Abstract
Purpose
Acute compartment syndrome (ACS) of the upper extremity is a rare but serious condition. The purpose of this study was to determine the etiology, diagnosis, treatment, and outcome of ACS of the upper extremity in a pediatric population.
Methods
We performed a retrospective chart review of all patients who underwent a decompressive fasciotomy for ACS of the upper extremity. Data collected included demographics, injury details, presenting symptoms, compartment measurements, time to diagnosis, time to treatment, and outcomes at the latest follow-up.
Results
Twenty-three children underwent fasciotomies for ACS of the forearm (15) and hand (8), at an average age of 9.3 years (range 0–17.8 years). The most common etiologies were fracture (13) and intravenous (IV) infiltration (3). The most common presenting symptoms were pain (83 %) and swelling (65 %). Compartment pressures were measured in 17/23 patients, and all but two patients had at least one compartment with a pressure >30 mmHg. The final two patients were diagnosed and treated for ACS based on clinical signs and symptoms. The average time from injury to fasciotomy was 32.8 h (3.7–158.0 h). Long-term outcome was excellent for 17 patients (74 %) and fair for 5 (22 %), based on the presence of loss of motor function, stiffness, or decreased sensation. One patient with brachial plexus injury and poor baseline function was excluded from functional outcome scoring. There was no association between the time from diagnosis to fasciotomy and functional outcome at the final follow-up (p = 1.000).
Conclusions
Although ACS of the upper extremity in children is often associated with a long delay between injury and fasciotomy, most children still achieve excellent outcomes. The majority of patients presented with pain and at least one additional symptom, but treatment was often delayed, implying that ACS of the upper extremity in children is a difficult diagnosis to establish and may be associated with a prolonged clinical time course.
Keywords: Acute compartment syndrome, Fasciotomy, Upper extremity, Children
Introduction
Acute compartment syndrome (ACS) in children is a rare but potentially devastating condition affecting orthopedic patients. ACS can result from a number of different injuries, including fractures (especially open), crush injuries, vascular problems, burns, and, rarely, infection [1–6]. It may also be iatrogenic in nature, due to casting complications or intravenous (IV) infiltrations [1–5, 7, 8]. Regardless of the specific etiology, the final common pathway of ACS is excessive pressure within muscle compartments, which leads to impaired perfusion. Left untreated, myonecrosis, contracture, neurologic dysfunction, and long-term disability can result [2, 4, 9–11].
The early identification and treatment of ACS has been associated with a decreased incidence of complications and improved outcomes [2, 4–6, 8, 12]. Early warning signs for ACS include significant swelling, paresthesias, and pain out of proportion to the injury [2, 7]. Diminished pulses, pallor, and progressive neurologic deficits are late findings that are less commonly seen. In children, in particular, increasing analgesic requirements and difficulty consoling the patient have been shown to be the best early indicators of ACS [7]. In spite of this, ACS can still be difficult to diagnose in the pediatric patient, especially those that are minimally communicative. As a result, several authors have advocated for heightened awareness and prolonged vigilance to better identify children at risk of ACS and to avoid complications from delayed treatment [1, 2, 4–8].
While several large studies have reviewed the etiology, management, and outcomes of ACS of the lower extremity in children [2, 3, 13], little data exist for the upper extremity [3]. The characterization of injuries and clinical scenarios that more often lead to upper extremity ACS would aid those treating and managing acute pediatric injuries. Therefore, the purpose of this study was to review the presentation, treatment, and outcome of ACS of the upper extremity in a pediatric population. The primary end point of the present study was correlation between outcome according to Flynn’s criteria and the time from injury to fasciotomy. The null hypothesis was that patients treated within 24 h from injury (early treatment group) and patients treated after more than 24 h from injury (delayed treatment group) would have comparable outcomes at follow-up.
Methods
Following institutional review board approval, we performed a retrospective chart review of all patients who were treated for ACS of the upper extremity from 2001 to 2011 at a single pediatric trauma center. Cases were identified by first querying our institution’s billing database for CPT codes for single or double fasciotomies of the upper extremity and then reviewing each resultant patient record to identify which patients had been diagnosed with ACS of the upper extremity.
