the obtained regression equations:
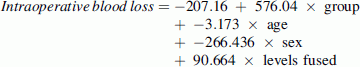 |
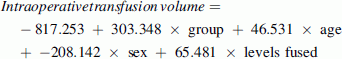 |
Group was coded as 0 for A and 1 for B. The mean age of the sample, which was 14.1 years, was entered for the age. Sex was coded as 1 for females and −1 for males. The mean number of levels fused for this sample, which was 11.6, was entered for the number of levels fused. The mean intraoperative blood loss prior to adjustment was significantly lower in Group A (537 ml) than Group B (1,245 ml) (r = 0.48, p = 0.001) (Table 1). The difference remained significant after controlling for age, sex, and number of levels fused (800 vs. 1,376 ml, respectively; r = 0.33, p = 0.027). The mean volume of blood transfused intraoperatively was also significantly lower for Group A (426 ml) than Group B (740 ml) (r = 0.34, p = 0.022). However, the difference in the volume of blood transfused intraoperatively was found to be nonsignificant after controlling for age, sex, and number of levels fused (598 vs. 902 ml, respectively; r = 0.27, p = 0.078). Four patients in Group A (16 %) and 7 patients in Group B (29 %) received a transfusion during the postoperative period.
Table 1.
Intraoperative data
| Group A | Group B | Difference | ||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | r | p-Value | |
| Intraoperative blood loss (ml) | 537 ± 320 | 1,245 ± 896 | 0.48 | 0.001* |
| Adjusted intraoperative blood loss (ml)a | 800 | 1,376 | 0.33 | 0.027* |
| Male | 1,066 | 1,642 | ||
| Female | 533 | 1,109 | ||
| Transfusion volume (ml) | 426 ± 247 | 740 ± 604 | 0.34 | 0.022* |
| Adjusted transfusion volume (ml)a | 598 | 902 | 0.27 | 0.078 |
| Male | 807 | 1,110 | ||
| Female | 390 | 694 | ||
SD standard deviation
aAdjusted based on a mean age of 14.1 and mean levels fused of 11.6
* p ≤ 0.05 indicates statistical significance
Discussion
TXA is a synthetic lysine analog similar to ε-aminocaproic acid that acts as an antifibrinolytic agent by competitively blocking the lysine binding site of plasminogen and plasmin, thus, stabilizing fibrin clots [16, 30]. Although TXA is approximately 6–10 times more potent than ε-aminocaproic acid [31, 32], there are no studies that directly compare the effectiveness of these two drugs on pediatric scoliosis surgery. Unlike aprotinin, a serine protease inhibitor, TXA has not been associated with cardiac or renal failure, myocardial infarction, stroke, or death [19, 20, 33]. TXA has been shown to decrease blood loss in many types of surgery, but few studies have been devoted to its use in scoliosis surgery [26–29]. The results of those few studies have been positive, but inconsistent. In a study of patients undergoing posterior spinal fusion for scoliosis, Sethna et al. [27] revealed a 41 % decrease in intraoperative blood loss after the administration of TXA. No difference was found in the amount of blood transfused. The major drawback of the study was the fact that a great number of patients had the diagnosis of Duchenne muscular dystrophy, a condition that typically results in higher intraoperative blood loss than idiopathic scoliosis [34]. In another study examining the difference in blood loss between scoliosis patients undergoing posterior spinal fusion receiving TXA versus placebo, Neilipovitz et al. [28] found no significant differences in intraoperative blood loss, but observed a 28 % decrease in transfusions. However, the authors studied a mixed population comprised of both idiopathic and nonidiopathic patients. The major difference in these two studies was in the dose of TXA used. Sethna et al. [27] used a higher dose of TXA at 100 mg/kg load followed by 10 mg/kg/h, while Neilipovitz et al. [28] used a lower dose loading at 10 mg/kg followed by 1 mg/kg/h. In a recent study, when two TXA dosing regimens were evaluated, a dose-dependent effect of TXA on the transfusion requirements was recorded [29]. Although only adolescent idiopathic scoliosis patients were examined, the number of patients included in the study was too low, while surgeries were performed by different surgeons. Shapiro et al. [26] found a 42 % decrease in blood loss and a 46 % decrease in transfusion volumes in a study retrospectively examining blood loss in spinal fusions performed for scoliosis secondary to Duchenne muscular dystrophy. When we studied only adolescent idiopathic scoliosis patients treated by the same surgeon with or without the use of TXA, there was significantly less blood loss for patients receiving an initial loading dose of TXA of 100 mg followed by a dose of 10 mg/h throughout the entire case.
