Abstract
Purpose
Children with multiple hereditary exostoses (MHE) have numerous osteochondromas, with the most prominent lesions typically over the appendicular skeleton. A recent report noted a high rate of intracanal lesions in this patient population and recommended preventative spinal screening with magnetic resonance imaging (MRI) or computed tomography (CT). We sought to evaluate the prevalence of spinal stenosis from intracanal osteochondromas at our pediatric orthopedic center in order to evaluate if routine screening is warranted.
Methods
All pediatric patients treated for MHE were retrospectively identified. Records were reviewed to determine demographics, previous orthopedic surgery, and indication and results of axial spine imaging (CT or MRI). Imaging studies were reviewed to evaluate the presence of intracanal and compressive spinal lesions.
Results
Between 1990 and 2011, axial imaging was performed in nine patients with MHE due to concerns of pain, weakness, and/or dizziness. These patients had moderate disease involvement, with a mean of 4.9 previous orthopedic surgeries to address skeletal osteochondromas. Two patients with MHE had cervical spinal stenosis secondary to intracanal osteochondromas. Both children successfully underwent spinal decompression. Thus, of our MHE population undergoing axial imaging, 22 % were noted to have intracanal lesions.
Conclusions
Our experience reveals a >20 % rate of compressive intracanal osteochondromas in MHE patients undergoing spinal imaging. These two patients represent 5 % of the MHE patients treated at our center. These lesions may be slow growing, and significant consequences can occur if not identified promptly. Thus, we confer that routine axial screening of the spinal canal may be warranted in these children.
Keywords: Pediatric, Multiple hereditary exostoses, Neurologic symptoms, Spinal impingement, Imaging, MRI, CT
Introduction
Multiple hereditary exostoses (MHE) is a skeletal dysplasia resulting in multiple cartilage-capped lesions. It is a genetic disorder inherited in an autosomal dominant fashion with incomplete penetrance in females [1, 2]. Although osteochondromas have a predilection for the metaphyses of long bones, the axial skeleton and vertebral column can also be involved [3–8].
There are numerous published reports of spinal cord impingement and neurologic compromise from intracanal osteochondromas [6, 9]. In most series, patients present with pain, weakness, or other significant neurologic findings [4, 8–23]. Roach et al. found that 12 out of 44 patients screened had lesions encroaching into the spinal canal and, thus, recommended routine magnetic resonance imaging (MRI) or computed tomography (CT) screening of the neuroaxis in patients with MHE to detect compressive lesions prior to the onset of neurologic deficits [6, 9]. However, the prevalence of asymptomatic compressive intracanal lesions in patients with MHE has not been confirmed at other centers [6]. Thus, we undertook a retrospective review to determine the prevalence of intracanal osteochondromas in children with MHE imaged at our center.
Materials and methods
A retrospective chart review was undertaken at a dedicated pediatric orthopedic hospital. The diagnostic registry was searched to identify all patients treated for a diagnosis of MHE between 1990 and 2011. Patients with solitary osteochondromas were excluded from this study, as were patients greater than 20 years of age. Records were reviewed for demographics, previous surgeries, and indication for axial imaging. A search of diagnostic codes yielded 67 possible patients. Of those, 44 were found to have a confirmed diagnosis of MHE. Nine patients had undergone axial imaging. All imaging studies were reviewed to determine the presence of an intracanal osteochondroma, associated neurologic symptoms and physical examination findings, and treatment course. Institutional review board approval was obtained for all aspects of this study.
Results
During the study period, axial imaging was performed in nine patients with MHE for a variety of indications, including back pain, routine screening, leg pain, spasticity, and headache (Table 1). The mean age at the time of scanning was 16.1 years (range 9–20 years). During the study period, the nine patients had undergone a mean of 4.9 orthopedic surgeries (range 2–10) under general anesthesia at our center.
Table 1.
Summary of patients undergoing axial imaging
| Patient number | Age at imaging | Gender | Reason for imaging | Area imaged | Findings |
|---|---|---|---|---|---|
| 1 | 20 | F | Back pain | MRI—spine | Normal |
| 2 | 19 | F | Osteochondroma on chest wall | MRI—spine | Normal |
| 3 | 16 | F | Back pain and MHE | MRI—spine | Normal |
| 4 | 9 | M | MHE | MRI—spine | Normal |
| 5 | 15 | F | Leg weakness, pain, lesion in right hip | MRI—spine | Osteochondroma on lamina of C5 compressing the spinal cord |
| 6 | 19 | M | Pain | MRI—spine | Normal |
| 7 | 18 | M | Spasticity, pain, and fasciculations | MRI—brain and spine | Normal |
| 8 | 14 | F | Rib and back pain | CT—spine | Normal |
| 9 | 15 | F | Dizziness, headaches needing ER visits | MRI—spine | Osteochondroma on C2 compressing the spinal cord |
Two patients with MHE with minimal symptoms were found to have cervical spine stenosis secondary to intracanal osteochondromas. Both children successfully underwent spinal decompression. Thus, of our MHE population, 20.5 % (9 of 44 patients) had symptoms that warranted axial imaging and 4.5 % (2 of 44 patients) were noted to have intracanal lesions.
The first patient was a 15-year-old female with known history of MHE who presented with right hip pain and sudden give-way on her right lower extremity, resulting in frequent falls. She had had multiple previous symptomatic osteochondromas resected from her lower extremities. She had missed a significant amount of school. She had no history of headaches or neck or back pain. On examination, she walked with a stable gait with no limp. She had 3+ knee jerk and ankle jerk, and downgoing toes bilaterally with no clonus. Sensation was intact to light touch throughout. She had 5/5 strength in her bilateral upper and lower extremities. While prone, right hip internal rotation was to 10°, compared to 35° on the left side. External rotation was 60° bilaterally. The patient had no pain with flexion internal rotation on the right hip. Hip flexion was to 120° bilaterally. Pain in the right hip was reproduced with extension and external rotation, with pain radiating down the leg. The spine appeared straight, with no evidence of scoliosis. There were no limitations in cervical, thoracic, or lumbar spine range of motion and no tenderness to palpation throughout her cervical, thoracic, or lumbar spine. Radiographs revealed two large osteochondromas in the right proximal femur, which were thought to be impinging on soft tissues or neurovascular structures. Due to concern about the atypical presentation and her history of MHE, she underwent MRI screening of her entire spine. This revealed several osteochondromas, including a large compressive lesion extending from the lamina at C5. This resulted in significant cord compression, with increased T2 signal on MRI (Fig. 1). She underwent emergent C5–C6 laminectomy. She recovered well with no complications, although her hip symptoms persisted. She underwent surgical hip dislocation with removal of two proximal femoral osteochondromas. She tolerated this procedure well, and her hip and leg symptoms have subsequently resolved.
Fig. 1.