Inclusion criteria were patients 0–18 years of age with a diagnosis of ACS (based on clinical findings, compartment pressure measurements, or a combination of the two, as determined by the treating attending surgeon). The minimum follow-up was 6 months or follow-up to complete clinical recovery. Exclusion criteria were age greater than 18 years at the time of diagnosis or fasciotomy for any diagnosis other than ACS.
Inpatient and outpatient records were reviewed to determine the demographics (gender, age at diagnosis), injury details, presenting symptoms, compartment pressure measurements, manner of treatment, time from injury to presentation, time to diagnosis, time to treatment, and total hospital stay. All compartment pressure measurements were performed using a Stryker Intra-Compartmental Pressure Monitor (Stryker, Kalamazoo, MI). Pressure was considered to be increased if it was either greater than 30 mmHg or if the difference between the diastolic blood pressure and the intra-compartmental pressure (ΔP) was less than 30 mmHg [14, 15].
As a standard protocol, once the fasciotomy sites were closed, occupational therapy was initiated. Rehabilitation consisted of both active and passive range of motion of the hand, wrist, and elbow. Edema control measures were instituted using compression stockings. Therapy continued until full range of motion was achieved or the patient plateaued.
Follow-up outpatient notes were then used to determine outcomes at the latest follow-up and the development of any complications. Functional outcome was graded as “excellent” (no loss of function or sensation), “fair” (minor permanent change), or “poor” (major loss of function) based on Flynn’s criteria [13]. Motor strength was based on the subjective testing of grip strength and elbow flexion and extension strength. Sensation was measured based on response to light touch.
Fisher’s exact testing was used to compare functional outcomes in patients with a time of ≤24 h from injury to fasciotomy (early treatment group) to patients with a time of >24 h from injury to fasciotomy (delayed treatment group).
Results
Over the study period, 23 children underwent fasciotomies for ACS of the upper extremity, including both the forearm (15) and hand (8). The average age of the patients at the time of injury was 9.3 years (range 0–17.8 years); there were 18 males and 5 females. The most common etiologies were fracture (13) and IV infiltration (4) (Table 1). Other mechanisms of injury included crush injury of the hand, infection, and neonatal compartment syndrome. Signs and symptoms at presentation included pain (83 %), swelling (65 %), weakness (26 %), paresthesia (22 %), and pulselessness (9 %). One patient had documented evidence of increasing analgesic requirements, but this information was not reliably recorded for the remaining patients. Three patients (13 %) were obtunded at the time of diagnosis. Compartment pressures were measured prior to fasciotomy in 17/22 patients, and all but two of these patients had at least one compartment with a pressure >30 mmHg (Table 2). The other two patients had diastolic blood pressures that were 35 and 37 mmHg greater than the measured compartmental pressures. These patients still underwent fasciotomy based on clinical signs and symptoms, as well as the use of vasopressors in one of the patients. All diagnoses of compartment syndrome were made by the attending orthopedic surgeon present at the time of evaluation. The patient clinical data are shown in Table 3, and timing, fasciotomy, and outcome data are shown in Table 4.
Table 1.
Summary of the injury characteristics
| Mechanism of injury | n |
|---|---|
| Fracture | 13 |
| IV infiltration | 4 |
| Crush injury of the hand | 3 |
| Infection | 2 |
| Neonatal compartment syndrome | 1 |
Table 2.
Compartment pressures measured intra-operatively
| Compartment | Number of patients | Mean pressure (mmHG) (SD) |
|---|---|---|
| Volar forearm | 16 | 44.6 (22.5) |
| Dorsal forearm | 9 | 44.4 (19.5) |
| Thenar | 7 | 35.0 (13.0) |
| Hypothenar | 7 | 47.1 (21.0) |
| Interosseous | 9 | 44.7 (13.8) |
| Mobile wad | 2 | 29 (4.2) |
| Mobile dorsal | 1 | 28 (–) |
Table 3.
