Abstract
Survival rates following traumatic cardiac arrest (TCA) are known to be poor but resuscitation is not universally futile. There are a number of potentially reversible causes to TCA and a well-defined group of survivors. There are distinct differences in the pathophysiology between medical cardiac arrests and TCA. The authors present some of the key differences and evidence related to resuscitation in TCA, and suggest a separate algorithm for the management of out-of-hospital TCA attended by a highly trained physician and paramedic team.
Introduction
Survival rates following traumatic cardiac arrest (TCA) are known to be poor from both civilian and military data [1,2]. However, the common misconception that resuscitation is futile for all patients is not well founded. Lockey and colleagues demonstrated that a well-trained physician and paramedic team attending out-of-hospital TCAs could achieve a 7.5% survival rate to hospital discharge, comparable with out-of-hospital cardiac arrest from any cause [1]. Survival rates are highly variable depending on the aetiology, and traumatic pathologies associated with an improved chance of successful resuscitation include hypoxia, tension pneumothorax and cardiac tamponade [1-3]. TCA is a unique disease in which clinicians are frequently confronted by a healthy heart that has arrested as a result of hypoxia, haemorrhage or obstructive shock [1-3]. Consequently, there are different management priorities in TCA and potential deviations from existing non-TCA algorithms. The authors developed an algorithm (Figure 1) to address these issues based on their own current practice and available published evidence.
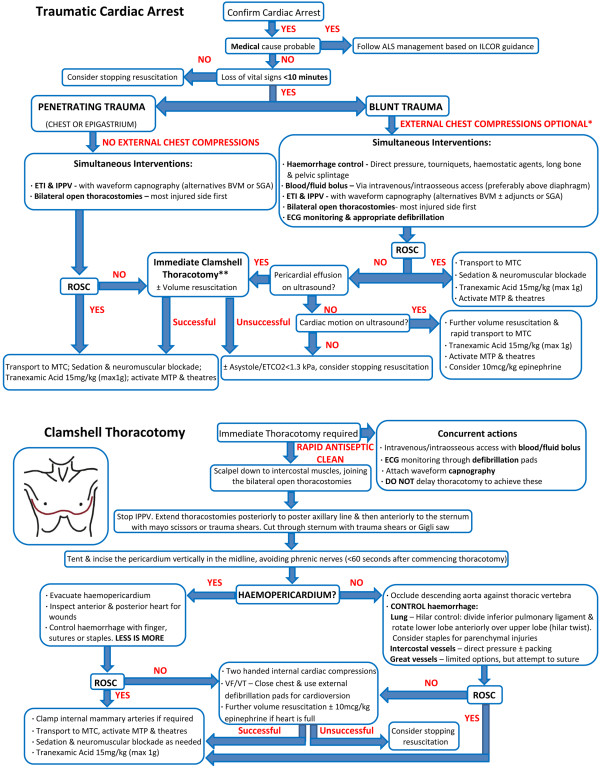
Figure 1.
Traumatic cardiac arrest and thoracotomy algorithm. *If signs of exsanguination or chest injuries, external chest compressions unlikely to be effective, and possibly detrimental. **In blunt trauma involving complex pathology, pericardiocentesis maybe a reasonable intermediate step. If ROSC not achieved, proceed to immediate thoracotomy. ALS, advanced cardiac life support; BVM, bag valve mask; ECG, electrocardiogram; ETCO2, end-tidal carbon dioxide partial pressure; ETI, endotracheal intubation; ILCOR, International Liaison Committee on Resuscitation; IPPV, intermittent positive pressure ventilation; MTC, major trauma centre; MTP, massive transfusion policy; ROSC, return of spontaneous circulation; SGA, supra-glottic airway; VF, ventricular fibrillation; VT, ventricular tachycardia.
Rationale for the traumatic cardiac arrest algorithm
Effective oxygenation is pivotal to good trauma care given that hypoxia has significant implications for morbidity and mortality [1,4]. Up to 44% of TCA survivors arrest primarily as a result of asphyxia, and consequently correcting hypoxia is essential [1]. There is much controversy over optimal airway management in the prehospital trauma patient, with a paucity of evidence for the benefit of advanced airway interventions over basic interventions [5]. The placement of a cuffed tracheal tube allows the delivery of high functional inspired oxygen concentrations, optimal positive pressure ventilation, prevention of gastric insufflation and protection against tracheal aspiration. Prehospital drug-facilitated tracheal intubation in a physician-led service has been shown to have a high success rate of between 98.8 and 100%, with minimal complications [6-8]. If tracheal intubation is to be used during TCA the tube should be placed by experienced clinicians, with appropriate manual inline stabilisation and mandatory confirmation by waveform capnography.
