Abstract
Measurement of cardiac output (CO) using minimally invasive devices has gained popularity. In 11 patients we compared CO values obtained using the bioreactance technique - a new continuous, totally non-invasive CO monitor - with those obtained by semi-continuous thermodilution using a pulmonary artery catheter. We obtained CO measurements at study inclusion and after any relevant change in hemodynamic status (spontaneous or during fluid challenge, inotrope or vasopressor infusions). There was a poor correlation between the two techniques (r = 0.145). These data suggest that caution should be applied when using bioreactance devices in critically ill patients.
Measurement of cardiac output (CO) requires use of invasive or minimally invasive devices; the use of noninvasive and minimally invasive devices has gained popularity in recent years. The bioreactance technique is a relatively new, continuous, totally non-invasive technique for measuring CO that is easily implemented. This new technique involves analyzing phase shifts of a delivered oscillating current that occur when the current traverses the thoracic cavity [1], and differs from traditional bioimpedance techniques that rely on analysis of changes in signal amplitude. Most validation studies in critically ill patients have shown good correlation and/or agreement of bioreactance values compared with CO values obtained using other devices in patients admitted after cardiac surgery [2-4]. However, validation in critically ill patients is lacking.
As part of the internal evaluation of a bioreactance device before its implementation in the unit (evaluation of new non-invasive monitoring systems before introduction in the unit does not require the approval of the ethics committee in our institution), we compared CO values obtained using the bioreactance technique (NICOM system; Cheetah Medical Inc., Portland, OR, USA) with those measured using semi-continuous cardiac output by thermodilution (CCO) with a pulmonary artery catheter (Vigilance, Edwards LifeSciences, Irvine, CA, USA). In 11 patients the CO values were compared at study inclusion and each time a relevant change in hemodynamics and/or in therapeutics (for example, fluid challenge, inotrope or vasopressor infusions) was observed (Table 1).
Table 1.
Patient characteristics
| Characteristic | n |
|---|---|
| Patients | 11 |
| Cardiogenic shock | 3 |
| Septic shock/distributive shock | 4 |
| Acute respiratory distress syndrome | 4 |
| Therapies | |
| Norepinephrine | 7 (8, 2 to 20) |
| Dobutamine | 4 (5, 5 to 8) |
| Mechanical ventilation | 5 |
| Hemofiltration | 2 |
Data in parentheses represent maximal dose, range (μg/minute for norepinephrine and μg/kg.min for dobutamine).
We recorded bioreactance CO (average of five values over a 5-minute period) just after obtaining the pulmonary artery catheter CCO (average of five CCO values over a 5-minute period). We collected 141 pairs of measurements (3 to 23 per patient); the duration of monitoring was at least 3 hours but never exceeded 24 hours. The pulmonary artery catheter CCO values ranged from 3.9 to 11 l/minute. There was poor correlation between the two techniques (correlation coefficient r = 0.145) (Figure 1). To limit the time effect, we randomly selected one pair of measurements for each patient - but this did not improve the results (r = 0.13). Bland and Altman analysis with correction for multiple measurements showed wide limits of agreement (Figure 2). The time course of CO was not well tracked either, sometimes with opposite trends between the two devices. We therefore decided to stop the evaluation.
Figure 1.
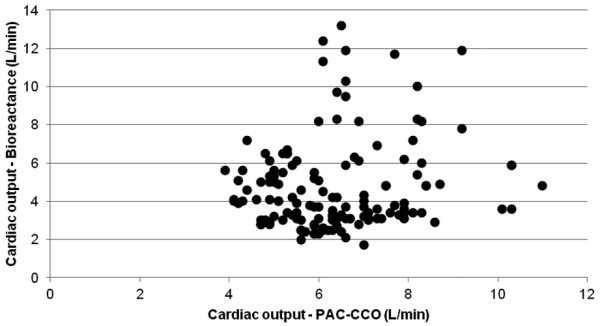
Correlation between pulmonary artery catheter semi-continuous cardiac output by thermodilution and bioreactance cardiac output. A total of 141 measurements in 11 patients, r = 0.1455. PAC-CCO, pulmonary artery catheter semi-continuous cardiac output by thermodilution.
Figure 2.
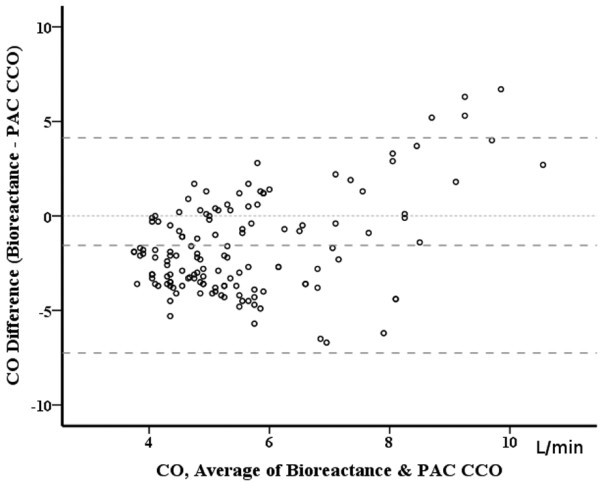
Pulmonary artery catheter semi-continuous cardiac output by thermodilution and bioreactance cardiac output: bias and agreement. A total of 141 pairs of measurements in 11 patients. Bias -1.6 L/min and limits of agreement 5.7 L/min. CO, cardiac output; PAC-CCO, pulmonary artery catheter semi-continuous cardiac output by thermodilution.
The bioreactance technique is dependent on diffusion of electrical current, so interstitial edema may interfere with measurements; we believe this is the most probable explanation for the poor correlation. Whatever the reason, these data suggest that caution should be applied when using bioreactance devices in critically ill patients.
Abbreviations
CCO: semi-continuous cardiac output by thermodilution; CO: cardiac output.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
David Fagnoul, Email: dfagnoul@ulb.ac.be.
Jean-Louis Vincent, Email: jlvincen@ulb.ac.be.
De Daniel Backer, Email: ddebacke@ulb.ac.be.
References
- Squara P, Denjean D, Estagnasie P, Brusset A, Dib JC, Dubois C. Noninvasive cardiac output monitoring (NICOM): a clinical validation. Intensive Care Med. 2007;16:1191–1194. doi: 10.1007/s00134-007-0640-0. [DOI] [PubMed] [Google Scholar]
- Marque S, Cariou A, Chiche JD, Squara P. Comparison between Flotrac-Vigileo and bioreactance, a totally noninvasive method for cardiac output monitoring. Crit Care. 2009;16:R73. doi: 10.1186/cc7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval NY, Squara P, Cleman M, Yalamanchili K, Winklmaier M, Burkhoff D. Multicenter evaluation of noninvasive cardiac output measurement by bioreactance technique. J Clin Monit Comput. 2008;16:113–119. doi: 10.1007/s10877-008-9112-5. [DOI] [PubMed] [Google Scholar]
- Benomar B, Ouattara A, Estagnasie P, Brusset A, Squara P. Fluid responsiveness predicted by noninvasive bioreactance-based passive leg raise test. Intensive Care Med. 2010;16:1875–1881. doi: 10.1007/s00134-010-1990-6. [DOI] [PubMed] [Google Scholar]