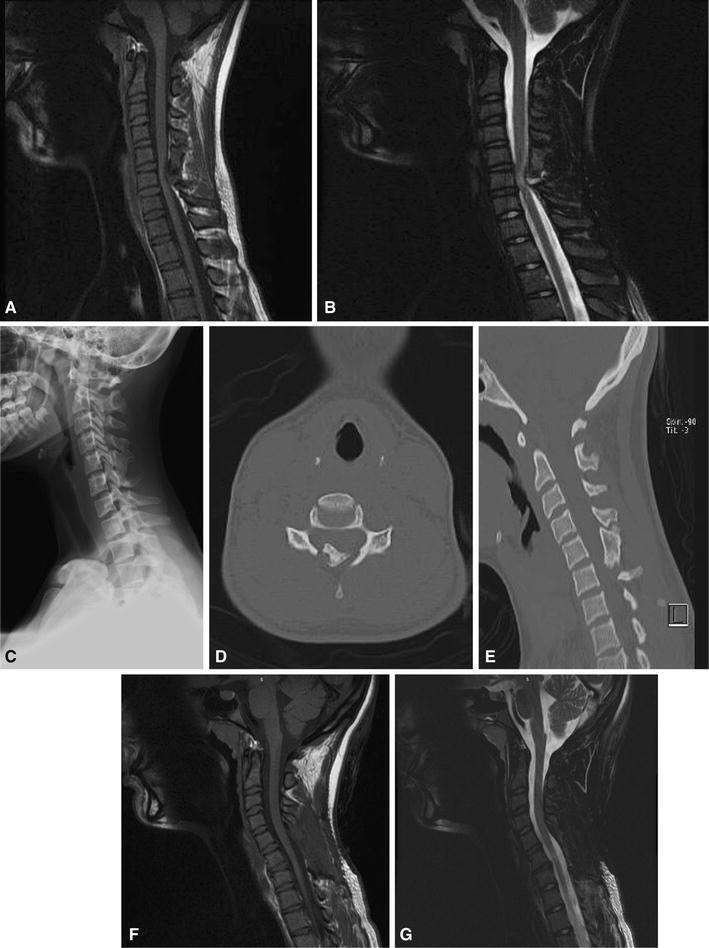
A 15-year-old female with multiple hereditary exostoses (MHE) presented with hip and posterior leg pain. Magnetic resonance imaging (MRI) screening of the entire spine was undertaken, given concern for radicular-type symptoms. a, b Sagittal T1 and T2 imaging of the cervical spine revealed a large posteriorly based osteochondroma compressing the spinal cord, with signal change on T2 imaging. c The lesion is not evident in plain radiographs. d, e Computed tomography (CT) underestimates the size of the lesion, given the large cartilage cap. Nevertheless, the appearance of the osteochondroma on computed tomography (CT) is concerning for spinal stenosis. This patient underwent surgical decompression, with no improvement in her hip and leg symptoms. f, g Sagittal T1 and T2 imaging of the cervical spine status post laminectomy. Note the resolution of the previous signal change on T2
The second patient underwent distal femoral osteotomy at age 15 years. Postoperatively, she complained of headaches. She subsequently did well in her postoperative course. She returned six months later with worsening headaches. The recorded physical examination at that visit was unremarkable, with no evidence of neurologic deficit. CT of her brain was obtained, which was unremarkable. MRI screening of her cervical spine was subsequently obtained, which revealed a compressive lesion at C2 (Fig. 2). She underwent urgent laminectomy with complete resolution of her headaches and no change in her neurologic function.
Fig. 2.
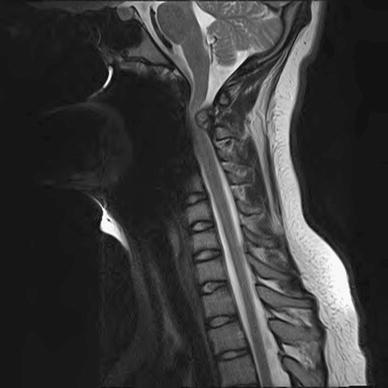
Another 15-year-old female presenting with severe headaches, who initially underwent CT head imaging, which was unremarkable. Eventually, MRI screening was obtained of her cervical spine, revealing a large compressive lesion at C2. She underwent surgical decompression with good relief of her symptoms
Discussion
Osteochondromas are among the most common musculoskeletal tumors. First described in 1814 by Boyer, MHE is due to a mutation in the tumor suppressor genes EXT1 (18q), EXT2 (11p), and EXT3 (19p), which codes for a protein that participates in the biosynthesis of heparan sulfate. In patients with MHE, heparan sulfate stays inside the cell and does not go through the membrane. This causes a disturbance in the negative feedback regulatory system, thus, leading to undifferentiated proliferation of chondrocytes in the metaphyseal region [23–27]. Their composition consists of healthy, lamellar bone and a cartilaginous cap, growing by endochondral bone formation at the metaphysis of long bones [4, 6, 28, 29]. Malignant transformation to a chondrosarcoma has been reported in both the appendicular and axial skeleton, primarily in adults [30–32].
The majority of the vertebral column lesions occur in the cervical (50–80 %) and thoracic (20–36 %) regions, and rarely in the lumbar spine (Table 2). Overall, C2 is the most commonly affected vertebrae [1, 8]. It has been hypothesized that the microtrauma from cervical spine mobility leads to the displacement and subsequent growth of small cartilaginous remains, thus, resulting in a predominance of cervical lesions [5, 7].
Table 2.