Once patients are intubated, attempts should be made to achieve adequate oxygenation while minimising minute ventilation and intrathoracic pressures. This avoids further reductions in venous return in patients with a shocked or arrested circulation [9]. There is much controversy over the impact of hyperoxia on ischaemic-reperfusion injury and mortality in traumatic brain injury and cardiac arrest survivors [10-12]. If return of spontaneous circulation (ROSC) is achieved, it would seem prudent to titrate the functional inspired oxygen concentration and ventilation to achieve an arterial oxygen saturation of 94 to 98% and normocapnia [4].
Proactive exclusion of tension pneumothoraces is an essential component of ensuring good oxygenation in TCA. Commonly taught needle thoracostomy decompression can potentially fail due to mechanical obstruction or kinking, can cause iatrogenic injury, can be incorrectly sited in 40% of patients and is expected to fail in 42.5% of patients due to the chest wall thickness [13-16]. Conversely, tube thoracostomy has been shown to be more effective at evacuating tension pneumothoraces during cardiac arrest [13], and prehospital placement is a predictor of survival in TCA (odds ratio = 0.3, 95% confidence interval = 0.13 to 0.8) [17]. The survival benefit of tube thoracostomy must be balanced against the notable risk of tube malposition in both the hospital and prehospital setting [18,19]. Prehospital open thoracostomy avoids the risk of tube malposition, and has been shown to be rapid, safe and effective during positive pressure ventilation [20-22]. An open thoracostomy also allows for complete lung re-expansion and easy thoracic reassessment. Cuffed tracheal tube insertion into the open thoracostomy should be considered when there is difficulty in maintaining patency due to excessive soft tissues [23]. The authors' opinion is that all TCA patients with chest injuries should have bilateral open thoracostomies to proactively exclude tension pneumothoraces.
Uncontrolled haemorrhage is the commonest cause of preventable death in trauma [24], and survival from exsanguination and true TCA is very rare [1,25]. The early control of catastrophic external haemorrhage with pressure dressings, haemostatic agents or tourniquets, combined with the traditional primary survey and damage control resuscitation, is rapidly becoming a standard of care [25,26]. In TCA, early control of catastrophic external haemorrhage should be accompanied by long bone splintage and application of a pelvic binder for blunt trauma patients.
Damage control resuscitation is a package of care that is composed of damage control surgery, haemostatic resuscitation and permissive hypotension [25]. Haemostatic resuscitation is the provision of balanced volume replacement in the haemorrhaging patient that targets trauma-induced coagulopathy [25]. This involves the early transfusion of packed red blood cells, fresh frozen plasma and platelets accompanied by tranexamic acid, while limiting the use of crystalloids and vasopressors [25,27]. The ideal ratio of fresh frozen plasma to packed red blood cells remains contentious; a ratio of 1:1 or greater may not confer additional benefit over ratios of 1:2 to 3:4 [28].
Prehospital blood transfusion has been infrequently described [29], and concerns regarding expense, transfusion reactions and difficult storage have limited the interest of prehospital providers. The authors recently reviewed 5 years of prehospital blood transfusions carried out by their own service. A total of 382 units of packed red blood cells were transfused to 147 trauma patients, with no documented transfusion reactions and acceptable wastage (presently unpublished data). The authors contend that blood is currently the resuscitation fluid of choice in TCA patients and should be utilised prehospital if available.
Volume resuscitation in TCA should ideally be achieved through large-bore peripheral intravenous access above the diaphragm. This allows desirable flow characteristics, low complication rates and reduced risk of extravasation through injured pelvic or abdominal vessels. However, intraosseous access has been shown to have a better first-pass success rate and time to vascular access in out-of-hospital cardiac arrest [30]. If intravenous access is not immediately available in TCA, the authors' current practice is to obtain proximal humerus intraosseous access given the desirable flow rates over other sites below the diaphragm [31].