Patient demographics and clinical data
| Patient ID | Gender | Age (years) | Mechanism of injury | Location of injury | Presenting symptom or sign (1 = Y, 0 = N, * = not reported) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Weakness | Paresthesia | Swelling | Pulselessness | Obtunded | |||||
| 01 | M | 11.5 | Fall from height (DH and BBFA fx) | R forearm | 1 | 0 | 1 | 1 | 0 | 0 |
| 02 | M | 0.0 | Neonatal compartment syndrome | R forearm | 0 | 1 | 1 | 1 | 1 | 0 |
| 03 | F | 10.2 | Fall from height (DH and BBFA fx) | L forearm | 1 | 0 | 0 | * | 0 | 0 |
| 04 | M | 0.3 | IV infiltration (brachial plexus birth injury) | R forearm | 0 | 1 | * | 0 | 0 | 0 |
| 05 | M | 0.2 | IV infiltration | L hand | 1 | * | * | 1 | 0 | 0 |
| 06 | M | 7.6 | Fall from height (open DH fracture) | R forearm | 1 | 1 | * | 1 | 0 | 0 |
| 07 | M | 8.6 | Fall from height (distal BBFA fx) | R forearm | 1 | 1 | 0 | * | * | 0 |
| 08 | F | 6.9 | Fall from height (type 3 SCH fx, ulnar shaft fx, distal BBFA fx) | R forearm | 1 | 0 | 0 | 0 | 0 | 0 |
| 09 | M | 4.6 | Fall from height (open BBFA fx) | L forearm | 1 | * | 0 | * | 0 | 0 |
| 10 | F | 17.8 | IV infiltration | L forearm | * | * | * | 1 | 0 | 1 |
| 11 | M | 15.7 | Fall from height (BBFA fx) | L forearm | 1 | 0 | 0 | 1 | 0 | 0 |
| 12 | M | 14.6 | Multi-trauma (sports injury; BBFA fx) | L forearm | 1 | 1 | 1 | * | 0 | 0 |
| 13 | M | 10.5 | Fall from height (radial neck fx) | L forearm | 1 | 0 | 1 | * | 0 | 0 |
| 14 | M | 14.3 | Fall from height (BBFA fx) | L forearm | 1 | 0 | 0 | 1 | 0 | 0 |
| 15 | M | 13.9 | Crush injury (DR fx) | R forearm | 1 | 0 | 0 | 1 | 0 | 0 |
| 16 | M | 15.8 | Multi-trauma (hit by train; multiple open MC fxs, open BBFA fx) | R hand | 1 | * | 0 | * | 0 | 1 |
| 17 | F | 0.5 | IV infiltration | R hand | 0 | 0 | 0 | 1 | * | 1 |
| 18 | M | 6.1 | Fall from height (type 3 SCH fx) | L forearm | 1 | 0 | 1 | 1 | 1 | 0 |
| 19 | M | 17.4 | Post-op. complication | L hand | 1 | 0 | 0 | 1 | 0 | 0 |
| 20 | M | 12.4 | Crush injury (MC fx) | L hand | 1 | * | * | 1 | 0 | 0 |
| 21 | F | 2.7 | Crush injury (multiple MC fx) | R hand | 1 | 1 | * | 1 | 0 | 0 |
| 22 | M | 11.8 | Infection (cellulitis) | L hand | 1 | 0 | 0 | 1 | 0 | 0 |
| 23 | M | 0.7 | Infection (abscess) | R hand | 1 | 0 | 0 | 1 | 0 | 0 |
fx fracture, DH distal humerus, DR distal radius, BBFA both-bone forearm, SCH supracondylar humerus, MC metacarpal
Table 4.