Summary of reported spinal osteochondromas in young patients (≤25 years of age)
| References | Number of patients | Mean age, years (range) | MHE or solitary | Number symptomatic | Number asymptomatic | Type of symptoms | Location of lesion |
|---|---|---|---|---|---|---|---|
| Tahasildar et al. [40] | 1 | 5 (5) | MHE | 1 | 0 | Restricted range of motion of the neck | Spinous process and lamina of C2 without spinal encroachment |
| Bonic and Kettner [33] | 1 | 21 (21) | Solitary | 1 | 0 | Neck pain and discomfort, tender palpable mass, limited range of motion of the neck | Right bifid tip of spinous process of C5 |
| Eap et al. [17] | 1 | 23 (23) | Solitary | 1 | 0 | Worsening of pre-existing right hemiparesis, appearance of left hemiparesis, bladder dysfunction and urgency | Posterior arch of C4 with spinal cord encroachment |
| Tian et al. [4] | 1 | 16 (16) | MHE | 1 | 0 | Difficulty walking, spastic paraparesis, hypesthesia of lower extremities, bilateral hyperactive deep tendon reflexes | Posterosuperior endplate of T6 vertebral body with spinal cord encroachment |
| Patel and Thacker [41] | 1 | 14 (14) | MHE | 1 | 0 | Painful restriction of neck movement | Posterior elements of C2 with spinal cord encroachment |
| Ezra et al. [11] | 1 | 4 (4) | MHE | 1 | 0 | Difficulty walking postfall, mildly reduced sensation to touch, decreased deep tendon reflexes in biceps, triceps, and brachioradialis, left Babinski sign, absent cremasteric reflex | Posterior elements of C7 and T1 with spinal cord encroachment |
| Gunay et al. [42] | 2 | 14 (9–19) | Solitary | 2 | 0 | Painful swelling on the neck, restricted neck range of motion | Spinous process of C5–C6, C5 spinous process, no spinal cord encroachment |
| Hassankhani [43] | 1 | 16 (16) | Solitary | 0 | 1 | Lumbar mass | Spinous process of L3 |
| Roach et al. [6] | 44 | 12.7 (4.2–19) | MHE | 27 | 3 | Neurological symptoms, acute quadriplegia following a minor trauma | 30 out of 44 patients had spinal lesions, 12 of the 44 had spinal canal encroaching lesions, 18 of 44 patients had lesions that did not encroach the spinal canal |
| Han and Kuh [44] | 1 | 7 (7) | MHE | 1 | 0 | Brown-Séquard syndrome development following mild trauma | Lamina of C7 with spinal cord encroachment |
| Rao and Jakheria [35] | 1 | 8 (8) | MHE | 0 | 1 | Swollen neck mass, no neurologic symptoms | Spinous process of C2–C6, no spinal canal encroachment |
| Chatzidakis et al. [45] | 1 | 22 (22) | Solitary | 0 | 1 | Asymptomatic, incidentally found on CT scan of the brain | Dens of C2 |
| Song and Lee [46] | 1 | 11 (11) | Solitary | 1 | 0 | Gait disturbance | Left superior articular process of T4 with spinal cord encroachment |
| Samartzis and Marco [47] | 1 | 11 (11) | Solitary | 1 | 0 | Right posterior thigh pain for 28 months, unresponsive to conservative treatment | Right anterior surface of S2 lamina, compressing the S2 root |
| Maheshwari et al. [48] | 1 | 20 (20) | Non-hereditary multiple exostoses | 1 | 0 | Increased muscle tone, exaggerated deep tendon reflexes and extensor plantar response, bilateral weakness below C6 myotome, motor loss below T2 | Left pedicle of C7 with spinal cord encroachment |
| McCall et al. [49] | 1 | 13 (13) | Solitary | 0 | 1 | Posterior neck mass | Lamina of C3, mild cord encroachment |
| Giudicissi-Filho et al. [10] | 1 | 18 (18) | MHE | 1 | 0 | Progressive weakness in lower limbs, quadriparesis, bilateral hyperactive deep tendon reflexes, bilateral Babinski sign | Lamina of C7 with spinal cord encroachment |
| Aldea et al. [50] | 1 | 24 (24) | MHE | 1 | 0 | Acute onset of difficulty in ambulating, bilateral hyperesthesia below C7 | Right C7 lamina with spinal cord encroachment |
| Faik et al. [18] | 2 | 18 (17–19) | 1 MHE and 1 solitary | 2 | 0 | Spastic paraparesis and a pyramidal syndrome in bilateral lower extremities, weakness and fatigue of right lower limb, pyramidal signs in right lower limb | Anterior and lateral part of the spinal canal at the T2–T3 level with spinal cord encroachment, costovertebral angle at the T4 level with spinal cord encroachment |
| Miyamoto et al. [51] | 1 | 23 (23) | MHE | 1 | 0 | Progressing tetraparesis | Pedicle of C2 on the left side, severe spinal cord encroachment |
| Chooi et al. [29] | 1 | 23 (23) | MHE | 1 | 0 | Weakness of right arm and both legs, difficulty ambulating, shock-like sensation radiating down right arm when head turned to right, urinary and bowel incontinence | Right anterior aspect of the posterior arch of C1 with spinal cord compression |
| Korinth et al. [52] | 1 | 12 (12) | MHE | 1 | 0 | Right-sided weakness and gait disturbance | C2 lamina with spinal cord encroachment |
| Fiechtl et al. [53] | 1 | 8 (8) | MHE | 1 | 0 | Difficulty with ambulation | L4–L5 facet joint with neural canal encroachment |
| Oga et al. [54] | 1 | 13 (13) | MHE | 1 | 0 | Slowly progressive gait disturbance | Lamina of C3 with spinal cord encroachment |
| Govender and Parbhoo [55] | 1 | 14 | MHE | 1 | 0 | Weakness in both lower extremities and urinary incontinence | Neural arch of T8 with spinal cord encroachment |
| Khosla et al. [56] | 2 | 11 (5–17) | Solitary | 2 | 0 | Weakness and atrophy of right arm, hyperreflexia of both lower extremities, difficulty ambulating, spastic gait | Right pedicle of C7 with encroachment, right posterior arch of T8 with encroachment |
| Ergün et al. [57] | 1 | 16 (16) | MHE | 1 | 0 | Quadriparesis | Left pedicles and intervertebral joints at the level of C5–C6 with spinal cord encroachment |
| Mikawa et al. [58] | 1 | 17 (17) | MHE | 1 | 0 | Progressive difficulty walking, spastic quadriparesis | Lamina and pedicle of C7 with spinal cord encroachment |
| Atabay et al. [15] | 1 | 17 (17) | MHE | 1 | 0 | Weakness in all extremities, neck pain and urinary dysfunction | Right C2 lamina with spinal cord encroachment and posterior arch of C4 |
| Labram and Mohan [59] | 1 | 9 (9) | MHE | 1 | 0 | Difficulty with ambulation and balance, nocturnal enuresis, quadriparesis | Lamina of C2 with encroachment and spinous process of T9 with no encroachment |
| Robbins et al. [60] | 1 | 15 (15) | MHE | 1 | 0 | Right arm weakness and reduced lateral rotation of the neck following fall from trampoline | Posterior elements of C3 with spinal cord encroachment |
| Morikawa et al. [61] | 1 | 21 (21) | Solitary | 1 | 0 | Intermittent numbness of both hands | Posterior arch of C1 with cord encroachment |
| Barros Filho et al. [62] | 1 | 16 (16) | MHE | 1 | 0 | Dysphagia | Anterior arch of C1 |
| Eder et al. [63] | 1 | 7 (7) | MHE | 1 | 0 | Gait disorder | Posterior arch of C2 with spinal cord encroachment |
| Albrecht et al. [7] | 1 | 16 (16) | Solitary | 0 | 1 | Asymptomatic | Right pedicle of T12 |
| Shapiro et al. [64] | 1 | 11 (11) | MHE | 1 | 0 | Difficulty with ambulation | Lamina of C2 with spinal cord encroachment |
| Moriwaka et al. [65] | 1 | 9 (9) | MHE | 1 | 0 | Progressive transverse myelopathy | C7–T1 vertebral bodies with spinal cord encroachment |