Early use of aggressive haemorrhage control, haemostatic resuscitation and damage control surgery in TCA may produce a number of unexpected survivors [2]. The British military have demonstrated that 75% of their TCA survivors had arrested as a result of haemorrhage [2]. This patient group demonstrates the importance of differentiating patients with true mechanical cardiac standstill (classical pulseless electrical activity or asystole) from those with cardiac motion but impalpable pulses due to profound hypotension (pseudo-pulseless electrical activity). The overwhelming majority of survivors present organised electrical activity on electrocardiogram or cardiac motion on ultrasound, and belong to the latter group [2,32,33].
Prehospital ultrasound has a multitude of applications in the unstable trauma patient, extending from simple thoracic ultrasound through to an extended focused assessment with sonography in trauma [34-36]. In TCA, the use of ultrasound to assist in the assessment of cardiac tamponade [37], pulse presence [37,38] and cardiac motion [2,32,33,37] forms an integral part of the authors' current practice.
Cardiac tamponade is a very real concern in penetrating trauma, but can also be seen in a small number of blunt trauma patients following cardiac rupture [39]. Physician-performed on-scene thoracotomy following penetrating TCA has been shown to have a survival rate to hospital discharge of 18% [3]. The primary pathology for all survivors in this series was cardiac tamponade secondary to knife-related cardiac wounds [3]. Such survival rates are similar to published data for in-hospital emergency department thoracotomy [40-42]. This dramatic intervention requires adequate training and clinical governance to ensure appropriate patient selection and optimal outcomes [43]. Given that the majority of physicians undertaking this procedure in the prehospital arena will be nonsurgeons, a simplified and standardised approach is required to maximise the chances of success, as described by Wise and colleagues [43]. As a result of this limited surgical experience and the high prevalence of cardiac tamponade in prehospital TCA survivors [3,43], the primary aim of the developed thoracotomy algorithm was to address this single pathology (Figure 1). Although the management of other thoracotomy findings are discussed, operators should be aware of the dismal prognosis in this group [3,41-43].
End-tidal carbon dioxide partial pressure (ETCO2) measurement has shown much promise in non-TCAs for confirming tracheal intubation, adequacy of chest compressions, ROSC and prognostication [44-46]. The difficulty comes when interpreting ETCO2 in cardiac arrests because it can be affected by the type of airway in situ, the presence of good quality chest compressions and the initial rhythm, cause and duration of cardiac arrest [46]. One of the most established and promising applications of ETCO2 is as a predictor of survival in non-TCAs [44-46]. What has proven more challenging has been defining a cutoff point for determining futility, and when it should be applied following cardiac arrest.
In one of the largest studies to date, Kolar and colleagues suggested that ETCO2 ≤1.9 kPa (14.3 mmHg) at 20 minutes in non-TCAs was able to predict ROSC with a sensitivity, specificity, positive predictive value and negative predictive value all of 100% [44]. There are obvious limitations when trying to extrapolate these findings to TCA patients for a number of reasons. The aetiologies clearly vary, and 20 minutes might be considered by some to be an excessive duration of resuscitation for TCA. Eckstein and colleagues showed that an initial ETCO2 >1.3 kPa (10 mmHg) and the absence of a fall >25% from the baseline were significantly associated with ROSC in out-of-hospital cardiac arrest [45].
No single ETCO2 value can be used in isolation in TCA. The authors feel that, following aggressive resuscitation, the combination of a downtime >10 minutes, no organised electrical activity on the electrocardiogram, a lack of cardiac motion on ultrasound and ETCO2 <1.3 kPa (10 mmHg) would suggest futility. Caution should be exercised when extrapolating the use of ETCO2 in TCA given the paucity of evidence in this setting and its reliance on good-quality chest compressions.
A gravid patient in TCA, known to be greater than 20 weeks gestation or with a fundal height higher than the umbilicus, may require a perimortem caesarean delivery (PMCD). PMCD will not only potentially result in a viableneonate, but is also vital to optimise maternal haemodynamics and outcomes [47]. PMCD should ideally be initiated within 4 minutes of TCA to improve maternaland neonatal outcomes; however, neonatal survival is still likely if delivery occurs within 10 to 15 minutes [47]. Prehospital PMCD has been undertaken by our service previously [48], and the authors feel it is an essential skill for prehospital physician-paramedic teams.
