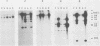Abstract
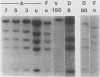
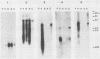
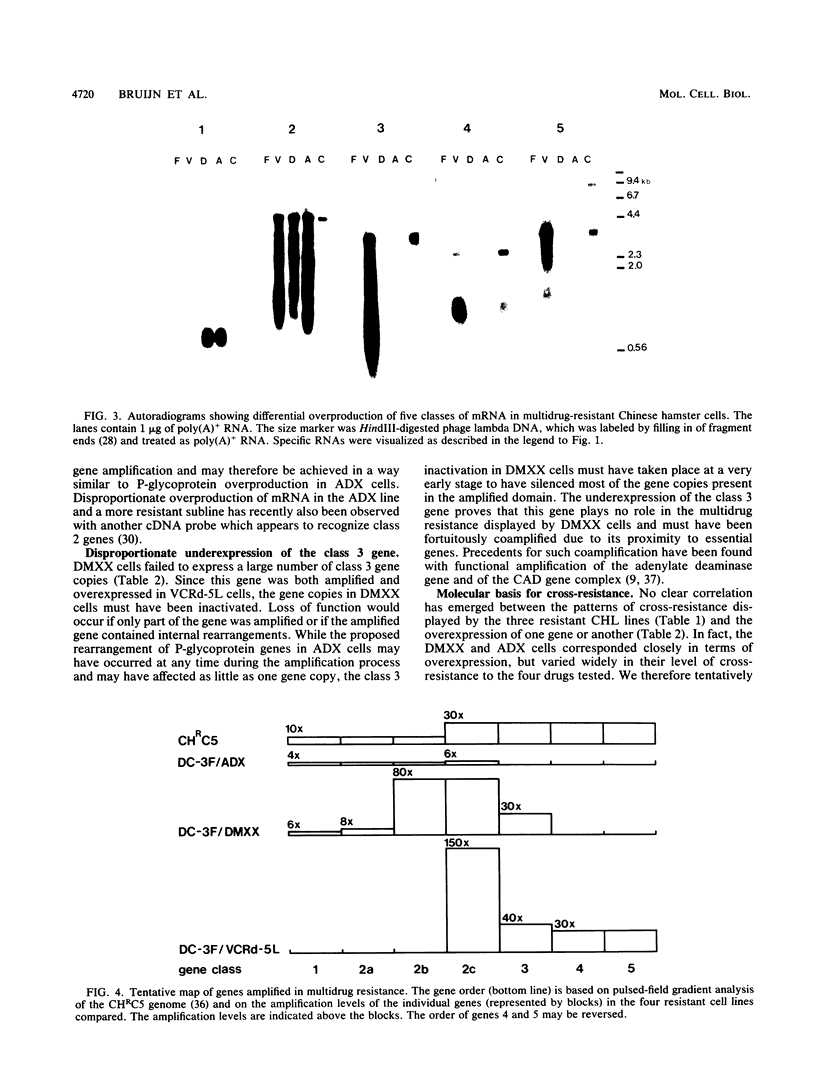
At least five linked genes are amplified in the multidrug-resistant Chinese hamster ovary cell line CHRC5, selected with colchicine (A. M. Van der Bliek, T. Van der Velde-Koerts, V. Ling, and P. Borst, Mol. Cell. Biol. 6:1671-1678, 1986). We report here that only a subset of these, encoding the 170-kilodalton P-glycoprotein, are consistently amplified in three different multidrug-resistant Chinese hamster lung cell lines, selected with vincristine, daunorubicin, or actinomycin D. Within each cell line, genomic sequences homologous to the P-glycoprotein cDNA probe were amplified to different levels. The pattern of differential amplification was consistent with the presence of at least two and possibly three P-glycoprotein genes. In the actinomycin D-selected cell line, these genes were disproportionately overexpressed relative to the associated levels of amplification. These results underline a central role for P-glycoprotein in multidrug resistance. In the daunorubicin-selected cell line, another, as yet uncharacterized, gene was amplified but disproportionately underexpressed. Its amplification was therefore fortuitous. We present a tentative map of the region in the hamster genome that is amplified in the multidrug-resistant cell lines which were analyzed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Chang T. D., Meyers M. B., Peterson R. H., Spengler B. A. Drug resistance in Chinese hamster lung and mouse tumor cells. Cancer Treat Rep. 1983 Oct;67(10):859–867. [PubMed] [Google Scholar]
- Biedler J. L., Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970 Apr;30(4):1174–1184. [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. Metaphase chromosome anomaly: association with drug resistance and cell-specific products. Science. 1976 Jan 16;191(4223):185–187. doi: 10.1126/science.942798. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P. DNA amplification and multidrug resistance. Nature. 1984 Jun 14;309(5969):580–580. doi: 10.1038/309580a0. [DOI] [PubMed] [Google Scholar]
- Carlsen S. A., Till J. E., Ling V. Modulation of membrane drug permeability in Chinese hamster ovary cells. Biochim Biophys Acta. 1976 Dec 14;455(3):900–912. doi: 10.1016/0005-2736(76)90059-6. [DOI] [PubMed] [Google Scholar]
- Debatisse M., de Saint Vincent B. R., Buttin G. Expression of several amplified genes in an adenylate-deaminase overproducing variant of Chinese hamster fibroblasts. EMBO J. 1984 Dec 20;3(13):3123–3127. doi: 10.1002/j.1460-2075.1984.tb02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Milbrandt J. D., Heintz N. H., Azizkhan J. C. DNA sequence amplification in mammalian cells. Int Rev Cytol. 1984;90:31–82. doi: 10.1016/s0074-7696(08)61487-4. [DOI] [PubMed] [Google Scholar]
- Inaba M., Fujikura R., Sakurai Y. Active efflux common to vincristine and daunorubicin in vincristine-resistant P388 leukemia. Biochem Pharmacol. 1981 Jul 1;30(13):1863–1865. doi: 10.1016/0006-2952(81)90027-7. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Martinsson T., Dahllöf B., Wettergren Y., Leffler H., Levan G. Pleiotropic drug resistance and gene amplification in a SEWA mouse tumor cell line. Complex relations revealed by drug uptake data, and lipid and protein analysis. Exp Cell Res. 1985 Jun;158(2):382–394. doi: 10.1016/0014-4827(85)90463-x. [DOI] [PubMed] [Google Scholar]