Patient timing, fasciotomy, and outcome data
| Patient | Time intervals (h) | Compartment pressure locations and values (mmHg) (if available) | Type of fasciotomy | Motor deficits at final follow-up | Sensory deficits at final follow-up | Stiffness at final follow-up | Functional outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Injury to ED admission | Injury to ACS diagnosis | ED admission to ACS diagnosis | ACS diagnosis to fasciotomy | Injury to fasciotomy | |||||||
| 01 | 7.2 | 42.5 | 35.3 | 1.7 | 44.2 | Volar FA and carpal tunnel | 0 | 0 | 0 | Excellent | |
| 02 | 3.0 | 5.3 | 2.3 | 1.1 | 6.4 | Volar FA | 0 | 0 | Lacks 20–30° pronation | Fair | |
| 03 | 4.8 | 34.0 | 29.3 | 1.5 | 35.5 | Volar FA | 0 | 0 | Lacking 10° pronation and 15–20° supination | Excellent | |
| 04 | N.A. | 1.4 | N.A. | 2.3 | N.A. | Volar FA: 38 Dorsal FA: 34 Thenar: 38 Hypothenar: 80 IO: ~45 |
Volar FA; thenar eminence; hypothenar eminence; 2nd and 4th IO; thumb abductor | Impaired strength and coordination; limiting ADL | 0 | Impaired PROM, AROM; limits participation in dressing, bathing, hygiene, play | Excluded from grading |
| 05 | N.A. | 1.8 | N.A. | 2.2 | N.A. | Volar FA: 112 | Volar FA and mobile wad | 0 | 0 | 0 | Excellent |
| 06 | 3.8 | 17.4 | 13.6 | 0.0 | 17.4 | Volar FA: 60 Dorsal FA: 20–25 |
Volar FA and carpal tunnel | 0 | 0 | 0 | Excellent |
| 07 | 29.0 | 39.1 | 10.1 | 2.9 | 42.0 | Superficial volar FA: 43 Deep volar FA: 44 Dorsal FA: 39 |
Volar FA; all dorsal compartments of the hand | 0 | 0 | 0 | Excellent |
| 08 | 5.0 | 17.3 | 12.3 | 0.0 | 17.3 | Superficial volar FA: 35 Deep volar FA: 34 Extensor: 74 |
Volar and dorsal FA | 0 | 0 | 0 | Excellent |
| 09 | 2.5 | 39.5 | 37.0 | 1.8 | 41.3 | Volar FA: 44 Mobile wad: 26 |
Volar and dorsal FA; carpal tunnel release | 0 | 0 | 0 | Excellent |
| 10 | N.A. | 3.3 | N.A. | 0.8 | N.A. | Volar FA: 65 Dorsal FA: 61 |
Volar and dorsal FA | 0 | 0 | 0 | Excellent |
| 11 | 1.1 | 48.5 | 47.4 | 3.0 | 51.5 | Superficial volar FA: 41 Deep volar FA: 50 Dorsal FA: 40 |
Volar and dorsal FA | 0 | 0 | 0 | Excellent |
| 12 | 17.0 | 56.0 | 39.0 | 2.0 | 58.0 | Volar and dorsal FA | 0 | 0 | Mild flexion of DIP joint of fifth finger on left; lacks 25° of full wrist supination/pronation | Fair | |
| 13 | 5.5 | 14.3 | 8.8 | 1.8 | 16.1 | Volar and dorsal FA; carpal tunnel release | 0 | 0 | 0 | Excellent | |
| 14 | 7.2 | 9.0 | 1.8 | 2.8 | 11.8 | Superficial volar FA: 35 Deep volar FA: 30 Mobile wad: 28 |
Volar FA; mobile wad; carpal tunnel release | 0 | Diminished median and radial nerve | 0 | Fair |
| 15 | 1.3 | 3.5 | 2.2 | 2.2 | 5.7 | Volar FA: 15 Dorsal FA: 65 Mobile wad: 32 |
Volar and dorsal FA | 0 | 0 | 0 | Excellent |
| 16 | 0.6 | 5.2 | 4.6 | 0.0 | 5.2 | Dorsal IO: 25 | Interossei and dorsal hand; thenar eminence; hypothenar eminence | Decreased grip and elbow strength; extensor muscle atrophy of forearm | Radial nerve palsy | 0 | Fair |
| 17 | N.A. | 62.0 | N.A. | 1.5 | N.A. | Hypothenar: 11 Thenar: 20 |
1–4 dorsal IO; palmar IO; thenar eminence; hypothenar eminence | 0 | 0 | 0 | Excellent |
| 18 | 4.4 | 15.8 | 11.3 | 1.7 | 17.5 | Volar FA | 0 | 0 | 0 | Excellent | |
| 19 | 55.7 | 56.5 | 0.8 | 1.7 | 58.2 | Thenar: 40 Hypothenar: 47 IO: 30–40 Dorsal FA: 16 Volar FA: 15 |
Thenar, hypothenar, dorsal IO, palmar IO, abductor pollicis | 0 | 0 | Pronation (20°) and supination deficit (5°) | Fair |
| 20 | 3.2 | 4.8 | 1.6 | 2.9 | 7.6 | Dorsal fifth: 35 Dorsal fourth: 70 Dorsal thumb: 55 Thenar: 30 Hypothenar: 55 |