Along with these subtle additions to managing TCA patients there are potentially a number of omissions from advanced cardiac life-support management that should be considered. The evidence for the benefit of external chest compressions in TCA is sparse. External chest compressions can deliver approximately one-third of the normal blood flow to the brain in non-TCAs and rely on adequate venous return [49]. In TCA the presence of hypovolaemia, tension pneumothorax or cardiac tamponade will all limit venous return, left ventricular end-diastolic volume and chest compression effectiveness [50]. External chest compressions may also delay or increase the procedural risk of definitive interventions for reversible causes, such as thoracostomy. Furthermore, in the presence of significant thoracic trauma, chest compressions could theoretically exacerbate underlying parenchymal injuries. Given these concerns, consideration should be given to omitting external chest compressions in TCA, or delaying them until preload and obstructive causes have been addressed. Such a decision should be clinically guided and is entirely at the discretion of individual team leaders and organisations.
There is limited evidence for the efficacy of any drugs during non-TCAs and, despite the continued use of epinephrine, a recent randomised control trial has questioned its role [51]. Jacobs and colleagues showed that although epinephrine was associated with increased incidence of ROSC, there was no improvement in survival to hospital discharge [51]. Epinephrine is also known to adversely affect cerebral microvascular blood flow during cardiopulmonary resuscitation, and may worsen cerebral ischaemia [52]. Combined with the fact that hypovolaemic TCA patients are already hypoperfused, severely acidaemic and maximally vasoconstricted, this may limit the role of epinephrine in this setting [53,54].
Post-resuscitation care
If ROSC is achieved, meticulous post-resuscitation care should be accompanied by expediting transport to an appropriate major trauma centre. A pre-alert should be delivered to the receiving major trauma centre with a request for activation of their massive transfusion policy if deemed necessary. Consideration should be given to bypassing the emergency department for the operating theatre or the interventional radiology suite for definitive haemorrhage control for select patients.
Ventilation should target normoxia and normocapnia, while minimising intrathoracic pressures [4]. This limits further secondary neurological insults and any reduction in venous return [4]. To assist with ventilation and neuroprotection, suitable sedation, analgesia and neuromuscular blockade should be titrated to effect. Sedation and analgesia also provide a degree of sympatholysis that allows continued haemostatic resuscitation, while maintaining permissive hypotension. The associated high-flow/low-pressure state promotes microvascular washout of previously hypoperfused areas with a theoretical improvement in lactate clearance and serum pH [55].
Physiological resuscitation endpoints post ROSC will depend on the underlying pathology and transport times involved [25,56]. The quality of evidence for permissive hypotension or targeted resuscitation is variable. For short transport times, however, the authors utilise the following endpoints for resuscitation: blunt trauma without traumatic brain injury - palpable radial pulse (systolic blood pressure >80 mmHg); blunt trauma with probable traumatic brain injury - systolic blood pressure >100 mmHg; and penetrating torso trauma - palpable central pulse or patient cerebration (systolic blood pressure >60 mmHg). If the transport time exceeds 1 hour, the detrimental effects of permissive hypotension may outweigh the benefits, and there may be a role for novel hybrid resuscitation [57].
The neuroprotective role of mild therapeutic hypothermia in traumatic brain injury and TCA survivors remains unclear [4,58,59]. The adverse impact of hypothermia on a trauma-induced coagulopathy could have catastrophic consequences prior to definitive haemorrhage control [60]. The authors' current practice is to maintain normothermia during transport through sufficient patient packaging to maximise passive warming, and to utilize active warming if necessary.
Conclusion
Greater Sydney Area Helicopter Emergency Medical Services undertakes prehospital and interhospital retrievals of critically ill and injured patients utilising a physician-paramedic team. TCA management by this service is guided by a standard operating procedure [61] that employs the evidence-based principles discussed above, and is summarised by the algorithm shown in Figure 1. This standardised approach for a highly trained physician-led prehospital team aims to maximise the number of neurologically intact survivors in out-of-hospital TCA. Further research is required before such an algorithm is to be more widely adopted.
Abbreviations
ETCO2: end-tidal carbon dioxide partial pressure; PMCD: perimortem caesarean delivery; ROSC: return of spontaneous circulation; TCA: traumatic cardiac arrest.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Peter Brendon Sherren, Email: petersherren@gmail.com.
Cliff Reid, Email: reidcg@me.com.
Karel Habig, Email: KHabig@ambulance.nsw.gov.au.
Brian J Burns, Email: bjburns@ambulance.nsw.gov.au.