| Wen et al. [66] | 1 | 23 (23) | MHE | 1 | 0 | Symptoms following a fall from chair: apnea, tetraplegia, reduced sensation on right side and left arm | Lamina of C1 with spinal cord encroachment |
| Tully et al. [67] | 1 | 12 (12) | MHE | 1 | 0 | Spastic quadriplegia | Vertebral body of C5 with spinal cord encroachment |
| Scher and Panje [68] | 1 | 10 (10) | Solitary | 1 | 0 | Hoarseness, neck swelling | Vertebral body of C2–C5 on the left posterior side |
| Kozlowski et al. [69] | 2 | 9.5 (6–13) | Solitary | 1 | 1 | Tenderness over coccyx, asymptomatic | Second segment of the coccyx, right pedicle of L5 with slight indentation of the dural sac |
| Cohn and Fielding [70] | 1 | 9 (9) | Solitary | 0 | 1 | Enlarged neck mass | Spinous process and lamina of C2, with no spinal canal encroachment |
| Karakaş and Patiroğlu [71] | 1 | 7 (7) | Solitary | 0 | 1 | Non-tender neck mass, limited cervical range of motion, and slight torticollis | Spinous process and lamina of C5 |
| O’Connor and Roberts [72] | 1 | 24 (24) | MHE | 1 | 0 | Numbness and weakness in right arm and leg | Lamina of C5 with cord encroachment |
| Misra et al. [73] | 1 | 25 (25) | MHE | 1 | 0 | Difficulty walking, weakness in arms and legs | C4 lamina with spinal cord encroachment |
| Novick et al. [74] | 2 | 8.5 (8–9) | Solitary | 0 | 2 | Painless neck mass | Spinous process of C5, left transverse process of C4 |
| Palmer and Blum [75] | 2 | 18 (14–22) | MHE and solitary | 2 | 0 | Incontinence, spastic gait, pain radiating down left arm | Body of C6 with encroachment, C7 with no encroachment |
| MacGee [76] | 1 | 16 (16) | Solitary | 1 | 0 | Transient quadriplegia for 30 min following trauma to chin | Posterior atlas of C2 with spinal cord encroachment |
| Ferrari et al. [77] | 1 | 23 (23) | MHE | 1 | 0 | Spastic paraparesis, gait disturbance, sphincter dysfunction | C2 right hemilamina with spinal cord encroachment |
| Glasauer [78] | 1 | 19 (19) | Solitary | 1 | 0 | Inverted supinator reflex, depressed right biceps reflex | Right lateral mass of C4–C5 |
| Urso et al. [79] | 1 | 9 | MHE | 1 | 0 | Bladder disturbance, lumbar lordosis, abnormal gait | Right vertebral lamina of L4 with spinal canal encroachment |
| Inglis et al. [80] | 1 | 8 (8) | Solitary | 0 | 1 | Painless neck mass | Spinous process of C5 |
| Twersky et al. [81] | 1 | 13 (13) | Solitary | 1 | 0 | Lumbar pain, bilateral sciatica | L4 with spinal canal encroachment |
| Madigan et al. [14] | 1 | 7 (7) | MHE | 1 | 0 | Paresis, gait disturbance, frequent falls, urinary incontinence | C2 lamina with anterior spinal cord encroachment |
| Fielding and Ratzan [82] | 1 | 14 (14) | Solitary | 0 | 1 | Painless neck mass | Spinous process of C3 |
| Crowell and Wepsic [83] | 2 | 15.5 (13–18) | MHE (1)/low-grade chondrosarcoma (1) | 2 | 0 | Weakness in both legs, impaired position sense in toes, ankles, and knees, episodic tingling in low back | Pathology revealed low-grade chondrosarcoma, T2 with spinal encroachment, T8 with spinal canal encroachment |
| Vinstein and Franken [84] | 1 | 14 (14) | MHE | 1 | 0 | Gait disturbance, left hemiparesis with clasp-knife spasticity | Arch of C2 with spinal cord encroachment |
| Hickey [85] | 3 | 16 (11–20) | Solitary and MHE | 2 | 1 | Large palpable mass at lumbosacral region, wasting and atrophy of left hand, bilateral Babinski, asymptomatic | Spinous process of L4, C6, and C7, pedicle of T7 |
| Decker and Wei [86] | 1 | 15 (15) | MHE | 1 | 0 | Bilateral spastic paraparesis, numbness and weakness of right leg | Right-sided paraspinal lesion at T10–T11 with spinal cord encroachment |
| Carmel and Cramer [87] | 1 | 13 (13) | MHE | 1 | 0 | Progressive weakness in right leg and right arm, loss of sensation in the left arm, neck pain on turning head to the right | Under the right hemilamina of C2 with spinal cord encroachment |
| Gokay and Bucy [88] | 1 | 24 (24) | MHE | 1 | 0 | Numbness and weakness of the left lower extremity, increased urgency and frequency of urine, incontinence | Lamina of L3 |
| Cannon [89] | 1 | 23 (23) | MHE | 1 | 0 | Diminished temperature sensation, numbness and tingling of legs, bilateral positive Babinski | T10 |
Extra-canal cervical osteochondromas may present with dysphagia, sleep apnea, or a palpable mass [33–37]. Intracanal lesions may cause pain, paresthesias, myelopathy, weakness, or gait disturbance [4, 6, 8–10, 23]. However, spinal cord compression can be difficult to detect in children. Neurologic findings such as pain or weakness are frequently a late manifestation of cord compression in children [6, 8]. Although spinal lesions tend to progress slowly, they can lead to acute neurological symptoms following minor trauma [5, 6, 8, 38, 39]. In Roach et al.’s series, two of the three patients with symptomatic lesions had permanent neurologic sequelae (presenting with paraplegia and quadriplegia), strengthening the case for screening and aggressive surgical management of asymptomatic lesions [6].
Our series is noteworthy in that the first patient had no symptoms attributable to the cervical spine lesion, and the second patient only had headaches. More concerning, the second patient had headaches following orthopedic surgery, then her symptoms resolved, only to recur 6 month later, finally resulting in a diagnosis. It is possible that hyperextension of the neck at the time of intubation resulted in worsening symptoms due to her C2 spinal stenosis, which, at the time, was undiagnosed. Thus, routine preoperative spinal screening may be warranted for MHE patients in anticipation of neck manipulation during the process of intubation, particularly given the high rate of surgical treatment in this population. According to a recent report, 77 % of children with MHE may be expected to undergo an orthopedic surgery related to their condition [32]. Children with MHE who had had axial imaging had undergone nearly five surgeries on average, typically for the removal of symptomatic osteochondromas or placement of guided growth plates for angular deformity.
In contrast to the rheumatoid population, routine plain radiographs will not necessarily show the pathology [6]. Roach et al. [6] demonstrated that only 17 % of lesions were identifiable on radiographs. Osteochondromas that occur in long bones tend to be pedunculated lesions, while in the spine, these lesions are sessile [4, 29]. Identifying spinal osteochondromas on plain films is difficult due to the complex anatomy of the spine [7]. Thus, axial imaging of MHE children would be required, which is a potentially costly undertaking, particularly if the study were to be obtained in young children who require sedation. Both patients in our series were adolescents. However, the youngest patient in Roach et al.’s series with spinal cord compromise was 5.8 years old, suggesting that imaging is necessary even in young patients.
