Abstract
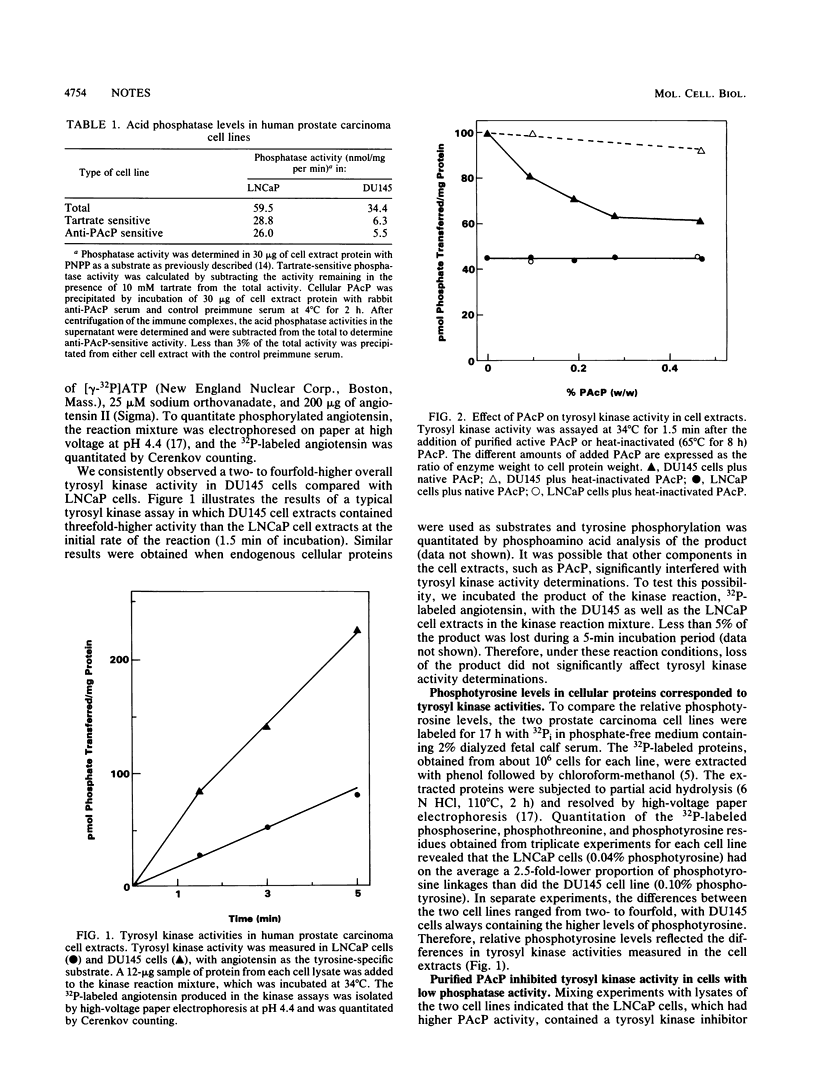
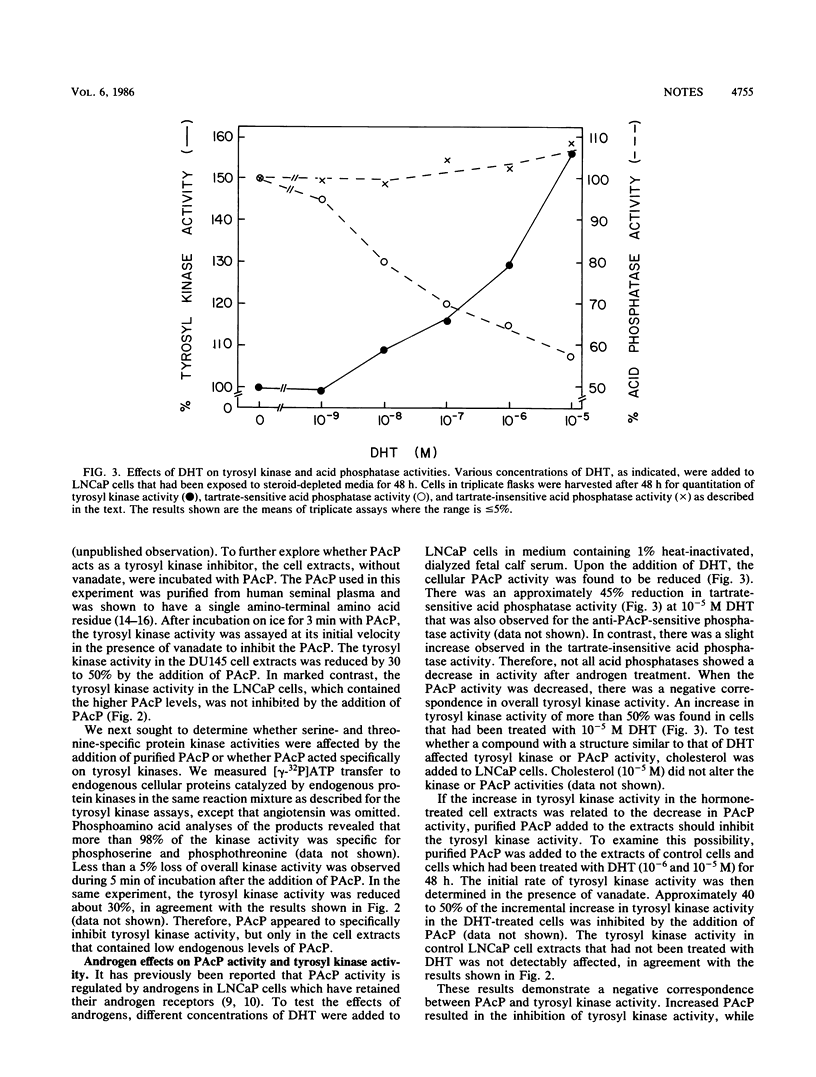
Alterations in prostatic acid phosphatase (PAcP), a phosphotyrosyl phosphatase, corresponded to changes in overall tyrosyl kinase activity. PAcP added to extracts of prostate carcinoma cells with a low endogenous level of PAcP activity and elevated tyrosyl kinase activity decreased the tyrosyl kinase activity. On the other hand, when PAcP activity was decreased by the addition of androgens to cells, there was a corresponding increase in tyrosyl kinase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Brown D. J., Gordon J. A. The stimulation of pp60v-src kinase activity by vanadate in intact cells accompanies a new phosphorylation state of the enzyme. J Biol Chem. 1984 Aug 10;259(15):9580–9586. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes J. G. Phosphotyrosyl-protein phosphatases. Curr Top Microbiol Immunol. 1983;107:163–180. doi: 10.1007/978-3-642-69075-4_5. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Chu T. M., Wajsman Z. L., Friedman M., Papsidero L., Kim U., Chai L. S., Kakati S., Arya S. K. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Kawinski E., Karr J. P., Rosenthal H., Chu T. M., Mirand E. A., Murphy G. P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983 Apr;43(4):1809–1818. [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Lam K. W., Li C. Y., Yam L. T., Smith R. S., Hacker B. Comparison of prostatic and nonprostatic acid phosphatase. Ann N Y Acad Sci. 1982;390:1–15. doi: 10.1111/j.1749-6632.1982.tb40300.x. [DOI] [PubMed] [Google Scholar]
- Li H. C., Chernoff J., Chen L. B., Kirschonbaum A. A phosphotyrosyl-protein phosphatase activity associated with acid phosphatase from human prostate gland. Eur J Biochem. 1984 Jan 2;138(1):45–51. doi: 10.1111/j.1432-1033.1984.tb07879.x. [DOI] [PubMed] [Google Scholar]
- Lin M. F., Clinton G. M. Human prostatic acid phosphatase has phosphotyrosyl protein phosphatase activity. Biochem J. 1986 Apr 15;235(2):351–357. doi: 10.1042/bj2350351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. F., Lee C. L., Li S. S., Chu T. M. Purification and characterization of a new human prostatic acid phosphatase isoenzyme. Biochemistry. 1983 Mar 1;22(5):1055–1062. doi: 10.1021/bi00274a009. [DOI] [PubMed] [Google Scholar]