Acknowledgements
The authors developed this algorithm to provide structured evidence-based guidance on the management of TCA. This algorithm should not be considered a standard of care and use of the algorithm is voluntary. Responsibility for the care of TCA patients lies with the treating physician, and is dependent on the clinical status of the patient, available resources and appropriate training.
This article was accepted for presentation at the 33rd International Symposium on Intensive Care and Emergency Medicine, Brussels, 19-22 March 2013, and submitted for presentation at Prehospital and Retrieval 2013, Glasgow, 25-26 April 2013.
Authors' contributions
PBS conceived the idea for the paper, and drafted the initial algorithm and manuscript. CR, KH and BJB devised the Greater Sydney Area Helicopter Emergency Medical Services standard operating procedure for TCA management. All authors were involved in the final editing of the algorithm and manuscript.
All authors have made substantial contributions to all of the following: the conception and design of the study, or acquisition of data or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be submitted.
References
- Lockey D, Crewdson K, Davies G. Traumatic cardiac arrest: who are the survivors? Ann Emerg Med. 2006;48:240–244. doi: 10.1016/j.annemergmed.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Tarmey NT, Park CL, Bartels OJ, Konig TC, Mahoney PF, Mellor AJ. Outcomes following military traumatic cardiorespiratory arrest: a prospective observational study. Resuscitation. 2011;82:1194–1197. doi: 10.1016/j.resuscitation.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Davies GE, Lockey DJ. Thirteen survivors of prehospital thoracotomy for penetrating trauma: a prehospital physician-performed resuscitation procedure that can yield good results. J Trauma. 2011;70:E75–E78. doi: 10.1097/TA.0b013e3181f6f72f. [DOI] [PubMed] [Google Scholar]
- Wijayatilake DS, Shepherd SJ, Sherren PB. Updates in the management of intracranial pressure in traumatic brain injury. Curr Opin Anaesthesiol. 2012;25:540–547. doi: 10.1097/ACO.0b013e328357960a. [DOI] [PubMed] [Google Scholar]
- Lossius HM, Røislien J, Lockey DJ. Patient safety in pre-hospital emergency tracheal intubation: a comprehensive meta-analysis of the intubation success rates of EMS providers. Crit Care. 2012;16:R24. doi: 10.1186/cc11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T, Ellis DY, Foster L, Lockey D. Cricoid pressure and laryngeal manipulation in 402 pre-hospital emergency anaesthetics: essential safety measure or a hindrance to rapid safe intubation? Resuscitation. 2010;81:810–816. doi: 10.1016/j.resuscitation.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Helm M, Hossfeld B, Schäfer S, Hoitz J, Lampl L. Factors influencing emergency intubation in the pre-hospital setting - a multicentre study in the German Helicopter Emergency Medical Service. Br J Anaesth. 2006;96:67–71. doi: 10.1093/bja/aei275. [DOI] [PubMed] [Google Scholar]
- Bloomer R, Burns BJ, Ware S. Improving documentation in prehospital rapid sequence intubation: investigating the use of a dedicated airway registry form. Emerg Med J. 2012. [Epub ahead of print] [DOI] [PubMed]
- Aufderheide TP, Lurie KG. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32(9 Suppl):S345–S351. doi: 10.1097/01.ccm.0000134335.46859.09. [DOI] [PubMed] [Google Scholar]
- Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, Shapiro NI, Trzeciak S. Emergency Medicine Shock Research Network (EMShockNet) Investigators. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123:2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Bailey M, Eastwood GM, Nichol A, Pilcher D, Hart GK, Reade MC, Egi M, Cooper DJ. Study of Oxygen in Critical Care (SOCC) Group. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15:R90. doi: 10.1186/cc10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Stein D, Hu P, Kufera J, Wooford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147:1042–1046. doi: 10.1001/archsurg.2012.1560. [DOI] [PubMed] [Google Scholar]