Roach et al.’s series was remarkable in that they noted that 9 out of 12 patients with lesions penetrating into the canal were asymptomatic. Three of the nine asymptomatic patients underwent prophylactic surgical decompression [6, 9]. Our series adds to these findings, reporting two additional children, one asymptomatic and one complaining only of headaches, who had compressive cervical spine lesions with T2 signal change requiring urgent decompression. It is important for caregivers to be familiar with the potential for spinal stenosis in this patient population, and for providers to have a low threshold for obtaining axial imaging. The primary weakness of our study is that not all 44 MHE patients in our cohort underwent axial imaging. This indicates that the prevalence of compression lesions may be higher than our reported rate of 4.5 % (2 of 44 patients). Given the retrospective nature of this study, we have not yet undertaken routine MRI screening of the neuroaxis in this patient population.
Thus, our series lends further support to routine axial imaging in children with MHE in order to detect compressive lesions and prevent neurologic compromise. Further work remains to determine whether a particular mutation or phenotype holds a greater propensity for large intracanal lesions. This may help refine the indications for screening. For now, we have begun screening all children with MHE at our center at one point during their childhood. Unless symptoms warrant it, screening is typically performed after the child is able to undergo an MRI scan without sedation in order to minimize the risk to the child. We prefer MRI over CT for screening children with MHE. With CT, the osseous center can be clearly visualized, but this often underestimates the size of the lesion. With MRI, the cartilaginous cap of the lesion is clearly visualized. It has been found that MRI and CT are comparable with respect to reliability in detecting an osteochondromas compressing the spinal cord. MRI has a greater advantage in growing children in that there is no radiation exposure, particularly in children with MHE, who have multiple lesions with potential for malignant transformation in adulthood.
In summary, the experience at our center reveals that at least 4.5 % of patients with MHE had compressive osteochondromas (2 of 44 patients). One patient was asymptomatic. Neurologic deficit in similar patients has been reported following minor trauma. Unlike patients with skeletal dysplasia and rheumatoids who undergo preoperative cervical evaluations, neck pathology in MHE patients may not be readily detectible in children with plain imaging. Thus, we confer with the recommendations of Roach et al. for the routine axial screening of these children. Further study is required in order to determine the true prevalence of these lesions and to formalize the indications and frequency of imaging, including preoperative and, potentially, preparticipation sports screening for MHE patients.
Acknowledgments
No external funding was obtained for this study.
Contributor Information
Ali Ashraf, Email: Ashraf.Ali@mayo.edu.
A. Noelle Larson, Phone: +1-507-2842995, FAX: +1-507-2664234, Email: larson.noelle@mayo.edu.
Gabriela Ferski, Email: Gferski@shrinenet.org.
Cary H. Mielke, Email: Cmielke@shrinenet.org
Nicholas M. Wetjen, Email: Wetjen.Nicholas@mayo.edu
Kenneth J. Guidera, Email: Kguidera@shrinenet.org
References
- 1.Murphey MD, Choi JJ, Kransdorf MJ, Flemming DJ, Gannon FH. Imaging of osteochondroma: variants and complications with radiologic–pathologic correlation. Radiographics. 2000;20(5):1407–1434. doi: 10.1148/radiographics.20.5.g00se171407. [DOI] [PubMed] [Google Scholar]
- 2.Legeai-Mallet L, Munnich A, Maroteaux P, Le Merrer M. Incomplete penetrance and expressivity skewing in hereditary multiple exostoses. Clin Genet. 1997;52(1):12–16. doi: 10.1111/j.1399-0004.1997.tb02508.x. [DOI] [PubMed] [Google Scholar]
- 3.Brastianos P, Pradilla G, McCarthy E, Gokaslan ZL. Solitary thoracic osteochondroma: case report and review of the literature. Neurosurgery. 2005;56(6):E1379. doi: 10.1227/01.NEU.0000159718.69601.AC. [DOI] [PubMed] [Google Scholar]
- 4.Tian Y, Yuan W, Chen H, Shen X. Spinal cord compression secondary to a thoracic vertebral osteochondroma. J Neurosurg Spine. 2011;15(3):252–257. doi: 10.3171/2011.4.SPINE10484. [DOI] [PubMed] [Google Scholar]
- 5.Quirini GE, Meyer JR, Herman M, Russell EJ. Osteochondroma of the thoracic spine: an unusual cause of spinal cord compression. AJNR Am J Neuroradiol. 1996;17(5):961–964. [PMC free article] [PubMed] [Google Scholar]
- 6.Roach JW, Klatt JW, Faulkner ND. Involvement of the spine in patients with multiple hereditary exostoses. J Bone Joint Surg Am. 2009;91(8):1942–1948. doi: 10.2106/JBJS.H.00762. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht S, Crutchfield JS, SeGall GK. On spinal osteochondromas. J Neurosurg. 1992;77(2):247–252. doi: 10.3171/jns.1992.77.2.0247. [DOI] [PubMed] [Google Scholar]
- 8.Zaijun L, Xinhai Y, Zhipeng W, Wending H, Quan H, Zhenhua Z, Dapeng F, Jisheng Z, Wei Z, Jianru X. Outcome and prognosis of myelopathy and radiculopathy from osteochondroma in the mobile spine: a report on 14 patients. J Spinal Disord Tech. 2011 doi: 10.1097/BSD.0b013e31823eb239. [DOI] [PubMed] [Google Scholar]
- 9.Roach JW. Hereditary multiple exostoses with spine involvement in a 4-year-old boy. Am J Med Genet A. 2010;152A(5):1263. doi: 10.1002/ajmg.a.33354. [DOI] [PubMed] [Google Scholar]
- 10.Giudicissi-Filho M, de Holanda CV, Borba LA, Rassi-Neto A, Ribeiro CA, de Oliveira JG. Cervical spinal cord compression due to an osteochondroma in hereditary multiple exostosis: case report and review of the literature. Surg Neurol. 2006;66(Suppl 3):S7–S11. doi: 10.1016/j.surneu.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 11.Ezra N, Tetteh B, Diament M, Jonas AJ, Dickson P. Hereditary multiple exostoses with spine involvement in a 4-year-old boy. Am J Med Genet A. 2010;152A(5):1264–1267. doi: 10.1002/ajmg.a.33345. [DOI] [PubMed] [Google Scholar]