Thenar, hypothenar, palmar fascia, 1st–4th IO, | 0 | 0 | 0 | Excellent |
| 21 | 3.8 | 5.0 | 1.3 | 1.9 | 6.9 | Thenar, hypothenar, IO: 50–65 | Thenar, hypothenar, all IO | 0 | 0 | 0 | Excellent |
| 22 | 148.9 | 157.0 | 8.1 | 1.0 | 158.0 | IO: all >40 Thenar: 20 Hypothenar: >40 |
Hypothenar, all IO | 0 | 0 | 0 | Excellent |
| 23 | 4.8 | 47.3 | 42.5 | 2.5 | 49.7 | Thenar, Hypothenar, IO: >40 | Thenar, hypothenar, all IO | 0 | 0 | 0 | Excellent |
FA forearm, IO interosseous
Sixteen patients developed ACS as a result of traumatic injuries and initially presented to our emergency room. Of these, ten patients had a fall from height, two patients sustained high-energy traumas (pedestrian versus train and sports-related injury, respectively), one patient presented with post-operative pain and swelling after surgical correction of a distal radius and ulna mal-union, and three patients sustained crush injuries to the hand. For these patients, the average time from injury to emergency department presentation was 9.5 h (σ = 14.2), the average time from emergency department presentation to ACS diagnosis was 16 h (σ = 15.9), and the average time from ACS diagnosis to fasciotomy was 1.7 h (σ = 1.0). The remaining seven patients were analyzed separately due to the atraumatic nature of their injuries. Four of the patients developed ACS from the infiltration of IV fluids while already hospitalized for an unrelated reason. Three occurred from the infiltration of standard replacement fluids [normal saline (NS) with KCL] and one resulted from the infiltration of NS plus insulin. Two patients developed ACS of the hand from an abscess or cellulitis, and the last patient was diagnosed with neonatal compartment syndrome of the forearm. Since these were atraumatic events, only their time from diagnosis to fasciotomy was considered, and this was found to be 1.6 h (σ = 0.6).
The average total hospital stay was 6.4 days (1–11 days). At the final follow-up, six patients had residual deficits (Table 4). One patient had an isolated sensory deficit and one patient had both a motor and a sensory deficit. Four patients had clinically significant stiffness at the final follow-up, largely consisting of limited forearm pronation and supination. Of the 23 patients in our series, the long-term outcome was excellent for 17 patients (77 %) and fair for 5 (23 %), based on the presence of decreased motor function, residual stiffness, or decreased sensation (Fig. 1) [13]. The remaining patient sustained a complete brachial plexus injury at birth and was, therefore, excluded from the functional grading based on his baseline level of function. There was no statistically significant difference between the functional outcome of patients with traumatic etiologies (12 excellent, 4 fair) and those with atraumatic etiologies (5 excellent, 1 fair) (p = 1.000).
Fig. 1.
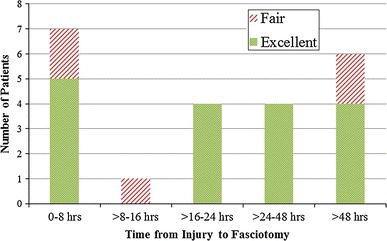
Functional outcome versus time from injury to fasciotomy
Of the patients treated early (≤24 h from injury to fasciotomy), nine had excellent outcomes and three had fair outcomes. Patients treated late (>24 h from injury to fasciotomy) had similar results: eight excellent and two fair final outcomes. Fisher’s exact testing revealed no statistically significant difference in the functional outcome when comparing those treated early to those who had a delay in treatment (p = 1.000, Fig. 1).