- Martin M, Satterly S, Inaba K, Blair K. Does needle thoracostomy provide adequate and effective decompression of tension pneumothorax? J Trauma Acute Care Surg. 2012;73:1412–1417. doi: 10.1097/TA.0b013e31825ac511. [DOI] [PubMed] [Google Scholar]
- Leigh-Smith S, Harris T. Tension pneumothorax - time for a re-think? Emerg Med J. 2005;22:8–16. doi: 10.1136/emj.2003.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrie EP, Collum N, McGovern S. The right place in the right space? Awareness of site for needle thoracocentesis. Emerg Med J. 2005;22:788–789. doi: 10.1136/emj.2004.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Ives C, McClure K, Branco BC, Eckstein M, Shatz D, Martin MJ, Reddy S, Demetriades D. Radiologic evaluation of alternative sites for needle decompression of tension pneumothorax. Arch Surg. 2012;147:813–818. doi: 10.1001/archsurg.2012.751. [DOI] [PubMed] [Google Scholar]
- Huber-Wagner S, Lefering R, Qvick M, Kay MV, Paffrath T, Mutschler W, Kanz KG. Working Group on Polytrauma of the German Trauma Society (DGU) Outcome in 757 severely injured patients with traumatic cardiorespiratory arrest. Resuscitation. 2007;75:276–285. doi: 10.1016/j.resuscitation.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Baldt MM, Bankier AA, Germann PS, Pöschl GP, Skrbensky GT, Herold CJ. Complications after emergency tube thoracostomy: assessment with CT. Radiology. 1995;195:539–543. doi: 10.1148/radiology.195.2.7724780. [DOI] [PubMed] [Google Scholar]
- Maybauer MO, Geisser W, Wolff H, Maybauer DM. Incidence and outcome of tube thoracostomy positioning in trauma patients. Prehosp Emerg Care. 2012;16:237–241. doi: 10.3109/10903127.2011.615975. [DOI] [PubMed] [Google Scholar]
- Deakin CD, Davies G, Wilson A. Simple thoracostomy avoids chest drain insertion in prehospital trauma. J Trauma. 1995;39:373–374. doi: 10.1097/00005373-199508000-00031. [DOI] [PubMed] [Google Scholar]
- Massarutti D, Trillò G, Berlot G, Tomasini A, Bacer B, D'Orlando L, Viviani M, Rinaldi A, Babuin A, Burato L, Carchietti E. Simple thoracostomy in prehospital trauma management is safe and effective: a 2-year experience by helicopter emergency medical crews. Eur J Emerg Med. 2006;13:276–280. doi: 10.1097/00063110-200610000-00006. [DOI] [PubMed] [Google Scholar]
- Mistry N, Bleetman A, Roberts KJ. Chest decompression during the resuscitation of patients in prehospital traumatic cardiac arrest. Emerg Med J. 2009;26:738–740. doi: 10.1136/emj.2008.065599. [DOI] [PubMed] [Google Scholar]
- Beer RG, Grimmett WG, Fraser JF. Appraisal of the endotracheal tube as an alternative to the intercostal catheter. Emerg Med Australas. 2010;22:573–574. doi: 10.1111/j.1742-6723.2010.01359.x. [DOI] [PubMed] [Google Scholar]
- Gruen RL, Jurkovich GJ, McIntyre LK, Foy HM, Maier RV. Patterns of errors contributing to trauma mortality: lessons learned from 2,594 deaths. Ann Surg. 2006;244:371–380. doi: 10.1097/01.sla.0000234655.83517.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T, Thomas GO, Brohi K. Early fluid resuscitation in severe trauma. BMJ. 2012;345:e5752. doi: 10.1136/bmj.e5752. [DOI] [PubMed] [Google Scholar]
- Sambasivan CN, Schreiber MA. Emerging therapies in traumatic hemorrhage control. Curr Opin Crit Care. 2009;15:560–568. doi: 10.1097/MCC.0b013e328331f57c. [DOI] [PubMed] [Google Scholar]
- CRASH-2 Collaborators. Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, Perel P, Prieto-Merino D, Woolley T. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–1101. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- Davenport R, Curry N, Manson J, De'Ath H, Coates A, Rourke C, Pearse R, Stanworth S, Brohi K. Hemostatic effects of fresh frozen plasma may be maximal at red cell ratios of 1:2. J Trauma. 2011;70:90–95. doi: 10.1097/TA.0b013e318202e486. discussion 95-96. [DOI] [PubMed] [Google Scholar]