- 12.Gille O, Pointillart V, Vital JM. Course of spinal solitary osteochondromas. Spine (Phila Pa 1976) 2005;30(1):E13–E19. [PubMed] [Google Scholar]
- 13.Aniba K, Aldea S, Gaillard S. Cervical cord compression by hereditary multiple exostosis: case report and review of literature. Neurochirurgie. 2011;57(2):85–87. doi: 10.1016/j.neuchi.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Madigan R, Worrall T, McClain EJ. Cervical cord compression in hereditary multiple exostosis. Review of the literature and report of a case. J Bone Joint Surg Am. 1974;56(2):401–404. [PubMed] [Google Scholar]
- 15.Atabay H, Kuyucu Y, Korkmaz O, Iplikcioğlu AC. Myelopathy due to hereditary multiple exostoses: CT and MR studies. Clin Neurol Neurosurg. 1996;98(2):186–188. doi: 10.1016/0303-8467(96)00005-4. [DOI] [PubMed] [Google Scholar]
- 16.Cirak B, Karabulut N, Palaoglu S. Cervical osteochondroma as a cause of spinal cord compression in a patient with hereditary multiple exostoses: computed tomography and magnetic resonance imaging findings. Australas Radiol. 2002;46(3):309–311. doi: 10.1046/j.1440-1673.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- 17.Eap C, Litré CF, Noudel R, Duntze J, Theret E, Rousseaux P. Spinal cord compression due to C4 vertebral arch osteochondroma. Orthop Traumatol Surg Res. 2011;97(1):94–97. doi: 10.1016/j.otsr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Faik A, Mahfoud Filali S, Lazrak N, El Hassani S, Hajjaj-Hassouni N. Spinal cord compression due to vertebral osteochondroma: report of two cases. Joint Bone Spine. 2005;72(2):177–179. doi: 10.1016/j.jbspin.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Er U, Simşek S, Yiğitkanlı K, Adabağ A, Kars HZ. Myelopathy and quadriparesis due to spinal cord compression of C1 laminar osteochondroma. Asian Spine J. 2012;6(1):66–70. doi: 10.4184/asj.2012.6.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratliff J, Voorhies R. Osteochondroma of the C5 lamina with cord compression: case report and review of the literature. Spine (Phila Pa 1976) 2000;25(10):1293–1295. doi: 10.1097/00007632-200005150-00017. [DOI] [PubMed] [Google Scholar]
- 21.Sharma MC, Arora R, Deol PS, Mahapatra AK, Mehta VS, Sarkar C. Osteochondroma of the spine: an enigmatic tumor of the spinal cord. A series of 10 cases. J Neurosurg Sci. 2002;46(2):66–70. [PubMed] [Google Scholar]
- 22.Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Cervical myelopathy caused by atlas osteochondroma and pseudoarthrosis between the osteochondroma and lamina of the axis: case report. Neurol Med Chir (Tokyo) 2010;50(4):346–349. doi: 10.2176/nmc.50.346. [DOI] [PubMed] [Google Scholar]
- 23.Burki V, So A, Aubry-Rozier B. Cervical myelopathy in hereditary multiple exostoses. Joint Bone Spine. 2011;78(4):412–414. doi: 10.1016/j.jbspin.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez C, Tredwell S, De Vera M, Hayden M. The genotype–phenotype correlation of hereditary multiple exostoses. Clin Genet. 2006;70(2):122–130. doi: 10.1111/j.1399-0004.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 25.Vanhoenacker FM, Van Hul W, Wuyts W, Willems PJ, De Schepper AM. Hereditary multiple exostoses: from genetics to clinical syndrome and complications. Eur J Radiol. 2001;40(3):208–217. doi: 10.1016/S0720-048X(01)00401-6. [DOI] [PubMed] [Google Scholar]
- 26.Trebicz-Geffen M, Robinson D, Evron Z, Glaser T, Fridkin M, Kollander Y, Vlodavsky I, Ilan N, Law KF, Cheah KS, Chan D, Werner H, Nevo Z. The molecular and cellular basis of exostosis formation in hereditary multiple exostoses. Int J Exp Pathol. 2008;89(5):321–331. doi: 10.1111/j.1365-2613.2008.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wuyts W, Van Hul W, De Boulle K, Hendrickx J, Bakker E, Vanhoenacker F, Mollica F, Lüdecke HJ, Sayli BS, Pazzaglia UE, Mortier G, Hamel B, Conrad EU, Matsushita M, Raskind WH, Willems PJ. Mutations in the EXT1 and EXT2 genes in hereditary multiple exostoses. Am J Hum Genet. 1998;62(2):346–354. doi: 10.1086/301726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sansur CA, Pouratian N, Dumont AS, Schiff D, Shaffrey CI, Shaffrey ME. Part II: spinal-cord neoplasms—primary tumours of the bony spine and adjacent soft tissues. Lancet Oncol. 2007;8(2):137–147. doi: 10.1016/S1470-2045(07)70033-5. [DOI] [PubMed] [Google Scholar]
- 29.Chooi YS, Siow YS, Chong CS. Cervical myelopathy caused by an exostosis of the posterior arch of C1. J Bone Joint Surg Br. 2005;87(2):257–259. doi: 10.1302/0301-620X.87B2.15560. [DOI] [PubMed] [Google Scholar]
- 30.Garrison RC, Unni KK, McLeod RA, Pritchard DJ, Dahlin DC. Chondrosarcoma arising in osteochondroma. Cancer. 1982;49(9):1890–1897. doi: 10.1002/1097-0142(19820501)49:9<1890::AID-CNCR2820490923>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Strovski E, Ali R, Graeb DA, Munk PL, Chang SD. Malignant degeneration of a lumbar osteochondroma into a chondrosarcoma which mimicked a large retropertioneal mass. Skeletal Radiol. 2012;41(10):1319–1322. doi: 10.1007/s00256-012-1405-6. [DOI] [PubMed] [Google Scholar]
- 32.Goud AL, de Lange J, Scholtes VA, Bulstra SK, Ham SJ. Pain, physical and social functioning, and quality of life in individuals with multiple hereditary exostoses in The Netherlands: a national cohort study. J Bone Joint Surg Am. 2012;94(11):1013–1020. doi: 10.2106/JBJS.K.00406. [DOI] [PubMed] [Google Scholar]
- 33.Bonic EE, Kettner NW. A rare presentation of cervical osteochondroma arising in a spinous process. Spine (Phila Pa 1976) 2012;37(1):E69–E72. doi: 10.1097/BRS.0b013e31821fcfde. [DOI] [PubMed] [Google Scholar]
- 34.Chandra J, Kirmi O, Woo EK. Radiology quiz case 2. Diagnosis: osteochondroma arising from the lateral process of C4 and hereditary multiple exostoses (HME) Arch Otolaryngol Head Neck Surg. 2010;136(9):925. doi: 10.1001/archoto.2010.141-a. [DOI] [PubMed] [Google Scholar]
- 35.Rao H, Jakheria S. Giant cervical exostosis: a case report with review of literature. J Pediatr Orthop B. 2009;18(2):103–105. doi: 10.1097/BPB.0b013e328329431c. [DOI] [PubMed] [Google Scholar]