Discussion
ACS of the upper extremity in children, while less common than of the lower extremity, still carries a risk of significant long-term sequelae [1, 2, 4, 12]. In spite of this, there are relatively few studies in the literature that focus on ACS of the upper extremity in children. Most studies of ACS are devoted to adult patients or to the lower extremity in children. We, therefore, sought to report on a single, large, consecutive series of pediatric patients treated at a major trauma center, with an emphasis on the etiology of this condition, the time course associated with its detection and management, and the long-term outcomes of these patients.
Several studies have explored the incidence, etiology, and outcomes of ACS of the lower extremity in children. Flynn et al. [13] evaluated 43 cases of acute traumatic compartment syndrome of the lower extremity in 42 children. The majority of the patients in this series had been injured in motor vehicle collisions (83 %), and the authors found that most children had excellent outcomes compared to adult patients, in spite of the delay to fasciotomy. Similarly, upper extremity compartment syndrome has been evaluated in the adult population. Dente et al. [12] reviewed 33 cases of upper extremity ACS in 27 adult patients. Only three of the patients in this series had fasciotomies that were delayed 6–48 h from the time of injury; however, functional outcome at the final follow-up was not discussed.
Reports of ACS of the upper extremity in children, however, have been much more infrequent [1–5, 8, 16–20]. The limited number of available large case series and systematic reviews on this subject is likely due to not only the rarity of the condition, but also possibly to missed or delayed diagnoses [2, 3, 5, 6]. In the largest series to date, Grottkau et al. [3] identified 131 cases of ACS in children over a period of 4 years, with 27 cases involving the upper extremity. The average age of these children was 12 years, with boys outnumbering girls in a ratio of 4:1. The study identified the most common mechanisms of injury to be forearm fractures (74 %), supracondylar humerus fractures (15 %), and carpal or metacarpal fractures (11 %). The incidence of compartment syndrome following forearm fracture was found to be 1.04 % (20/1,905), with a significantly increased risk in open fractures (relative risk = 2.2) [3].
Previous reports have suggested that the most common causes of ACS in the upper extremity in children are traumatic (e.g., supracondylar humerus fractures, both-bone forearm fractures) [1, 2, 11, 21]. In our series, 15 patients (65 %) suffered traumatic injuries, with the majority being due to a fall from a height or a crush injury of the hand. Non-traumatic cases of ACS have also been reported in the literature. Prasarn et al. [4] identified 13 cases of ACS in children in the absence of fractures. Fifty-eight percent of these cases were iatrogenic in nature (IV infiltration, IV medication administration, tourniquet retention), and three patients required amputation, all of which were being cared for in the intensive care unit (ICU).
The iatrogenic injuries from our series consisted of four IV infiltrations and one post-operative complication following surgical correction of a distal radial/ulnar malunion. Three of the IV infiltrations had excellent outcomes at final follow-up and one had severe deficits in active and passive range of motion, as well as diminished strength and coordination, which interfered with activities of daily living. The patient with a post-operative complication following malunion revision had a fair outcome, with loss of range of motion of the affected wrist.
The most common early presenting signs of compartment syndrome in adults include increasing pain, swelling, and pain with passive stretch. Later findings include paralysis, pulselessness, and neurologic symptoms. However, in children, these symptoms are often unreliable. In a study by Bae et al., pain, pallor, paresthesia, paralysis, and pulselessness were found to be inconsistent indicators of impending compartment syndrome in children [2]. The authors suggested instead that the physician should look for increasing analgesic needs as an early warning sign for potential problems. Because the early presentation of ACS can be subtle, several authors recommend the routine measurement of compartment pressures for certain patients, namely, those who are uncooperative, have altered mental status, are very young, or have unreliable or inconsistent clinical symptoms [16]. Our study suggests that pain (83 %) and swelling (65 %) were the most common presenting symptoms, with few patients exhibiting motor or sensory deficits (26 and 22 %, respectively) or pulselessness (9 %). This likely exemplifies the difficulties inherent in assessing children for subjective symptoms. While paresthesias were only documented for 22 % of the patients, this number may likely have been higher with an older or more cooperative patient group. Unfortunately, as this study was retrospective, we did not have accurate data on analgesic requirements and are unable to comment on its reliability in the early detection of ACS in children.