- Barkana Y, Stein M, Maor R, Lynn M, Eldad A. Prehospital blood transfusion in prolonged evacuation. J Trauma. 1999;46:176–180. doi: 10.1097/00005373-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Reades R, Studnek JR, Vandeventer S, Garrett J. Intraosseous versus intravenous vascular access during out-of-hospital cardiac arrest: a randomized controlled trial. Ann Emerg Med. 2011;58:509–516. doi: 10.1016/j.annemergmed.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Warren DW, Kissoon N, Sommerauer JF, Rieder MJ. Comparison of fluid infusion rates among peripheral intravenous and humerus, femur, malleolus, and tibial intraosseous sites in normovolemic and hypovolemic piglets. Ann Emerg Med. 1993;22:183–186. doi: 10.1016/S0196-0644(05)80199-4. [DOI] [PubMed] [Google Scholar]
- Cureton EL, Yeung LY, Kwan RO, Miraflor EJ, Sadjadi J, Price DD, Victorino GP. The heart of the matter: utility of ultrasound of cardiac activity during traumatic arrest. J Trauma Acute Care Surg. 2012;73:102–110. doi: 10.1097/TA.0b013e3182569ebc. [DOI] [PubMed] [Google Scholar]
- Aichinger G, Zechner PM, Prause G, Sacherer F, Wildner G, Anderson CL, Pocivalnik M, Wiesspeiner U, Fox JC. Cardiac movement identified on prehospital echocardiography predicts outcome in cardiac arrest patients. Prehosp Emerg Care. 2012;16:251–255. doi: 10.3109/10903127.2011.640414. [DOI] [PubMed] [Google Scholar]
- Ketelaars R, Hoogerwerf N, Scheffer GJ. Prehospital chest ultrasound by a Dutch helicopter emergency medical service. J Emerg Med. 2013. pii: S07364679(12)01417-5. [DOI] [PubMed]
- Heegaard W, Hildebrandt D, Spear D, Chason K, Nelson B, Ho J. Prehospital ultrasound by paramedics: results of field trial. Acad Emerg Med. 2010;17:624–630. doi: 10.1111/j.1553-2712.2010.00755.x. [DOI] [PubMed] [Google Scholar]
- Kim CH, Shin SD, Song KJ, Park CB. Diagnostic accuracy of focused assessment with sonography for trauma (FAST) examinations performed by emergency medical technicians. Prehosp Emerg Care. 2012;16:400–406. doi: 10.3109/10903127.2012.664242. [DOI] [PubMed] [Google Scholar]
- Price S, Uddin S, Quinn T. Echocardiography in cardiac arrest. Curr Opin Crit Care. 2010;16:211–215. doi: 10.1097/MCC.0b013e3283399d4c. [DOI] [PubMed] [Google Scholar]
- Tsung JW, Blaivas M. Feasibility of correlating the pulse check with focused point-of-care echocardiography during pediatric cardiac arrest: a case series. Resuscitation. 2008;77:264–269. doi: 10.1016/j.resuscitation.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Press GM, Miller S. Utility of the cardiac component of FAST in blunt trauma. J Emerg Med. 2013;44:9–16. doi: 10.1016/j.jemermed.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Mollberg NM, Glenn C, John J, Wise SR, Sullivan R, Vafa A, Snow NJ, Massad MG. Appropriate use of emergency department thoracotomy: implications for the thoracic surgeon. Ann Thorac Surg. 2011;92:455–461. doi: 10.1016/j.athoracsur.2011.04.042. [DOI] [PubMed] [Google Scholar]
- Moore EE, Knudson MM, Burlew CC, Inaba K, Dicker RA, Biffl WL, Malhotra AK, Schreiber MA, Browder TD, Coimbra R, Gonzalez EA, Meredith JW, Livingston DH, Kaups KL. WTA Study Group. Defining the limits of resuscitative emergency department thoracotomy: a contemporary Western Trauma Association perspective. J Trauma. 2011;70:334–339. doi: 10.1097/TA.0b013e3182077c35. [DOI] [PubMed] [Google Scholar]
- Rhee PM, Acosta J, Bridgeman A, Wang D, Jordan M, Rich N. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg. 2000;190:288–298. doi: 10.1016/S1072-7515(99)00233-1. [DOI] [PubMed] [Google Scholar]