- Lin M. F., Lee C. L., Sharief F. S., Li S. S., Chu T. M. Glycoprotein exhibiting immunological and enzymatic activities of human prostatic acid phosphatase. Cancer Res. 1983 Aug;43(8):3841–3846. [PubMed] [Google Scholar]
- Lin M. F., Lee P. L., Clinton G. M. Characterization of tyrosyl kinase activity in human serum. J Biol Chem. 1985 Feb 10;260(3):1582–1587. [PubMed] [Google Scholar]
- Loor R., Wang M. C., Valenzuela L., Chu T. M. Expression of prostatic acid phosphatase in human prostate cancer. Cancer Lett. 1981 Oct;14(1):63–69. doi: 10.1016/0304-3835(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Nelson R. L., Branton P. E. Identification, purification, and characterization of phosphotyrosine-specific protein phosphatases from cultured chicken embryo fibroblasts. Mol Cell Biol. 1984 Jun;4(6):1003–1012. doi: 10.1128/mcb.4.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Wells S. K., Collett M. S. Increase in the phosphotransferase specific activity of purified Rous sarcoma virus pp60v-src protein after incubation with ATP plus Mg2+. Mol Cell Biol. 1983 Sep;3(9):1589–1597. doi: 10.1128/mcb.3.9.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Shriner C. L., Brautigan D. L. Cytosolic protein phosphotyrosine phosphatases from rabbit kidney. Purification of two distinct enzymes that bind to Zn2+-iminodiacetate agarose. J Biol Chem. 1984 Sep 25;259(18):11383–11390. [PubMed] [Google Scholar]
- Sparks J. W., Brautigan D. L. Specificity of protein phosphotyrosine phosphatases. Comparison with mammalian alkaline phosphatase using polypeptide substrates. J Biol Chem. 1985 Feb 25;260(4):2042–2045. [PubMed] [Google Scholar]
- Stone K. R., Mickey D. D., Wunderli H., Mickey G. H., Paulson D. F. Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer. 1978 Mar 15;21(3):274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- Weinmaster G., Zoller M. J., Smith M., Hinze E., Pawson T. Mutagenesis of Fujinami sarcoma virus: evidence that tyrosine phosphorylation of P130gag-fps modulates its biological activity. Cell. 1984 Jun;37(2):559–568. doi: 10.1016/0092-8674(84)90386-6. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. In vitro phosphorylation of angiotensin analogs by tyrosyl protein kinases. J Biol Chem. 1983 Jan 25;258(2):1022–1025. [PubMed] [Google Scholar]
- Yam L. T. Clinical significance of the human acid phosphatases: a review. Am J Med. 1974 May;56(5):604–616. doi: 10.1016/0002-9343(74)90630-5. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]