- 36.Grivas TB, Polyzois VD, Xarchas K, Liapi G, Korres D. Seventh cervical vertebral body solitary osteochondroma. Report of a case and review of the literature. Eur Spine J. 2005;14(8):795–798. doi: 10.1007/s00586-005-0890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang V, Chou D. Anterior C1–2 osteochondroma presenting with dysphagia and sleep apnea. J Clin Neurosci. 2009;16(4):581–582. doi: 10.1016/j.jocn.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Marchand EP, Villemure JG, Rubin J, Robitaille Y, Ethier R. Solitary osteochondroma of the thoracic spine presenting as spinal cord compression. A case report. Spine. 1986;11(10):1033–1035. doi: 10.1097/00007632-198612000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Lotfinia I, Vahedi P, Tubbs RS, Ghavame M, Meshkini A. Neurological manifestations, imaging characteristics, and surgical outcome of intraspinal osteochondroma. J Neurosurg Spine. 2010;12(5):474–489. doi: 10.3171/2009.11.SPINE0980. [DOI] [PubMed] [Google Scholar]
- 40.Tahasildar N, Sudesh P, Goni V, Tripathy SK. Giant osteochondroma of axis in a child with multiple hereditary exostoses: case report and review of literature. J Pediatr Orthop B. 2012;21(3):280–285. doi: 10.1097/BPB.0b013e32834c3186. [DOI] [PubMed] [Google Scholar]
- 41.Patel A, Thacker MM. Cervical spinal canal compromise in a 14-year-old girl with hereditary multiple exostoses. Pediatr Radiol. 2010;40(Suppl 1):S158. doi: 10.1007/s00247-010-1751-2. [DOI] [PubMed] [Google Scholar]
- 42.Gunay C, Atalar H, Yildiz Y, Saglik Y. Spinal osteochondroma: a report on six patients and a review of the literature. Arch Orthop Trauma Surg. 2010;130(12):1459–1465. doi: 10.1007/s00402-010-1058-7. [DOI] [PubMed] [Google Scholar]
- 43.Hassankhani EG. Solitary lower lumbar osteochondroma (spinous process of L3 involvement): a case report. Cases J. 2009;2:9359. doi: 10.1186/1757-1626-2-9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han IH, Kuh SU. Cervical osteochondroma presenting as brown-sequard syndrome in a child with hereditary multiple exostosis. J Korean Neurosurg Soc. 2009;45(5):309–311. doi: 10.3340/jkns.2009.45.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chatzidakis E, Lypiridis S, Kazdaglis G, Chatzikonstadinou K, Papatheodorou G. A rare case of solitary osteochondroma of the dens of the C2 vertebra. Acta Neurochir (Wien) 2007;149(6):637–638. doi: 10.1007/s00701-007-1151-z. [DOI] [PubMed] [Google Scholar]
- 46.Song KJ, Lee KB. Solitary osteochondroma of the thoracic spine causing myelopathy. Eur J Pediatr Surg. 2007;17(3):210–213. doi: 10.1055/s-2007-965124. [DOI] [PubMed] [Google Scholar]
- 47.Samartzis D, Marco RA. Osteochondroma of the sacrum: a case report and review of the literature. Spine (Phila Pa 1976) 2006;31(13):E425–E429. doi: 10.1097/01.brs.0000220222.63828.d3. [DOI] [PubMed] [Google Scholar]
- 48.Maheshwari AV, Jain AK, Dhammi IK. Osteochondroma of C7 vertebra presenting as compressive myelopathy in a patient with nonhereditary (nonfamilial/sporadic) multiple exostoses. Arch Orthop Trauma Surg. 2006;126(10):654–659. doi: 10.1007/s00402-006-0211-9. [DOI] [PubMed] [Google Scholar]
- 49.McCall TD, Liu JK, Kestle JR. Sporadic osteochondroma of the cervical spine. Case illustration. J Neurosurg. 2006;104(4 Suppl):293. doi: 10.3171/ped.2006.104.4.293. [DOI] [PubMed] [Google Scholar]
- 50.Aldea S, Bonneville F, Poirier J, Chiras J, George B, Carpentier A. Acute spinal cord compression in hereditary multiple exostoses. Acta Neurochir (Wien) 2006;148(2):195–198. doi: 10.1007/s00701-005-0680-6. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto K, Sakaguchi Y, Hosoe H, Mori A, Yamazaki S, Hattori S, Shimizu K. Tetraparesis due to exostotic osteochondroma at upper cervical cord in a patient with multiple exostoses–mental retardation syndrome (Langer–Giedion syndrome) Spinal Cord. 2005;43(3):190–194. doi: 10.1038/sj.sc.3101690. [DOI] [PubMed] [Google Scholar]
- 52.Korinth MC, Ramaekers VT, Rohde V. Cervical cord exostosis compressing the axis in a boy with hereditary multiple exostoses. Case illustration. J Neurosurg. 2004;100(2 Suppl Pediatrics):223. doi: 10.3171/ped.2004.100.2.0223. [DOI] [PubMed] [Google Scholar]
- 53.Fiechtl JF, Masonis JL, Frick SL. Spinal osteochondroma presenting as atypical spinal curvature: a case report. Spine (Phila Pa 1976) 2003;28(13):E252–E255. doi: 10.1097/00007632-200307010-00026. [DOI] [PubMed] [Google Scholar]
- 54.Oga M, Nakatani F, Ikuta K, Tamaru T, Arima J, Tomishige M. Treatment of cervical cord compression, caused by hereditary multiple exostosis, with laminoplasty: a case report. Spine (Phila Pa 1976) 2000;25(10):1290–1292. doi: 10.1097/00007632-200005150-00016. [DOI] [PubMed] [Google Scholar]
- 55.Govender S, Parbhoo AH. Osteochondroma with compression of the spinal cord. A report of two cases. J Bone Joint Surg Br. 1999;81(4):667–669. doi: 10.1302/0301-620X.81B4.9248. [DOI] [PubMed] [Google Scholar]
- 56.Khosla A, Martin DS, Awwad EE. The solitary intraspinal vertebral osteochondroma. An unusual cause of compressive myelopathy: features and literature review. Spine (Phila Pa 1976) 1999;24(1):77–81. doi: 10.1097/00007632-199901010-00019. [DOI] [PubMed] [Google Scholar]
- 57.Ergün R, Okten AI, Beşkonakli E, Akdemir G, Taşkin Y. Cervical laminar exostosis in multiple hereditary osteochondromatosis: anterior stabilization and fusion technique for preventing instability. Eur Spine J. 1997;6(4):267–269. doi: 10.1007/BF01322449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mikawa Y, Watanabe R, Nakashima Y, Hayashida T. Cervical spinal cord compression in hereditary multiple exostoses. Report of a case and a review of the literature. Arch Orthop Trauma Surg. 1997;116(1–2):112–115. doi: 10.1007/BF00434114. [DOI] [PubMed] [Google Scholar]
- 59.Labram EK, Mohan J. Diaphyseal aclasis with spinal cord compression. Report of two cases and review of the literature. J Neurosurg. 1996;84(3):518–521. doi: 10.3171/jns.1996.84.3.0518. [DOI] [PubMed] [Google Scholar]