- Wise D, Davies G, Coats T, Lockey D, Hyde J, Good A. Emergency thoracotomy: 'how to do it'. Emerg Med J. 2005;22:22–24. doi: 10.1136/emj.2003.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar M, Krizmaric M, Klemen P, Grmec S. Partial pressure of end-tidal carbon dioxide successful predicts cardiopulmonary resuscitation in the field: a prospective observational study. Crit Care. 2008;12:R115. doi: 10.1186/cc7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M, Hatch L, Malleck J, McClung C, Henderson SO. End-tidal CO2 as a predictor of survival in out-of-hospital cardiac arrest. Prehosp Disaster Med. 2011;26:148–150. doi: 10.1017/S1049023X11006376. [DOI] [PubMed] [Google Scholar]
- Heradstveit BE, Sunde K, Sunde GA, Wentzel-Larsen T, Heltne JK. Factors complicating interpretation of capnography during advanced life support in cardiac arrest - a clinical retrospective study in 575 patients. Resuscitation. 2012;83:813–818. doi: 10.1016/j.resuscitation.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Einav S, Kaufman N, Sela HY. Maternal cardiac arrest and perimortem caesarean delivery: evidence or expert-based? Resuscitation. 2012;83:1191–1200. doi: 10.1016/j.resuscitation.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Bloomer R, Reid C, Wheatley R. Prehospital resuscitative hysterotomy. Eur J Emerg Med. 2011;18:241–242. doi: 10.1097/MEJ.0b013e328344f2c5. [DOI] [PubMed] [Google Scholar]
- Hock Ong ME, Fook-Chong S, Annathurai A, Ang SH, Tiah L, Yong KL, Koh ZX, Yap S, Sultana P. Improved neurologically intact survival with the use of an automated, load-distributing band chest compression device for cardiac arrest presenting to the emergency department. Crit Care. 2012;16:R144. doi: 10.1186/cc11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna GK, Pavlin EG, Kirkman T, Copass MK, Rice CL. Hemodynamic effects of external cardiac massage in trauma shock. J Trauma. 1989;29:1430–1433. doi: 10.1097/00005373-198910000-00022. [DOI] [PubMed] [Google Scholar]
- Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: a randomised double-blind placebo-controlled trial. Resuscitation. 2011;82:1138–1143. doi: 10.1016/j.resuscitation.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Ristagno G, Tang W, Huang L, Fymat A, Chang YT, Sun S, Castillo C, Weil MH. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med. 2009;37:1408–1415. doi: 10.1097/CCM.0b013e31819cedc9. [DOI] [PubMed] [Google Scholar]
- Voelckel WG, Raedler C, Wenzel V, Lindner KH, Krismer AC, Schmittinger CA, Herff H, Rheinberger K, Königsrainer A. Arginine vasopressin, but not epinephrine, improves survival in uncontrolled hemorrhagic shock after liver trauma in pigs. Crit Care Med. 2003;31:1160–1165. doi: 10.1097/01.CCM.0000060014.75282.69. [DOI] [PubMed] [Google Scholar]
- Sperry JL, Minei JP, Frankel HL, West MA, Harbrecht BG, Moore EE, Maier RV, Nirula R. Early use of vasopressors after injury: caution before constriction. J Trauma. 2008;64:9–14. doi: 10.1097/TA.0b013e31815dd029. [DOI] [PubMed] [Google Scholar]
- Dawes R, Thomas GO. Battlefield resuscitation. Curr Opin Crit Care. 2009;15:527–535. doi: 10.1097/MCC.0b013e32833190c3. [DOI] [PubMed] [Google Scholar]
- Bicknell WH, Wall MJ, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- Doran CM, Doran CA, Woolley T, Carter A, Male K, Midwinter MJ, Mahoney PF, Watts S, Kirkman E. Targeted resuscitation improves coagulation and outcome. J Trauma Acute Care Surg. 2012;72:835–843. doi: 10.1097/TA.0b013e318248347b. [DOI] [PubMed] [Google Scholar]
- Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Syst Rev. 2009;2:CD001048. doi: 10.1002/14651858.CD001048.pub3. [DOI] [PubMed] [Google Scholar]
- Finkelstein RA, Alam HB. Induced hypothermia for trauma: current research and practice. J Intensive Care Med. 2010;25:205–226. doi: 10.1177/0885066610366919. [DOI] [PubMed] [Google Scholar]
- Watts DD, Trask A, Soeken K, Perdue P, Dols S, Kaufmann C. Hypothermic coagulopathy in trauma: effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma. 1998;44:846–854. doi: 10.1097/00005373-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Traumatic Cardiac Arrest. Helicopter Operating Procedure. http://nswhems.files.wordpress.com/2011/09/traumatic-cardiac-arrest-c-06.pdf