- Meyers M. B., Biedler J. L. Increased synthesis of a low molecular weight protein in vincristine-resistant cells. Biochem Biophys Res Commun. 1981 Mar 16;99(1):228–235. doi: 10.1016/0006-291x(81)91736-8. [DOI] [PubMed] [Google Scholar]
- Meyers M. B., Spengler B. A., Chang T. D., Melera P. W., Biedler J. L. Gene amplification-associated cytogenetic aberrations and protein changes in vincristine-resistant Chinese hamster, mouse, and human cells. J Cell Biol. 1985 Feb;100(2):588–597. doi: 10.1083/jcb.100.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. H., Meyers M. B., Spengler B. A., Biedler J. L. Alteration of plasma membrane glycopeptides and gangliosides of Chinese hamster cells accompanying development of resistance to daunorubicin and vincristine. Cancer Res. 1983 Jan;43(1):222–228. [PubMed] [Google Scholar]
- Richert N., Akiyama S., Shen D., Gottesman M. M., Pastan I. Multiply drug-resistant human KB carcinoma cells have decreased amounts of a 75-kDa and a 72-kDa glycoprotein. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2330–2333. doi: 10.1073/pnas.82.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehm H., Biedler J. L. Cellular resistance to daunomycin in Chinese hamster cells in vitro. Cancer Res. 1971 Apr;31(4):409–412. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985;28(1):51–75. doi: 10.1016/0163-7258(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Robertson S. M., Ling V., Stanners C. P. Co-amplification of double minute chromosomes, multiple drug resistance, and cell surface P-glycoprotein in DNA-mediated transformants of mouse cells. Mol Cell Biol. 1984 Mar;4(3):500–506. doi: 10.1128/mcb.4.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland P., 3rd, Pfeilsticker J., Hoffee P. A. Adenosine deaminase gene amplification in deoxycoformycin-resistant mammalian cells. Arch Biochem Biophys. 1985 Jun;239(2):396–403. doi: 10.1016/0003-9861(85)90705-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Scotto K. W., Biedler J. L., Melera P. W. Amplification and expression of genes associated with multidrug resistance in mammalian cells. Science. 1986 May 9;232(4751):751–755. doi: 10.1126/science.2421411. [DOI] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Su T. S., Bock H. G., O'Brien W. E., Beaudet A. L. Cloning of cDNA for argininosuccinate synthetase mRNA and study of enzyme overproduction in a human cell line. J Biol Chem. 1981 Nov 25;256(22):11826–11831. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Van der Velde-Koerts T., Ling V., Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1986 May;6(5):1671–1678. doi: 10.1128/mcb.6.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Vitto L., Rubnitz J. Co-amplification of rRNA genes with CAD genes in N-(phosphonacetyl)-L-aspartate-resistant Syrian hamster cells. Mol Cell Biol. 1983 Nov;3(11):2066–2075. doi: 10.1128/mcb.3.11.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]