All types of blood transfusion have an inherent level of risk, even preoperatively donated autologous blood [1, 5–7]. Popovsky et al. [7] examined over 4 million American Red Cross whole-blood donation records and noted that autologous donors were 12 times as likely as general blood donors to undergo an adverse event serious enough to require hospitalization. Several studies have also found that autologous blood is also transfused at a higher rate than homologous blood [1, 8, 35]. Simpson et al. [1] reviewed 5 years of autologous blood transfusions at Children’s Hospital of Denver and found that 11 % of autologous and 4 % of homologous transfusions were given when not indicated. Fifty-four of the 55 nonindicated autologous transfusions were given postoperatively. The reasons for this was unclear, although they may have been transfused simply because autologous blood was available and thought to be safe. Ridgeway et al. [36] examined the use of autologous blood transfusion in scoliosis surgery and, similarly, demonstrated that 95 % of autologous units were infused, while only 51 % of cross-matched homologous units were infused, despite there being no significant differences in blood loss or laboratory values between the groups.
The overall risk of infection from blood products is declining due to improved screening methods, but awareness of the number of transmissible pathogens is increasing [9]. Although rare, documented transmission of variant Creutzfeldt–Jakob disease, West Nile Virus, babesiosis, and sleeping sickness has occurred via blood transfusion [9]. Additionally, there is evidence to support that uninfected allogenic transfusion causes immunosuppression and increases the rate of postoperative infection in orthopedic surgery [10–14]. Triulzi et al. [10] found a markedly higher rate of infection in postoperative spinal fusion patients who received allogenic transfusions (20.8 %) as opposed to those who did not (3.8 %). The mechanism behind transfusion immunomodulation remains unclear [10–13].
The primary concern with the use of antifibrinolytic agents, such as TXA, is the increased risk of thromboembolic events. Vascular thrombosis has been described in nonsurgical patients treated with TXA for bleeding control, but these were isolated case reports [16]. Prospective control trials in surgically treated children receiving TXA have shown a very low incidence of intravascular thrombosis [28, 37, 38]. None of our patients had intravascular thrombosis after treatment with a high-dose TXA protocol. Although the results of the present and previous studies suggest the safety of TXA use in patients undergoing adolescent idiopathic scoliosis surgery, vigilance for deep venous thrombosis is recommended.
In the present study, although the use of TXA led to a significant decrease in intraoperative blood loss, it did not appear to affect the amount or volume transfused. This can be attributed to no adherence in standardized criteria established for a blood transfusion threshold. Nevertheless, the transfusion of available autologous blood is often based on factors other than hemodynamics, hematocrit, or base deficit. Transfusions may be given to “stay ahead” of blood loss that previous experience may have lead the anesthesiologist to anticipate.
The results of this study indicate that TXA can be safely used to decrease intraoperative blood loss in posterior spinal fusions for adolescent idiopathic scoliosis. Although no significant difference in transfusion rates was found, our results suggest that the need for transfusion could be decreased by using TXA if standardized guidelines for the transfusion threshold are established and followed. A prospective trial with established transfusion guidelines would be indicated in order to assess the impact on transfusion requirements.
Conflict of interest
None.