Several studies have shown that the time from injury to surgery has an effect on long-term outcomes [4, 5, 22]. In a study conducted by Prasarn et al. [4], favorable outcomes were found in patients that had decompression surgery less than 6 h from the time of diagnosis. Finkelstein reviewed five patients that had fasciotomies more than 35 h from the time of injury [23]. Of these patients, one died of multi-organ failure and septicemia, and the remaining four required limb amputations. Our statistical analysis, however, revealed no significant difference in the functional outcome of patients who underwent fasciotomy within 24 h of injury compared to patients treated after greater than 24 h of delay. The reason for this finding is unclear, but it may be due to insufficient power. As a result, we still advocate for the urgent treatment of ACS in order to minimize the risk of neurologic injury and myonecrosis.
The limitations of this study include its retrospective nature and small sample size, and potentially inexact time recordings in the medical records. Due to the rare frequency of this complication, a prospective study is impractical and would require the monitoring of thousands of fracture patients in order to gather a sample large enough for adequate analysis. With regards to the variability in time measurements, this is largely unavoidable due to the nature of these data. We made every attempt to correlate multiple time recordings if present, and to use only official recordings as noted in paramedic records, emergency department notes, attending surgeon progress notes, anesthesia records, and operative reports. Similarly, while the time from injury to fasciotomy and the time from diagnosis to fasciotomy is, indeed, interesting, the time of elevated pressure is more relevant. Continuous monitoring of compartment pressure, however, is neither practical nor realistic.
In summary, ACS of the upper extremity is a rare but serious condition in the pediatric population that remains difficult to diagnose. The majority of patients in our series presented with pain and at least one other symptom, similar to adults and ACS of the lower extremity in children. Yet, the delay in diagnosis in our series was longer than that which has been reported for both of these patient populations [13]. In spite of this, most children still achieve excellent outcomes. The results of this study should raise awareness of the potential for late and often ambiguous presentation of this condition in children.
Conflict of interest
No outside funding was used for this investigation. The authors have no conflicts of interest to report with respect to this investigation.
Footnotes
This study was performed at The Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
This was an IRB-approved study.
References
- 1.Blakemore LC, Cooperman DR, Thompson GH, Wathey C, Ballock RT. Compartment syndrome in ipsilateral humerus and forearm fractures in children. Clin Orthop Relat Res. 2000;376:32–38. doi: 10.1097/00003086-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bae DS, Kadiyala RK, Waters PM. Acute compartment syndrome in children: contemporary diagnosis, treatment, and outcome. J Pediatr Orthop. 2001;21(5):680–688. [PubMed] [Google Scholar]
- 3.Grottkau BE, Epps HR, Di Scala C. Compartment syndrome in children and adolescents. J Pediatr Surg. 2005;40(4):678–682. doi: 10.1016/j.jpedsurg.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Prasarn ML, Ouellette EA, Livingstone A, Giuffrida AY. Acute pediatric upper extremity compartment syndrome in the absence of fracture. J Pediatr Orthop. 2009;29(3):263–268. doi: 10.1097/BPO.0b013e31819c3d54. [DOI] [PubMed] [Google Scholar]