- 60.Robbins SE, Laitt RD, Lewis T. Hereditary spinal osteochondromas in diaphyseal aclasia. Neuroradiology. 1996;38(1):59–61. doi: 10.1007/BF00593223. [DOI] [PubMed] [Google Scholar]
- 61.Morikawa M, Numaguchi Y, Soliman JA. Osteochondroma of the cervical spine. MR findings. Clin Imaging. 1995;19(4):275–278. doi: 10.1016/0899-7071(94)00063-I. [DOI] [PubMed] [Google Scholar]
- 62.Barros Filho TE, Oliveira RP, Taricco MA, Gonzalez CH. Hereditary multiple exostoses and cervical ventral protuberance causing dysphagia. A case report. Spine (Phila Pa 1976) 1995;20(14):1640–1642. doi: 10.1097/00007632-199507150-00015. [DOI] [PubMed] [Google Scholar]
- 63.Eder HG, Oberbauer RW, Ranner G. Cervical cord compression in hereditary multiple exostoses. J Neurosurg Sci. 1993;37(1):53–56. [PubMed] [Google Scholar]
- 64.Shapiro SA, Javid T, Putty T. Osteochondroma with cervical cord compression in hereditary multiple exostoses. Spine (Phila Pa 1976) 1990;15(6):600–602. doi: 10.1097/00007632-199006000-00033. [DOI] [PubMed] [Google Scholar]
- 65.Moriwaka F, Hozen H, Nakane K, Sasaki H, Tashiro K, Abe H. Myelopathy due to osteochondroma: MR and CT studies. J Comput Assist Tomogr. 1990;14(1):128–130. doi: 10.1097/00004728-199001000-00025. [DOI] [PubMed] [Google Scholar]
- 66.Wen DY, Bergman TA, Haines SJ. Acute cervical myelopathy from hereditary multiple exostoses: case report. Neurosurgery. 1989;25(3):472–475. doi: 10.1227/00006123-198909000-00028. [DOI] [PubMed] [Google Scholar]
- 67.Tully RJ, Pickens J, Oro J, Levine C. Hereditary multiple exostoses and cervical cord compression: CT and MR studies. J Comput Assist Tomogr. 1989;13(2):330–333. doi: 10.1097/00004728-198903000-00029. [DOI] [PubMed] [Google Scholar]
- 68.Scher N, Panje WR. Osteochondroma presenting as a neck mass: a case report. Laryngoscope. 1988;98(5):550–553. doi: 10.1288/00005537-198805000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Kozlowski K, Scougall J, Stevens M. Solitary osteochondroma of the spine. Report of two unusual cases in children. Rofo. 1987;146(4):462–464. doi: 10.1055/s-2008-1048522. [DOI] [PubMed] [Google Scholar]
- 70.Cohn RS, Fielding JW. Osteochondroma of the cervical spine. J Pediatr Surg. 1986;21(11):997–999. doi: 10.1016/S0022-3468(86)80123-3. [DOI] [PubMed] [Google Scholar]
- 71.Karakaş ES, Patiroğlu TE. Osteochondroma of the cervical spine. Turk J Pediatr. 1986;28(2):133–135. [PubMed] [Google Scholar]
- 72.O’Connor GA, Roberts TS. Spinal cord compression by an osteochondroma in a patient with multiple osteochondromatosis. Case report. J Neurosurg. 1984;60(2):420–423. doi: 10.3171/jns.1984.60.2.0420. [DOI] [PubMed] [Google Scholar]
- 73.Misra UK, Nag D, Dave VS, Shukla R, Kar AM. Cervical cord compression due to chondromatous change in a patient with metaphysial aclasis. J Neurol Neurosurg Psychiatry. 1983;46(12):1155–1157. doi: 10.1136/jnnp.46.12.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Novick GS, Pavlov H, Bullough PG. Osteochondroma of the cervical spine: report of two cases in preadolescent males. Skeletal Radiol. 1982;8(1):13–15. doi: 10.1007/BF00361361. [DOI] [PubMed] [Google Scholar]
- 75.Palmer FJ, Blum PW. Osteochondroma with spinal cord compression: report of three cases. J Neurosurg. 1980;52(6):842–845. doi: 10.3171/jns.1980.52.6.0842. [DOI] [PubMed] [Google Scholar]
- 76.MacGee EE. Osteochondroma of the cervical spine: a cause of transient quadriplegia. Neurosurgery. 1979;4(3):259–260. doi: 10.1227/00006123-197903000-00013. [DOI] [PubMed] [Google Scholar]
- 77.Ferrari G, Taddei L, Vivenza C, Rossi G. Paraparesis in hereditary multiple exostoses: case report. Neurology. 1979;29(7):973–977. doi: 10.1212/WNL.29.7.973. [DOI] [PubMed] [Google Scholar]
- 78.Glasauer FE. Benign lesions of the cervical spine. Acta Neurochir (Wien) 1978;42(3–4):161–175. doi: 10.1007/BF01405331. [DOI] [PubMed] [Google Scholar]
- 79.Urso S, Carfagni A, Amorese V. Vertebral compression syndrome in multiple exostoses (case report) Ital J Orthop Traumatol. 1977;3(3):333–340. [PubMed] [Google Scholar]
- 80.Inglis AE, Rubin RM, Lewis RJ, Villacin A. Osteochondroma of the cervical spine. Case report. Clin Orthop Relat Res. 1977;126:127–129. [PubMed] [Google Scholar]
- 81.Twersky J, Kassner EG, Tenner MS, Camera A. Vertebral and costal osteochondromas causing spinal cord compression. Am J Roentgenol Radium Ther Nucl Med. 1975;124(1):124–128. doi: 10.2214/ajr.124.1.124. [DOI] [PubMed] [Google Scholar]
- 82.Fielding JW, Ratzan S. Osteochondroma of the cervical spine. J Bone Joint Surg Am. 1973;55(3):640–641. [PubMed] [Google Scholar]
- 83.Crowell RM, Wepsic JG. Thoracic cord compression due to chondrosarcoma in two cousins with hereditary multiple exostoses. Report of two cases. J Neurosurg. 1972;36(1):86–89. doi: 10.3171/jns.1972.36.1.0086. [DOI] [PubMed] [Google Scholar]
- 84.Vinstein AL, Franken EA., Jr Hereditary multiple exostoses. Report of a case with spinal cord compression. Am J Roentgenol Radium Ther Nucl Med. 1971;112(2):405–407. doi: 10.2214/ajr.112.2.405. [DOI] [PubMed] [Google Scholar]
- 85.Hickey CH. Osteochondroma of the vertebra. Henry Ford Hosp Med J. 1969;17(1):53–58. [PubMed] [Google Scholar]
- 86.Decker RE, Wei WC. Thoracic cord compression from multiple hereditary exostoses associated with cerebellar astrocytoma. Case report. J Neurosurg. 1969;30(3):310–312. doi: 10.3171/jns.1969.30.3part1.0310. [DOI] [PubMed] [Google Scholar]
- 87.Carmel PW, Cramer FJ. Cervical cord compression due to exostosis in a patient with hereditary multiple exostoses. Case report. J Neurosurg. 1968;28(5):500–503. doi: 10.3171/jns.1968.28.5.0500. [DOI] [PubMed] [Google Scholar]
- 88.Gokay H, Bucy PC. Osteochondroma of the lumbar spine; report of a case. J Neurosurg. 1955;12(1):72–78. doi: 10.3171/jns.1955.12.1.0072. [DOI] [PubMed] [Google Scholar]
- 89.Cannon JF. Hereditary multiple exostoses. Am J Hum Genet. 1954;6(4):419–425. [PMC free article] [PubMed] [Google Scholar]