References
- 1.Simpson MB, Georgopoulos G, Orsini E, et al. Autologous transfusions for orthopaedic procedures at a children’s hospital. J Bone Joint Surg Am. 1992;74:652–658. [PubMed] [Google Scholar]
- 2.Meert KL, Kannan S, Mooney JF. Predictors of red cell transfusion in children and adolescents undergoing spinal fusion surgery. Spine. 2002;27:2137–2142. doi: 10.1097/00007632-200210010-00012. [DOI] [PubMed] [Google Scholar]
- 3.Guay J, Haig M, Lortie L, et al. Predicting blood loss in surgery for idiopathic scoliosis. Can J Anaesth. 1994;41:775–781. doi: 10.1007/BF03011583. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro F, Sethna N. Blood loss in pediatric spine surgery. Eur Spine J. 2004;13:S6–S17. doi: 10.1007/s00586-004-0760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14:671–676. doi: 10.1097/MOH.0b013e3282e38e8a. [DOI] [PubMed] [Google Scholar]
- 6.Tasaki T, Ohto H. Nineteen years of experience with autotransfusion for elective surgery in children: more troublesome than we expected. Transfusion. 2007;47:1503–1509. doi: 10.1111/j.1537-2995.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popovsky MA, Whitaker B, Arnold NL. Severe outcomes of allogeneic and autologous blood donation: frequency and characterization. Transfusion. 1995;35:734–737. doi: 10.1046/j.1537-2995.1995.35996029156.x. [DOI] [PubMed] [Google Scholar]
- 8.Benavides S, Nicol K, Koranyi K, et al. Yersinia septic shock following an autologous transfusion in a pediatric patient. Transfus Apher Sci. 2003;28:19–23. doi: 10.1016/S1473-0502(02)00096-4. [DOI] [PubMed] [Google Scholar]
- 9.Sculco TP, Gallina J. Blood management experience: relationship between autologous blood donation and transfusion in orthopedic surgery. Orthopedics. 1999;22:s129–s134. doi: 10.3928/0147-7447-19990102-05. [DOI] [PubMed] [Google Scholar]
- 10.Triulzi DJ, Vanek K, Ryan DH, et al. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32:517–524. doi: 10.1046/j.1537-2995.1992.32692367194.x. [DOI] [PubMed] [Google Scholar]
- 11.Innerhofer P, Walleczek C, Luz G, et al. Transfusion of buffy coat-depleted blood components and risk of postoperative infection in orthopedic patients. Transfusion. 1999;39:625–632. doi: 10.1046/j.1537-2995.1999.39060625.x. [DOI] [PubMed] [Google Scholar]
- 12.Innerhofer P, Klingler A, Klimmer C, et al. Risk for postoperative infection after transfusion of white blood cell-filtered allogeneic or autologous blood components in orthopedic patients undergoing primary arthroplasty. Transfusion. 2005;45:103–110. doi: 10.1111/j.1537-2995.2005.04149.x. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;45:33S–40S. doi: 10.1111/j.1537-2995.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 14.Vamvakas EC. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev. 2002;16:144–160. doi: 10.1053/tmrv.2002.31463. [DOI] [PubMed] [Google Scholar]
- 15.Blanchette CM, Wang PF, Joshi AV, et al. Cost and utilization of blood transfusion associated with spinal surgeries in the United States. Eur Spine J. 2007;16:353–363. doi: 10.1007/s00586-006-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005–1032. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 17.Longstaff C. Studies on the mechanisms of action of aprotinin and tranexamic acid as plasmin inhibitors and antifibrinolytic agents. Blood Coagul Fibrinolysis. 1994;5:537–542. [PubMed] [Google Scholar]
- 18.Wu CC, Ho WM, Cheng SB, et al. Perioperative parenteral tranexamic acid in liver tumor resection. A prospective randomized trial toward a “blood transfusion”-free hepatectomy. Ann Surg. 2006;243:173–180. doi: 10.1097/01.sla.0000197561.70972.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 20.Mangano DT, Miao Y, Vuylsteke A, et al. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA. 2007;297:471–479. doi: 10.1001/jama.297.5.471. [DOI] [PubMed] [Google Scholar]