- 5.Erdös J, Dlaska C, Szatmary P, Humenberger M, Vécsei V, Hajdu S. Acute compartment syndrome in children: a case series in 24 patients and review of the literature. Int Orthop. 2011;35(4):569–575. doi: 10.1007/s00264-010-1016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalyani BS, Fisher BE, Roberts CS, Giannoudis PV. Compartment syndrome of the forearm: a systematic review. J Hand Surg Am. 2011;36(3):535–543. doi: 10.1016/j.jhsa.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Kadiyala RK, Waters PM. Upper extremity pediatric compartment syndromes. Hand Clin. 1998;14(3):467–475. [PubMed] [Google Scholar]
- 8.Ramos C, Whyte CM, Harris BH. Nontraumatic compartment syndrome of the extremities in children. J Pediatr Surg. 2006;41(12):e5–e7. doi: 10.1016/j.jpedsurg.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Prasarn ML, Ouellette EA. Acute compartment syndrome of the upper extremity. J Am Acad Orthop Surg. 2011;19(1):49–58. doi: 10.5435/00124635-201101000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Heckman MM, Whitesides TE, Jr, Grewe SR, Judd RL, Miller M, Lawrence JH., 3rd Histologic determination of the ischemic threshold of muscle in the canine compartment syndrome model. J Orthop Trauma. 1993;7(3):199–210. doi: 10.1097/00005131-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Whitesides TE, Heckman MM. Acute compartment syndrome: update on diagnosis and treatment. J Am Acad Orthop Surg. 1996;4(4):209–218. doi: 10.5435/00124635-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Dente CJ, Feliciano DV, Rozycki GS, Cava RA, Ingram WL, Salomone JP, Nicholas JM, Kanakasundaram D, Ansley JP. A review of upper extremity fasciotomies in a level I trauma center. Am Surg. 2004;70(12):1088–1093. [PubMed] [Google Scholar]
- 13.Flynn JM, Bashyal RK, Yeger-McKeever M, Garner MR, Launay F, Sponseller PD. Acute traumatic compartment syndrome of the leg in children: diagnosis and outcome. J Bone Joint Surg Am. 2011;93(10):937–941. doi: 10.2106/JBJS.J.00285. [DOI] [PubMed] [Google Scholar]
- 14.Blick SS, Brumback RJ, Poka A, Burgess AR, Ebraheim NA. Compartment syndrome in open tibial fractures. J Bone Joint Surg Am. 1986;68(9):1348–1353. [PubMed] [Google Scholar]
- 15.McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99–104. [PubMed] [Google Scholar]
- 16.Matsen FA., 3rd Compartmental syndromes. Hosp Pract. 1980;15(2):113–117. doi: 10.1080/21548331.1980.11946559. [DOI] [PubMed] [Google Scholar]
- 17.Kowtharapu DN, Thabet AM, Holmes L, Jr, Kruse R. Osteochondral flap avulsion fracture in a child with forearm compartment syndrome. Orthopedics. 2008;31(8):805. doi: 10.3928/01477447-20080801-09. [DOI] [PubMed] [Google Scholar]
- 18.Mai MC, Beck R, Gabriel K, Singh KA. Posterior arm compartment syndrome after a combined supracondylar humeral and capitellar fractures in an adolescent: a case report. J Pediatr Orthop. 2011;31(3):e16–e19. doi: 10.1097/BPO.0b013e31820fc8c9. [DOI] [PubMed] [Google Scholar]
- 19.Pettitt DA, McArthur P. A boy with a painful arm. BMJ. 2011;342:c5972. doi: 10.1136/bmj.c5972. [DOI] [PubMed] [Google Scholar]
- 20.Mubarak SJ, Carroll NC. Volkmann’s contracture in children: aetiology and prevention. J Bone Joint Surg Br. 1979;61-B(3):285–293. doi: 10.1302/0301-620X.61B3.479251. [DOI] [PubMed] [Google Scholar]
- 21.McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome. Who is at risk? J Bone Joint Surg Br. 2000;82(2):200–203. doi: 10.1302/0301-620X.82B2.9799. [DOI] [PubMed] [Google Scholar]
- 22.Sheffler LC, Lattanza L, Hagar Y, Bagley A, James MA. The prevalence, rate of progression, and treatment of elbow flexion contracture in children with brachial plexus birth palsy. J Bone Joint Surg Am. 2012;94(5):403–409. doi: 10.2106/JBJS.J.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelstein JA, Hunter GA, Hu RW. Lower limb compartment syndrome: course after delayed fasciotomy. J Trauma. 1996;40(3):342–344. doi: 10.1097/00005373-199603000-00002. [DOI] [PubMed] [Google Scholar]