- 21.Yaniv E, Shvero J, Hadar T. Hemostatic effect of tranexamic acid in elective nasal surgery. Am J Rhinol. 2006;20:227–229. [PubMed] [Google Scholar]
- 22.Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96:576–582. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 23.Yamasaki S, Masuhara K, Fuji T. Tranexamic acid reduces postoperative blood loss in cementless total hip arthroplasty. J Bone Joint Surg Am. 2005;87:766–770. doi: 10.2106/JBJS.D.02046. [DOI] [PubMed] [Google Scholar]
- 24.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78:434–440. [PubMed] [Google Scholar]
- 25.Phillips SJ, Chavan R, Porter ML, et al. Does salvage and tranexamic acid reduce the need for blood transfusion in revision hip surgery? J Bone Joint Surg Br. 2006;88:1141–1142. doi: 10.1302/0301-620X.88B9.17605. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro F, Zurakowski D, Sethna NF. Tranexamic acid diminishes intraoperative blood loss and transfusion in spinal fusions for Duchenne muscular dystrophy scoliosis. Spine. 2007;32:2278–2283. doi: 10.1097/BRS.0b013e31814cf139. [DOI] [PubMed] [Google Scholar]
- 27.Sethna NF, Zurakowski D, Brustowicz RM, et al. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 2005;102:727–732. doi: 10.1097/00000542-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Neilipovitz DT, Murto K, Hall L, et al. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. 2001;93:82–87. doi: 10.1097/00000539-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Grant JA, Howard J, Luntley J, et al. Perioperative blood transfusion requirements in pediatric scoliosis surgery: the efficacy of tranexamic acid. J Pediatr Orthop. 2009;29:300–304. doi: 10.1097/BPO.0b013e31819a85de. [DOI] [PubMed] [Google Scholar]
- 30.Neilipovitz DT. Tranexamic acid for major spinal surgery. Eur Spine J. 2004;13:S62–S65. doi: 10.1007/s00586-004-0716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrons RW, Jahr JS. A review of post-cardiopulmonary bypass bleeding, aminocaproic acid, tranexamic acid, and aprotinin. Am J Ther. 1996;3:821–838. doi: 10.1097/00045391-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Hardy JF, Desroches J. Natural and synthetic antifibrinolytics in cardiac surgery. Can J Anaesth. 1992;39:353–365. doi: 10.1007/BF03009046. [DOI] [PubMed] [Google Scholar]
- 33.Schneeweiss S, Seeger JD, Landon J, et al. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358:771–783. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 34.Bess RS, Lenke LG. Blood loss minimization and blood salvage techniques for complex spinal surgery. Neurosurg Clin N Am. 2006;17:227–234. doi: 10.1016/j.nec.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Kannan S, Meert KL, Mooney JF, et al. Bleeding and coagulation changes during spinal fusion surgery: a comparison of neuromuscular and idiopathic scoliosis patients. Pediatr Crit Care Med. 2002;3:364–369. doi: 10.1097/00130478-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Ridgeway S, Tai C, Alton P, et al. Pre-donated autologous blood transfusion in scoliosis surgery. J Bone Joint Surg Br. 2003;85:1032–1036. doi: 10.1302/0301-620X.85B7.13466. [DOI] [PubMed] [Google Scholar]
- 37.Zonis Z, Seear M, Reichert C, et al. The effect of preoperative tranexamic acid on blood loss after cardiac operations in children. J Thorac Cardiovasc Surg. 1996;111:982–987. doi: 10.1016/S0022-5223(96)70374-4. [DOI] [PubMed] [Google Scholar]
- 38.Reid RW, Zimmerman AA, Laussen PC, et al. The efficacy of tranexamic acid versus placebo in decreasing blood loss in pediatric patients undergoing repeat cardiac surgery. Anesth Analg. 1997;84:990–996. doi: 10.1097/00000539-199705000-00008. [DOI] [PubMed] [Google Scholar]


